Abstract
Gastric cancer, a disease of disparity associated with Helicobacter pylori (H. pylori) infection, is the world's second leading cause of cancer deaths. The pathogen H. pylori target the epithelial adhesion receptors, E-cadherin and β1-integrin, to modulate the host cytoskeleton via disruption of the epithelial cell polarity necessary for maintaining the infection, but how this leads to the development of the carcinoma is widely unclear. While Rho family GTPases’ signaling to the cytoskeleton and these receptors is required for initiating and maintaining the infection, the responsible effectors, and how they might influence the etiology of the carcinomas are currently unknown. Here we discuss the potential role of the Cdc42-IQGAP1 axis, a negative regulator of the tumor suppressors E-cadherin and β1-integrin, as a potential driver of H. pylori-induced gastric carcinoma and propose avenues for addressing its disparity. Chronic dysfunction of the IQGAP1-signaling pathway, resulting from H. pylori-induced disruption of cell polarity, can explain the pathogenesis of the carcinoma, at least, in subsets of infected population, and thus could provide a potential means for personalized medicine.
Keywords: IQGAP, Gastric cancer, Cdc42, H. Pylori, Akt, mTOR, Erk1/2
Development and disparity of gastric carcinomas
Gastric carcinomas (hereon referred to as GC) are malignant tumors that arise from hyperproliferation of the stomach epithelial cells in Helicobacter pylori (H. Pylori)-infected patients, and are accompanied by hypochlorhydria (low-acid secretion), and atrophic gastritis, which is believed to be the precursor of the carcinoma [Fox et al., 2006]. The ulcerogenic bacterium H. pylori, which infect about half of the world’s population, is a gram-negative spiral flagellated bacteria designated by the World Health Organization as a class-I carcinogen. Epithelial cells are differentiated in a distinctive apical-basal axis of polarity required for polarized secretion, and loss of this polarity represents the first hallmark feature of oncogenic transformation leading to carcinoma [Tanos and Rodriguz-Boulan, 2008, Nelson, 2009]. Level of epithelial cell differentiation distinguishes the two major anatomic types of gastric carcinoma; the tubular/intestinal and the diffuse [Lauren, 1965]. Unlike the intestinal-type, the diffuse-type is characterized by poorly differentiated (polarized) cells and has potential for high metastasis, rapid disease progression, and poor prognosis. Not only that the molecular basis of this difference is poorly defined, but also the basis of tumorigenesis remains unknown. While there has been a worldwide decline in the incidence of the intestinal-type, there has been a rise in the incidence of the diffuse-type, which is mainly found in children [Fox et al., 2006, Bauer and Meyer, 2011]. Remarkably, whereas ~50% of the world population is infected, only about 5% of this group develop GC and account for the second leading cause of cancer deaths worldwide [Fox et al., 2006, Bauer and Meyer, 2011], thus presenting GC as a prototype of cancer disparity.
Intriguingly, GC afflicts twice as many males as females worldwide, and it burdens poorer populations and ethnic minorities in the United States [Ward et al., 2004, Fox et al., 2006]. Thus, the disparity in GC incidence and mortality has been attributed to variable factors, including race, gender, age, individual behavior, socio-economical status, cultural beliefs, health care systems, poor nutrition, obesity and tobacco use [Ward, et al., 2004, Kamangar, et al., 2006, Wiredu and Armah, 2006]. The discrepancy between the rates of high-infection and low incidence of carcinoma in Africa inspired an unrealized pursuit of the mechanism of the “African enigma” [Fox et al., 2006], and led to the proposition of a protective value of H. pylori infection to some indigenous diseases [Bauer and Meyer, 2011]. The important caveats, however, are the lack of accurate population statistics, limited cancer registry and diagnostics, which could also explain the large regional variation of GC incidence in Sub-Saharan Africa [Ferlay, et al., 2010, Buffart, et al. 2011].
Another factor imparting on this disparity is that H. pylori display a remarkable allelic and intraspecies diversity even in the same patient at different times [in Fox et al., 2006]. Similarly, epigenetic modifications of the bacterial proteins that mediate the infection vary according to race and geographic regions. One such example is the differential phosphorylation of the putative oncogenic EPIYA (Glu-Pro-Ile-Tyr-Ala) motifs of the H. pylori virulent factor, cytotoxin-associated gene A (CagA) [Fox et al., 2006, Bauer and Meyer, 2011]. Genetic variations in patients also exhibit ethnic and regional differences that reflect on susceptibility to infection and clinical outcome [Fox et al., 2006, Buffart et al., 2011]. Add to this the effect of rising antibiotic resistance, these factors clearly represent a challenge for understanding the etiology of the disease and a barrier for effective diagnosis and treatment.
Accordingly, it has been suggested that environment-host genetic interactions factor strongly in the pathogenesis of the carcinoma [Fox et al., 2006, Bauer and Meyer, 2011]. This type of interaction typically involves signaling pathways responsive to extracellular cues. Dysfunction of such pathways can potentially help explain the etiology and the high disparity of GC, and the observation why it develops in only 5–10% of the infected population after a long duration of infection. Below, we discuss and argue the role of H. pylori infection as a primer by disrupting cell polarity, that alone may be insufficient for tumorigenesis, but that consequent chronic dysfunction of upstream signaling pathways that integrate the machineries of host cell polarity and homeostasis, is the real culprit.
The H. pylori CagA as a catalyst, not a direct cause, of GC etiology
It is still unclear whether H. pylori promote GC by direct epithelial invasion, by injection of its presumptive oncoprotein CagA, or by some combination of the two. Although the bacteria are not invasive and they hide in the mucus layer to avoid the stomach acidity, about 10% adhere to cells and a few have been observed intracellularly [Fox et al., 2006], but the significance of these to GC etiology remains unknown. One line of evidence implicates CagA as an oncoprotein in promoting human GC. CagA is a 120–145kDa H. pylori-unique effector protein encoded on the 40kb cag pathogenicity island, which also encodes the specialized type IV secretion system (T4SS). CagA is injected into the host gastric epithelial cells via the T4SS, leading to the disruption of epithelial differentiation, defined by loss of the apico-basal polarity and cell-cell adhesion [Bagnoli et al., 2005].
The cascade of molecular events presumed to lead to tumorigenesis has been extensively reviewed [Fox et al., 2006, Bauer and Meyer, 2011, Wessler et al, 2011], and is described as to begin with T4SS forming a needle-like membrane protrusion carrying CagA into the host stomach epithelia [Kwok et al., 2007, Kaplan et al., 2012]. CagA utilizes the α5β1 integrin as receptors to enter into the cells by interacting with membrane phosphatidylserine [Murata-Kamiya et al., 2010]. Following internalization, CagA undergoes tyrosine phosphorylation by nonreceptor tyrosine kinases, first by Src then by Abl [Tammer, et al., 2007, Mueller et al., 2012]. Whereas the interplay and specifics of Src and Abl phosphorylation of CagA are unknown, CagA-Abl interaction appears to impede the endocytosis-mediated downregulation of the epidermal growth factor receptor, EGFR [Bauer et al., 2009], apparently to attenuate acid secretion in the stomach. Indeed, the EGFR ligand EGF is a known inhibitor of acid secretion in gastric epithelia [Lewin et al, 1999]. Constitutive EGFR activity, a known oncogenic factor, would not only attenuate acid secretion, but also would predispose to oncogenic transformation, thus, instead, supporting deregulation of host signaling as a direct cause of carcinogenesis. Moreover, association of CagA with PAR1, a member of the PAR polarity complex, appears to disrupt epithelial cell polarity, favoring a CagA-SHP-2 (tyrosine phosphatase) interaction, which activates the host extracellular signal-regulated kinases (ERK1/2) and promotes an Epithelial-Mesenchymal Transition (EMT)-like phenotype marked by considerable actin polymerization and cellular elongation [Bauer and Meyer, 2011]. Thus, it appears that CagA mimics host adaptor proteins [Schneider et al., 2008] and hijacks signaling pathways for maintaining a nonpolarized phenotype with attenuated acid secretion favorable for bacterial survival.
Indeed, several lines of evidence argue against the CagA-mediated process being the direct mechanism of tumorigenesis. First, CagA has also been detected on the surface of vesicles secreted by H. pylori [Olofsson, et al., 2010]. Although the role of microvesicles and exosomes in tumorigenesis has been established, they also play a more general role in cell-cell communication and signaling. Second, the adhesion integrins are located on the inaccessible basolateral side of epithelia. To gain access, H. pylori disrupt epithelial adherens junctions (AJs) complexes, in a CagA-independent manner, by displacing E-cadherin, and thus releasing the oncogenic β-catenin via Akt-dependent inactivation of the β-catenin inhibitor, GSK3β [Murata-Kamiya, et al., 2007, Sokolova, et al., 2008]. To cleave E-cadherin, H. pylori utilize its high-temperature requirement A (HtrA), a chaperone and serine protease important for stress response and protein quality control, and thus it access the epithelial intercellular space [Hoy et al., 2010, 2012]. Disruption of the epithelial apical junctional complex and formation of ectopic complexes at the site of the bacterial attachment has also been reported to occur via CagA interaction with the junctional molecule ZO1[Bauer and Meyer, 2011]. Whereas the significance of this to tumorigenesis is unknown, it points to the dynamic interaction between apical and basolateral proteins in maintaining epithelial polarity and secretion, which are the main target of H. pylori. Third, CagA interacts in both a phosphorylation-dependent and -independent manner with a wide range of host signaling proteins [Bauer and Meyer, 2011, Wessler et al, 2011], so it may play a more pleiotropic role to maintain the non-polarized state of the host cell. Indeed, the functional outcome of the vast majority of the CagA-binding partners input into the induction of the scatter, non-polarized, phenotype [Wessler et al, 2011]. Fourth, whereas the drastic EMT-like shape of host cells depends on CagA, H. pylori-induced cell motility is largely CagA-independent [Wessler et al, 2011], predicting that host-mediated mechanisms in motility and invasive growth are ultimately responsible for cancer development and progression. Importantly, strains lacking CagA, have been found in patients with gastric cancer, and are capable of promoting transformation in cell culture [Fox, et al., 2006, Wessler et al, 2011]. Interestingly, the bacteria are undetectable by the onset of gastric carcinoma, thus supporting the notion that H. pylori infection alone is insufficient for preneoplasia [Fox, et al., 2006] and perhaps explaining the discrepancy whereby nearly half of the world population is infected while only about 5% develop carcinoma. Therefore, to date, the mechanisms by which CagA might lead to carcinoma are still being debated and remain elusive [Fox, et al., 2006, Wessler et al, 2011, Bauer and Meyer, 2011].
It would appear that H. pylori, in order to access nutrients and control the pH in its environment, utilize two antagonistic mechanisms [Bauer and Meyer, 2011]. First, it employs CagA to reduce acid secretion from host epithelia by disrupting cell polarity, which predisposes to tumorigenesis [Tanos and Rodriguz-Boulan, 2008]. Second, it uses its vacuolating cytotoxin A (VacA) to induce apoptosis in gut epithelia, perhaps to release nutrients or to kill immune cells and locally inhibit host immunity [Fox et al., 2006, Bauer and Meyer, 2011]. It would appear that maintaining this balance-- between loss of epithelial cell polarity and apoptosis -- serves the requirement of H. pylori’s survival and chronic colonization of the stomach. However in ~5% of infected cases, imbalance of this process leads to tumorigenesis, and the consequent demise of H. pylori, which may explain the reported clearance of the bacteria from gastric adenoma. Alternatively, the small number of infected individuals who develop GC seems reminiscent of the small incidence of internalized bacteria [Fox et al., 2006], and could provide an explanation for the development of carcinoma in that population. Thus, while the mechanisms of H. pylori infection are rapidly unfolding, it is still unclear whether neoplasia arises from activity of the internalized CagA or whole bacteria.
Analysis of genetic changes in GC tumors has not produced definitively responsible genetic lesions beside those explaining deregulated tumor genomes, like p53 suppression, and DNA aneuploidy, or upregulated tumor metabolism and vascularization, like those linked to Cox-2 and VEGF overexpression [Fox et al., 2006]. However, suppression of the adenomatous polyposis coli (APC) protein in 50% of cases, upregulation of β-catenin in 25% of cases [Fox et al., 2006], as well as upregulation of Akt and consequent inhibition of GSK3β [Murata-Kamiya, et al., 2007, Sokolova, et al., 2008], support the concept that H. pylori-induced chronic deregulation of host cell signaling networks ultimately accounts for neoplastic transformation. Specifically, because these are downstream events controlled by IQGAP1-signaling [Tekletsadik, et al., 2012], it appears attractive to propose that IQGAP1, which also controls many aspects of epithelial cell adhesion and polarity such as actin filament dynamics, microtubule function, and secretion and proliferation, has a major role both in mediating the infection and in driving the GC pathogenesis, which is discussed below.
Potential role of IQGAP1-mTORC1-Akt-signal as a driver of gastric carcinoma
Human IQGAP1 is one of a three-member (IQGAP1–3) family of widely conserved proteins with considerable sequence homology and structural similarities, but they differ in tissue distribution and cellular functions [Mateer et al, 2003, Osman 2010, Malarkannan et al., 2012]. The modular structure of IQGAP1 and signaling as a phosphorylation-sensitive conformational-switch, positions it as an integrator of signaling pathways involved in cell polarity, growth and proliferation (Fig. 1). Given that the hallmarks associated with H. pylori infection depend on the activation of Rho GTPases [Watanabe et al., 2009], the Cdc42-IQGAP1-signaling axis must play a major role. Involvement of IQGAP1 can provide mechanistic explanations to nearly all aspects of the H. pylori-induced effects on epithelial polarity (Fig. 2) such as the dissociation of junctional complexes, including AJ and tight junctions (TJ), leading to the distinctive cell elongation morphology, and the associated alterations in actin polymerization. It can also explain development of the hypochlorhydria, and associated atrophy, and chronic dysregulation of IQGAP1-mTORC1-Akt-MAPK signaling would explain the onset of gastric neoplasia, as discussed in the following three sections.
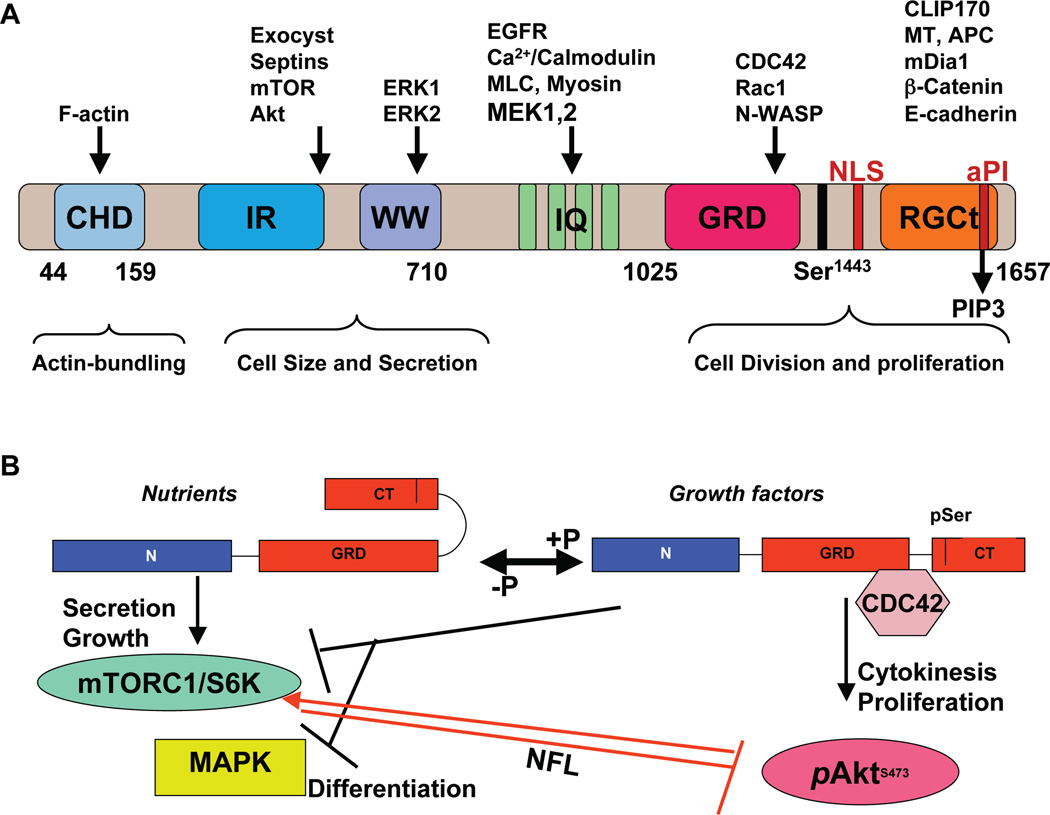
Figure 1. Model of IQGAP1 as a phosphorylation-sensitive molecular rheostat of mTORC-Akt and MAPK signaling controling epithelial homeostasis.
(A) Schematic of IQGAP1 domain structure, relevant binding partners, and cellular functional The amino acids encompassing the domains are indicated beneath the structure. CHD; calponin homology domain; IR-WW: IQGAP1-repeats (IR) and the tryptophan (WW) repeats; IQ: four isoleucine and glutamine rich motifs; GRD: Ras GTPase-activating protein-related domain; RGCT: RasGAP-C terminus (RGCT) domain; the critical Ser-1443 is indicated; NLS: nuclear localization signal; aPI: C2/PH-like domain, which binds PIP3. (B) IQGAP1 modulates mTORC1/S6K1-Akt and MAPK signaling in a phosphorylation-dependent manner. In presence of nutrients, dephosphorylated IQGAP1 has an autoinhibited form by folding of the Cterminal domain, thus masking the Cdc42-binding site to bind mTORC1 and promote secretion and cell growth [Rittmeyer etal., 2008, Wang et al., 2009]. In response to growth factors, phosphorylated pIQGAP1S1443 has an open form that binds Cdc42-GTP and attenuates (black lines) MAPK (Erk1/2) signaling and the mTORC1/S6K negative feedback loop (NFL, red line) inhibition of Akt, thus elevating pAktS473 signal and promoting cell proliferation, while suppressing cell differentiation, and inhibiting secretion [Wang et al., 2009, Tekletsadik et al., 212]. Inability of IQGAP1 to cycle between the two phosphoforms deregulates cell homeostasis leading to transformed phenotypes and neoplasia, if the pathway is tilted to the right, as in cells expressing the phosphomimetic mutant IQGAP1S1443E or cytokinesis arrest and apoptosis, if the pathway is tilted to the left, as in cells expressing IQGAP1IR-WW or the phosphodefective mutant IQGAP1S1443A [Wang et al., 2009, Tekletsadik, et al., 212]
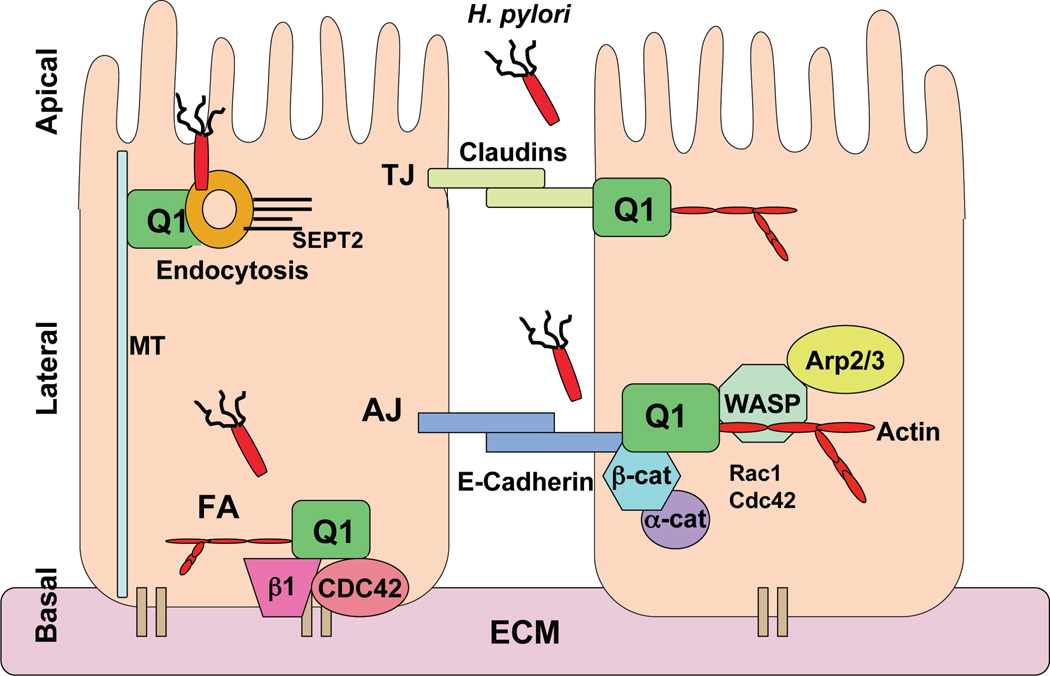
Figure 2. IQGAP1 as a potential target of H. pylori in disrupting epithelial polarity.
Schematic representation of polarized epithelia and the role of IQGAP1 in organizing tight junctions (TJ), which separate the apical and basal-lateral membrane, adheren junctions (AJ), which facilitate communication with adjacent cells and the focal adhesion (FA) complex, which facilitate interactions with the extracellular basement membrane (ECM). E-Cadherin is a transmembrane glycoprotein with an extracellular domain that mediates cell-cell adhesion and a cytoplasmic tails that directly binds β-catenin, which serves as a scaffold to anchor α-catenin and actin polymerization machinery (e.g. N-WASP and Arp2/3). IQGAP1 interplays with Rac1 and Cdc42 to regulate this complex and facilitate actin dynamics, thus regulating AJ formation and epithelial polarity. IQGAP1 also binds MT and septins, and regulates endocytic cup formation (endocytosis). These four IQGAP1-based cellular structures provide potential gateways for the entry of H.pylori (red rod with flagella) directly or via its effector protein CagA, into gut epithelia, leading to disruption of cell polarity and deregulation of IQGAP1-signaling, which may ultimately lead to gastric carcinoma.
1-Potential role for IQGAP1 in H. pylori-induced loss of cell polarity
The H. pylori-infected stomach tissues display dramatic morphology and changes in the cytoskeleton, but understanding the molecular basis of this requires the recapitulation of in vitro studies in human tissues, which to our knowledge has not yet been done. Also, H. pylori manipulate the host actin cytoskeleton and adhesion complexes to induce an EMT-like morphology, which could promote cell migration and invasion in tumorigenesis [Wessler et al, 2011]. However, recent evidence supports the notion that H. pylori prevents cell migration by subverting the dynamics of focal adhesions (FAs) [Tsutsumi et al., 2006, Schneider, et al., 2008], clearly to maintain non-polarized, but immotile, phenotype with reduced acid secretion, as a refuge, in which targeting IQGAP1 function can play a major role.
IQGAP1 resides upstream of the signaling cascade that regulates cell adhesion and involves the Rho GTPases Cdc42 and Rac1, AJ and TJ molecules, MAPK, and the extracellular matrix (ECM)-integrin system [Mateer et al., 2003] that is hijacked by H. pylori for sustaining infection. First, consistent with AJs being specific targets of H. pylori, the infection increases IQGAP1 transcript level and leads to the translocation of E-cadherin and IQGAP1 from the AJ membranes to intracellular tubular vesicles/structures [Conlin et al., 2004]. The nature of these vesicular structures is unknown, but it may provide a likely mechanism for cell scattering and induction of the elongated phenotype, and/or denotes changes in IQGAP1-signal. Increasing IQGAP1 expression level enhances IQGAP1’s serine phosphorylation and binding to activated Cdc42-GTP [Rittmeyer et al., 2008] and promotes cell migration and invasion [Wang et al., 2009]. Activated IQGAP1-Cdc42 dissociates AJ by delocalizing α-catenin from the E-cadherin-α-catenin-β-catenin complex, leading to the translocation of β-catenin to the nucleus and the initiation of oncogenic transcriptional events that sustain cell scattering, increased migration and invasion [reviewed in Noritake, et al., 2005], and the induction of transformed phenotypes [Wang et al., 2009, Tekletsadik et al., 2012]. Genetic and pharmacologic studies, using dominant-negative and -active mutants, and GTPase-specific inhibitors, identified Rac1, but not RhoA or Cdc42 as crucial components leading to H. pylori-induced cell elongation [Brandt, et al., 2007a]. IQGAP1, being a major regulator of epithelial adhesion, interacts with Rac1 or Cdc42 to regulate AJ negatively through dissociation of the E-cadherin-α/β-catenin complex [Noritake, et al., 2005], and positively through interactions with nectins [Katata et al., 2003], which are adhesion molecules involved in TJ and AJ formation [Sakisaka et al., 2007].
Second, in order to disrupt epithelial polarity and invade the inter-epithelial space, H. pylori also manipulate the FA complexes. The integrin-based FA, composed of the α and β heterodimers, bridges the actin cytoskeleton with the ECM and mediates dynamic cell motility [Schneider, et al., 2008, Horwitz, 2012]. Internalization of CagA entails direct interaction with β1 integrin [Kaplan et al 2012]. Thereafter, CagA-SHP-2 specifically dephosphorylates the FA kinase (FAK) leading to the disruption of FA-ECM and cell polarity, and thus producing the characteristic elongated shape [Tsutsumi et al., 2006] due to failure of cells to release their back end while attempting motility in vitro [Schneider, et al., 2008]. As this process likely occurs in vivo, it is tempting to speculate that while it is in the survival interest of H. pylori to disrupt cell polarity by dissociating AJ, it must inactivate the FAs’ dynamics to block cell motility in vivo and sustain infection by residing in the intercellular space of immotile cells. While the H. pylori may create localized disjunction of FAs from actin, it must manipulate the actin cytoskeleton to maintain host cell integrity for sustained infection.
Polarized epithelial cells maintain a dynamic interdependence among the cytoskeleton, AJs and the cell matrix FAs. Actin filament dynamics in the junctional cytoskeleton regulate cadherin junctions and perturbation of either one disrupts the other, and consequently cell and tissue morphogenesis [Ratheesh and Yap, 2012]. E-cadherin binds the F-actin nucleation machinery composed of N-WASP, Arp2/3 and cortactin [Ratheesh and Yap, 2012], and this entire machinery couples to the FA proteins, creating a dynamic cross-talk essential for maintaining cell-cell junctions [Quadri, 2012, Ratheesh and Yap, 2012]. One of the molecules mediating such cross talk is vinculin, an actin-binding protein that associates with both the cadherin complex and the integrins FAs [Ziegler et al., 2006]. Thus, the assumption that disruption of FA dynamics alone accounts for the characteristic elongated shape of pylori-infected epithelia may require further evaluation. Indeed, CagA-mediated vinculin dephosphorylation disturbs the interaction with and recruitment of Arp2/3 complex, resulting in a reduction of FA complexes [Mose et al., 2006]. Moreover, in response to H. pylori infection, activation of MAPK via Rap1 GTPases [Wessler et al, 2011] or protein kinases C (PKCs) [Brandt et al., 2009] leads to ERK1/2-mediated activation of cortactin, which is also a substrate for FAK tyrosine kinase. Active (serine-phosphorylated) cortactin binds and sequesters FAK, leading to dissociation of FAs, and thus contributing to cell elongation [Tegtmeyer et al., 2011]. Therefore, in all likelihood, these findings represent the tip of the iceberg as to the overall number of signaling molecules co-opted by CagA to manipulate the host cell for the bacterial continued survival.
Indeed these molecules are regulated by the interplay of Cdc42 and its regulator-effector IQGAP1. Furthermore, IQGAP1 serves as a regulatory scaffold for ERK1/2 [Tekletsadik et al., 2012], binds Rap1 [Awasthi et al., 2010, Malarkannan et al., 2012] and is regulated by PKC-mediated phosphorylation [Grohmanova et al., 2004]. IQGAP1, being termed a master regulator of actin dynamics, serves as a scaffold regulator for both the E-cadherin-catenin junctional complex and the actin bundling and nucleation machinery composed of N-WASP, Arp2/3 complex and the formin mDia [Mateer et al, 2002, 2003, Bensenor et al, 2007, Le Clainche et al., 2007, Brandt and Grosse, 2007]. It modulates cell migration and invasion in a Cdc42-dependant manner [Wang et al., 2009], and Cdc42 regulates cell adhesion by driving the expression of the adhesion receptor β1 integrin, implicated in metastasis [Reymond et al 2012] and as a crucial gateway for H. pylori CagA entry into the host cytoplasm.
Although the observed H. pylori internalization into cells [Fox et al., 2006] has received little attention, and thus the mechanism is unknown, IQGAP1-septin can provide a gateway for bacterial entry, as well as a substrate for CagA-disruption of cell polarity (Fig. 2). Septins (SEPT1–14) comprise an evolutionarily conserved family of GTPases, which form filamentous heteropolymers [Kinoshita, 2003, Sirajuddin et al. 2007]. They are considered novel cytoskeletal scaffolds that interact with membranes, actin filaments, and microtubules (MT) for assembling and activating components of cell signaling. Recent evidence implicates SEPT2 in bacterial invasion via a process dependent on PI3K-Akt activation [Mostowy et al., 2012]. IQGAP1 interacts with SEPT2 in different epithelial cell lines to influence exocytosis and cell shape [Rittmeyer et al., 2008]. SEPT2 interaction with MT is required for the biogenesis of the polarized columnar-shaped epithelia and its depletion causes fibroblast-shaped epithelia [Spiliotis et al., 2008], perhaps reminiscent of the H. pylori-induced shape. Given IQGAP1’s interactions with actin, MT, septins and Akt, an investigation into its role as a major player in H. pylori-mediated disruption of gut epithelial polarity is now warranted. This view is further encouraged by a recent study showing that IQGAP1 mediates β-catenin dissociation from AJs to promote actin polymerization required for E. coli K1 invasion, leading to brain oedema in neonatal meningitis [Krishnan et al., 2012].
Importantly, IQGAP1-mediated phagocytosis (Fig. 2) can explain the paradox of invasion when the host cell lacks receptors or the pathogen lacks the ligand. Phagocytosis constitutes an alternative route for invasion, and both IQGAP1 [Brandt, et al., 2007b] and its partner SEPT2 [Huang et al., 2008] are required for phagocytic cup formation in an F-actin polymerization-dependent process, where IQGAP1 is a major regulator.
IQGAP1 also modulates epithelial polarity through regulation of TJs, which H. pylori must disrupt first to access the AJs. Formation of cadherins-AJ facilitates the assembly of TJs, which are found directly above the AJs, and interference with cadherin-based adhesion disturbs TJs [Fig. 2, Niessen, 2007]. The core components of TJs include the claudins, a 24-member family of proteins with specific organ and tissue distribution, often abnormally expressed in cancer [Valle and Morin, 2010]. Claudin-3, -4 and -6 serve as receptors for Clostridium perfringens enterotoxin (CPE), leading to rapid cytolysis of cells expressing these proteins, and thus CPE has been sought as therapy for claudin-marked cancers [Takahashi et al., 2011, Lal-Nag et al., 2012, Walther et al., 2012]. Recently we found that IQGAP1 antagonizes the role of TJ claudin-4 via a Cdc42-JNK pathway [Tanos et al., in press]. Further investigation should reveal the mechanism of claudins in cancer, as well as the significance of IQGAP1 regulation of these proteins. However it is attractive to envision that H. pylori, directly or through CagA, may interact with TJs via IQGAP1. It is also curious whether interplay of CagA-VacA involves interplay of IQGAP1-claudins or other members of TJs or AJs in regulating apoptosis (atrophy) in stomach epithelia. Unraveling such mechanisms may position VacA as an anticancer therapeutic.
Accordingly, IQGAP1 likely represents a facile target for H. pylori-mediated modulation of cell-cell contacts and host actin cytoskeleton (Fig. 2), a hypothesis that warrants investigation. Moreover, the second hallmark of H. pylori infection, which is the decreased acid secretion “hypochlorhydria”, can also be explained by IQGAP1’s actions.
2- Potential role for IQGAP1 in H. pylori-induced hypochlorhydria
It has been well documented that reduced acid secretion (hypochlorhydria) is linked to colonization by H. pylori, and results in atrophic gastritis, and thus an increased risk of gastric cancer. The molecular mechanism behind the hypochlorhydria development is unclear, but it was explained on the basis that the T4SS-integrins interaction, which leads to the dissociation of the metalloproteinase ADAM17, inhibits the expression of the proton pump enzyme H+, K+-ATPase [Saha et al., 2010], thus inhibiting acid secretion. While the mechanism leading to atrophic gastritis is also unclear, it has been widely attributed to inflammation via alterations in the cytokine TNF-α and the transcription factor NF-κB [Bauer and Meyer, 2011], which reside downstream of signaling pathways, whose identity is unknown currently. However, reduced hypochlorhydria can be explained based on altered IQGAP1-signal and function.
Cell-cell contacts are active sites of regulated exocytosis, which require the concerted action of the Rho GTPases and their downstream effectors, including the cytoskeleton and the exocytic proteins [Nelson, 2009]. As discussed earlier, decreased AJ would attenuate acid secretion and increase epithelial permeability, allowing nutrient accessibility and increased H. pylori survival. Hydrochloric acid secretion in stomach epithelia is signal-controlled via receptors residing on the basolateral membrane that ultimately lead to the insertion of the H+, K+-ATPase into the apical membranes of parietal cells [Yao and Forte, 2003]. Knockout of IQGAP1, and not IQGAP2, in mice perturbs stomach epithelial cell growth [Li et al., 2000]. In gastric parietal cells, IQGAP1 localizes to the basolateral membrane whereas Cdc42 and IQGAP2 reside in the apical membrane, with IQGAP2 having no effect on acid secretion [Zhou et al., 2003]. In several epithelial cell lines, IQGAP1 localizes to cell-cell contacts and regulates secretion with active Cdc42 acting as a negative regulator [Rittmeyer, et al., 2008, Osman, 2010], which may explain their differential polarized distribution as an additional regulatory mechanism.
IQGAP1 regulation of secretion also appears to involve phosphorylation-mediated differential interactions with partner and signaling proteins, where reversible phosphorylation drives a conformational-switch via intra-domain interaction (Fig. 1b). Unphosphorylated IQGAP1 has a folded C-terminal domain, which masks the Cdc42-binding region, in which case IQGAP1 promotes secretion and cell growth via interactions with the membrane targeting and tethering machineries, the exocyst and the t-SNARE Syntaxin 1A, and with the nutrient sensor mTORC1 [Rittmeyer et al 2008, Wang et al., 2009, Tekletsadik et al., 2012]. EGF-mediated phosphorylation of IQGAP1 allows it to unfold and bind active Cdc42, leading to attenuated secretion [Rittmeyer, et al., 2008]. If H. pylori were to target IQGAP1, during bacterial or CagA invasion, it would explain the observed delocalization of IQGAP1 to cytoplasmic tubular structures in H. pylori-infected cells [Conlin, et al., 2004], the attenuation of acid secretion, and the loss of epithelial polarity and its accompanied morphogenetic changes. Furthermore, it would explain the reported deregulation of Akt and ERK1/2 signaling pathways, as IQGAP1, serving as a regulatory scaffold, modulates mTORC1-Akt and ERK1/2 signaling to control cell homeostasis [Wang, et al., 2009, Tekletsadik et al., 2012]. Thus, as IQGAP1 inversely regulates secretion and growth, and cell proliferation and transformation by different domains and by modulating mTORC1-Akt and MAPK signaling, this mechanism would underlie its potential role in driving gastric neoplasm, which is discussed below.
3- Potential role for IQGAP1-signaling pathway in gastric carcinoma
IQGAP1 associates with a wide range of human carcinomas [Johnson et al., 2009], and can induce transformed phenotypes, accelerate the cell cycle, and promote cytokinesis, cell migration and invasion in cell culture [Wang et al., 2009, Tekletsadik et al., 2012]. These features support the view that association of IQGAP1 with human carcinomas represents a cause rather than a consequence of tumorigenesis [Wang et al., 2009], which could be the case in GC. Whereas IQGAP1-knockout mice exhibit gastric hyperplasia and dysplasia [Li et al., 2000], screens for SNPs in IQGAP1 gene in diffuse-type gastric carcinomas identified in-frequent mutations in the GRD, encompassing part of the binding site for the activated Cdc42 and Rac1 [Morris et al., 2005]. The significance of these mutations in GC development has not been analyzed, but they may represent mutations impacting IQGAP1 signal in a subset of GC in that particular population and could be useful for personalized medicine. Indeed, genetic mutations or presence of SNPs in IQGAP1 do not seem to account for its association with carcinomas, thus supporting the notion that IQGAP1-signal dysfunction must be the culprit in cancer [Wang et al., 2009, Tekletsadik et al., 2012].
IQGAP1 localization appears to correlate with GC tumor differentiation or H. pyloriinfection . In one study, it diffusely localized to the cytoplasm in a subset of intestinal-type tumors, and to the membranes in a subset of diffuse-type tumors, inversely correlating with Ecadeherins-α-catenin localization [Takemoto et al 2001]. However, delocalization from the plasma membrane to cytoplasmic vesicles also was observed in H. pylori-infected cultured cells [Conlin 2004], implying changes in IQGAP1’s activity at the cellular level. While more work will be required for sorting out the basis and the significance of these changes, IQGAP1-phosphorylation (activity) likely determines its subcellular distribution and, thus signaling partners [Osman, 2010, Tekletsadik et al., 2012]. IQGAP1 binds EGFR [McNulty et al., 2011], and in response to EGF, robustly activates Akt, while attenuating the activities of mTORC1, GSK3α/β and ERK1/2, thus inhibiting secretion and cell growth and promoting cell transformation [Wang, et al., 2009, Tekletsadik et al., 2012]. IQGAP1’s inactivation of GSK3α/β and dissociation of E-cadherin-α/β-catenin complex, would liberate the oncogenic α-catenin from its inhibitory complex to the nucleus where it can trigger neoplastic transformation. Thus, H. pylori infection may mimic mitogen signals, which could explain why it chronically activates EGFR [Bauer et al., 2009] and why the tumor-suppressor APC, a part of the inhibitory complex of α-catenin [Wu and Pan, 2010] and a binding-partner of IQGAP1 [Watanabe, et al., 2004], is suppressed in 50% of gastric carcinomas [Fox et al., 2006]. Interestingly, CagA-transfected cells in vitro, also increased the levels of IQGAP2, R-Ras, and B-Raf and the activity of Erk1/2, independent of Ras activation [Zhu, et al., 2005], which is known to activate the MAPK cascade. Whereas the significance of IQGAP2 increase requires further investigation, the enhancement of Ras-independent Erk1/2 activity predicts deregulation of IQGAP1-signal. Comparative analysis of IQGAP1-signaling pathway in diffuse- and intestinal-type carcinomas likely will reveal differences in dysregulation of certain effector and/or regulator proteins, or differences in the degree to which the pathway might be up- or down-regulated. Such analysis could be useful for devising rational strategies for detection and treatment.
Deregulation of signaling pathways can explain cancer disparity
Currently there is no reliable molecular markers for GC aside from HER2 (EGFR), and current treatment regimens rely on histopathologic and anatomic criteria, and neglect possible molecular differences [Shah et al., 2011], which may explain the high mortality rate of GC worldwide. However, there is a current shift towards identifying deregulated signaling pathways [Ooi et al., 2009] as prerequisite for personalized medicine [Yeatman et al., 2012]. Not surprisingly, several studies have identified the Wnt-E-cadhern-β-catenin pathway, which reside downstream of IQGAP1, as a potential oncogenic pathway in the majority of GC [Tanaka, et al., 2002, Ooi et al., 2009, Shah et al., 2011]. In this context, analysis of signaling pathways has two advantages. First protein markers are considered more superior to nucleic acid markers, as they represent the actual players in cell function [Mishra and Verma, 2010]. Second, they are likely to identify distinct disparity markers, as signaling pathways are modulated according to the specific external environmental cues in each individual or population case.
Indeed, whereas the American Association for Cancer Research (AACR) has projected that tumors will no longer be defined by their site-of-origin, but rather by their genetic driverlesion or profile, and that there will be commonalities among different tumors, findings from The Cancer Genome Atlas (TCGA) have revealed that cancer comprises hundreds of distinct molecular diseases. Clearly, this warrants investigation of epigenetic changes arising from gene-environment interactions-- typically manifested at the signaling, and not the genetic level -- for devising more personalized approaches to diagnostics and treatment. Delineating the IQGAP1-Cdc42-mTORC1-Akt-MAPK pathway in gastric carcinoma will likely meet this goal. Being a central pathway for cell growth control, and a nutrient- and growth factor-sensor that cross-talks with essential pathways that regulate cell homeostasis, this pathway will likely yield environment-specific markers. Indeed, it appears that this pathway, if tilted to the right, promotes tumorigenesis via Akt, and to the left it could promote cytokinesis arrest and apoptosis via ERK1/2/GSK3αβ, with IQGAP1 serving as a molecular rheostat [Fig. 1b, Wang et al., 2009; Tekletsadik et al., 2012]. Thus, the factors that modulate the pathway-signal-dynamics will also be important. Furthermore, bioinformatic analyses (Osman, unpublished) predict that a number of epigenetic processes including various microRNAs (miRs) regulate the diversity of the pathway cellular functions. These miRs can represent facile diagnostics and targeted therapeutics [Rottiers and Näär 2012], likely reflective of ethnicity, gender and geographic location, and can be valuable as disparity biomarkers.
Acknowledgement
Work in the MO lab was supported by grants from NIH-NCI (CA104285) and from ACS.
Footnotes
The authors declare no competing interests.
References
- Awasthi A, Samarakoon A, Chu H, Kamalakannan R, Quilliam LA, Chrzanowska-Wodnicka M, Gilbert C, White GC, II, Malarkannan S. Rap1b facilitates NK cell functions via IQGAP1-mediated signalosomes. JEM. 2010;207:1923–1938. doi: 10.1084/jem.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert S, Clyne M, Tegtmeyer N. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Communication and Signaling. 2011:9–28. doi: 10.1186/1478-811X-9-28. http://www.biosignaling.com/content/9/1/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnoli F, Buti L, Tompkins A, Covacci A, Aieva MR. Helicobacter pyloriCagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci U S A. 2005;102:16339–16344. doi: 10.1073/pnas.0502598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Bartfeld S, Meyer TF. H. pylori selectively blocks EGFR endocytosis via the non-receptor kinase c-Abl and CagA. Cell Microbiol. 2009;11:156–169. doi: 10.1111/j.1462-5822.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- Bauer B, Meyer TF. The Human Gastric Pathogen Helicobacter pylori and Its Association with Gastric Cancer and Ulcer Disease. Ulcers. 2011 Article ID 340157, 23 pages. [Google Scholar]
- Bensenor L, Kan H-M, Wang N, Wallrabe H, Davidson LA, Cai Y, Schafer DA, Bloom GS. IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J. Cell Sci. 2007;120:658–669. doi: 10.1242/jcs.03376. [DOI] [PubMed] [Google Scholar]
- Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S, Wessler S, Hartig R, Backert S. Helicobacter pylori activates protein kinase C delta to control Raf in MAP kinase signalling: role in AGS epithelial cell scattering and elongation. Cell Motil Cytoskeleton. 2009;66:874–892. doi: 10.1002/cm.20373. [DOI] [PubMed] [Google Scholar]
- Brandt S, Shafikhani S, Balachandran P, Jin S, Hartig R, König W, Engel J, Backert S. Use of a novel coinfection system reveals a role for Rac1, H-Ras, and CrkII phosphorylation in Helicobacter pylori-induced host cell actin cytoskeletal rearrangements. FEMS Immunol Med Microbiol. 2007a;50:190–205. doi: 10.1111/j.1574-695X.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007b;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffart TE, Louw M, van Grieken NCT, Tijssen M, Carvalho B, Ylstra B, Grabsch H, Mulder CJJ, van de Velde CJH, van der Merwe SW, Gerrit A, Meijer GA. Gastric cancers of Western European and African patients show different patterns of genomic instability. BMC Medical Genomics. 2011;2011:4–7. doi: 10.1186/1755-8794-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlin VS, Curtis SB, Zhao Y, Moore EDW, Smith VC, Meloche RM, Finlay BB, Buchan AMJ. Helicobacter pylori Infection Targets Adherens Junction Regulatory Proteins and Results in Increased Rates of Migration in Human Gastric Epithelial Cells. Infection And Immunity. 2004;72:5181–5192. doi: 10.1128/IAI.72.9.5181-5192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. IntJCancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Fox GJ, Wang CT, Parsonnet J. Helicobacter, Chronic inflammation, and cancer. In: Hausen ZH, editor. Infections Causing Human Cancer. Wiley-VCH; 2006. pp. 386–467. [Google Scholar]
- Grohmanova K, Schlaepfer D, Hess D, Gutierrez P, Beck M, Kroschewski R. Phosphorylation of IQGAP1 modulates its binding to Cdc42, revealing a new type of Rho-GTPase regulator. J. Biol. Chem. 2004;279:48495–48504. doi: 10.1074/jbc.M408113200. [DOI] [PubMed] [Google Scholar]
- Johnson M, Manisha Sharmaa M, Henderson BR. IQGAP1 regulation and roles in cancer. Cell Signal. 2009;10:1471–1478. doi: 10.1016/j.cellsig.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Horwitz AR. The origins of the molecular era of adhesion research. Nature Rev Molec Cell Biol. 2012;13:805–812. doi: 10.1038/nrm3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy B, Löwer M, Weydig C, Carra G, Tegtmeyer N, Geppert T, Schröder P, Sewald N, Backert S, Schneider G, Wessler S. Helicobcter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy BB, Geppert TT, Boehm MM, et al. Distinct Roles of Secreted HtrA Proteases from Gram-negative Pathogens in Cleaving the Junctional Protein and Tumor Suppressor E-cadherin. J. Biol. Chem. 2012;287:10115–10120. doi: 10.1074/jbc.C111.333419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-W, Yan M, Collins RF, DiCiccio JE, Grinstein S, et al. Mammalian Septins Are Required for Phagosome Formation. Mol Biol Cell. 2008;19:1717–1726. doi: 10.1091/mbc.E07-07-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamangar F, Dores GM, Anderson WF. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- Katata T, Irie K, Fukuhara A, Kawakatsu T, Yamada A, Shimizu K, Takai Y. Involvement of nectin in the localization of IQGAP1 at the cell-cell adhesion sites through the actin cytoskeleton in Madin–Darby canine kidney cells. Oncogene. 2003;22:2097–2109. doi: 10.1038/sj.onc.1206255. [DOI] [PubMed] [Google Scholar]
- Kaplan-Türköz B, Jiménez-Soto LF, Dian C, Ertl C, Remaut H, Louche A, Tosi T, Haas R, Terradot L. Structural insights into Helicobacter pylori oncoprotein CagA interaction with β1 integrin. Proc Natl Acad Sci U S A. 2012;109:14640–14645. doi: 10.1073/pnas.1206098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M. The septins. Genome Biology. 2003;4:236. doi: 10.1186/gb-2003-4-11-236. http://genomebiology.com/2003/4/11/236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Fernandez GE, Sacks DB, Prasadarao NV. IQGAP1 mediates the disruption of adherens junctions to promote Escherichia coli K1 invasion of brain endothelial cells. Cell Microbiol. 2012;14:1415–1433. doi: 10.1111/j.1462-5822.2012.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- Lal-Nag M, Battis M, Santin AD, Morin PJ. Claudin-6: a novel receptor for CPE-mediated cytotoxicity in ovarian cancer. Oncogenesis. 2012;1:e33. doi: 10.1038/oncsis.2012.32. www.nature.com/oncsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren T. The two histologic main types of gastric carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Le Clainche C, Schlaepfer D, Ferrari A, Klingauf M, Grohmanova K, Veligodskiy A, Dirdy D, Le D, Egile C, Carlier M-F, et al. IQGAP1 stimulates actin assembly through the N-Wasp-Arp2/3 pathway. J. Biol. Chem. 2007;282:426–435. doi: 10.1074/jbc.M607711200. [DOI] [PubMed] [Google Scholar]
- Lewin D, Lewin K. Stomach: normal anatomy and histology. In: Graham DY, Genta RM, Dixon F, editors. Gastritis. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 765–773. 1999. [Google Scholar]
- Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol. Cell Biol. 2000;20:697–701. doi: 10.1128/mcb.20.2.697-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkannan S, Awasthi A, Rajasekaran K, Kumar P, Schuldt KM, Bartoszek A, Manoharan N, Goldner NK, Umhoefer CM, Thakar MS. IQGAP1: a regulator of intracellular spacetime relativity. J Immunol. 2012;188:2057–2063. doi: 10.4049/jimmunol.1102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002;277:12324–12333. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- Mateer SC, Wang N, Bloom GS. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil. Cytoskeleton. 2003;55:147–155. doi: 10.1002/cm.10118. [DOI] [PubMed] [Google Scholar]
- Mateer SC, Morris LE, Cromer DA, Bensenor LB, Bloom GS. Actin filament binding by a monomeric IQGAP1 fragment with a single calponin homology domain. Cell Motil Cytoskeleton. 2004;58:231–241. doi: 10.1002/cm.20013. [DOI] [PubMed] [Google Scholar]
- McNulty DE, et al. MAPK scaffold IQGAP1 binds the EGF receptor and modulates its activation. J Biol Chem. 2011;286:15010–15021. doi: 10.1074/jbc.M111.227694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Verma M. Cancer Biomarkers: Are We Ready for the Prime Time? Cancers. 2010;2:190–208. doi: 10.3390/cancers2010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moese S, Selbach M, Brinkmann V, Karlas A, Haimovich B, Backert S, Meyer TF. The Helicobacter pylori CagA protein disrupts matrix adhesion of gastric epithelial cells by dephosphorylation of vinculin. Cellular Microbiology. 2006;9:1148–1161. doi: 10.1111/j.1462-5822.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- Morris LE, Bloom GS, Frierson HF, Powell SM. Nucleotide variants within the IQGAP1 gene in diffuse-type gastric cancers. Genes Chromosomes Cancer. 2005;42:280–286. doi: 10.1002/gcc.20150. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Nam Tham T, Danckaert A, Guadagnini S, Boisson-Dupuis S, et al. Septins Regulate Bacterial Entry into Host Cells. PLoS ONE. 2009;4(1):e4196. doi: 10.1371/journal.pone.0004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Tegtmeyer N, Brandt S, Yamaoka Y, De Poire E, Sgouras D, Wessler S, Torres J, Smolka A, Backert S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest. 2012;122:1553–1566. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host and Microbe. 2010;7:399–411. doi: 10.1016/j.chom.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Nelson JW. Remodeling Epithelial Cell Organization: Transitions Between Front–Rear and Apical–Basal Polarity. Cold Spring Harb Perspect Biol. 2009 Jul;1(1):a000513. doi: 10.1101/cshperspect.a000513. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM. Tight Junctions/Adherens Junctions: Basic Structure and Function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- Noritake U, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J. Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- Olofsson A, Vallström A, Petzold K, et al. Biochemical and functional characterization of Helicobacter pylori vesicles. Molecular Microbiology. 2010;77:1539–1555. doi: 10.1111/j.1365-2958.2010.07307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLos Genet. 2009;5:e1000676. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman MA. An emerging role for IQGAP1 in regulating protein traffic. TheScientificWorldJOURNAL. 2010;10:944–953. doi: 10.1100/tsw.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri SK. Cross talk between focaladhesion kinase and cadherins: Role in regulating endothelial barrier function. Microvascular Research. 2012;1:3–11. doi: 10.1016/j.mvr.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh A, Yap AS. A bigger picture: classical cadherins and the dynamic actin cytoskeleton. Nat Rev Molec Cell Biol. 2012;13:673–679. doi: 10.1038/nrm3431. [DOI] [PubMed] [Google Scholar]
- Reymond N, Jae Hong Im, Ritu Garg, Francisco M Vega, Barbara Borda d'Agua, Philippe Riou, Susan Cox, Ferran Valderrama, Ruth J Muschel, Ridley AJ. Cdc42 promotes transendothelial migration of cancer cells through {beta}1 integrin. J. Cell Biol. 2012;199:653–668. doi: 10.1083/jcb.201205169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmeyer EN, Daniel S, Hsu SC, Osman MA. A dual role for IQGAP1 in regulating exocytosis. J Cell Sci. 2008;121:391–408. doi: 10.1242/jcs.016881. [DOI] [PubMed] [Google Scholar]
- Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nature Reviews. Mol Cell Biol. 2012;13:239–251. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A, Backert S, Hammond CE, Gooz M, Smolka AJ. Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPaseα subunit. Gastroenterology. 2010;139:239–248. doi: 10.1053/j.gastro.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Yoshimi Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr. Opin. Cell Biol. 2007;19:593–602. doi: 10.1016/j.ceb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Schneider S, Weydig C, Wessler S. Targeting focal adhesions: Helicobacter pylori-host communication in cell migration. Cell Communication and Signaling. 2008;6:2. doi: 10.1186/1478-811X-6-2. http://www.biosignaling.com/content/6/1/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular Classification of Gastric Cancer: A New Paradigm. Clin. Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, et al. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita K, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180:295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kondoh M, Suzuki H, Yagi K. Claudin as a target for drug development. Curr Med Chem. 2011;18:1861–1865. doi: 10.2174/092986711795496809. [DOI] [PubMed] [Google Scholar]
- Takemoto H, Yuchiro D, Shiozaki H, Imamura H, Utsunomya T, Miyata H, Yano M, Inoue M, Fujiwara Y, Monden M. Localization of IQGAP1 is inversely correlated with intercellular adhesion mediated by E-cadherin in gastric cancers. Int. J. Cancer. 2001;91:783–788. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1121>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Tammer I, Brandt S, Hartig R, König W, Backert S. Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology. 2007;132:1309–1319. doi: 10.1053/j.gastro.2007.01.050. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kitajima Y, Edakuni G, Sato S, Miyazaki K. Abnormal expression of Ecadherin and beta-catenin may be a molecular marker of submucosal invasion and lymph node metastasis in early gastric cancer. Br J Surg. 2002;89:236–244. doi: 10.1046/j.0007-1323.2001.01985.x. [DOI] [PubMed] [Google Scholar]
- Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer N, Wittelsberger R, Hartig R, Wessler S, Martinez-Quiles N, Backert S. Serine Phosphorylation of Cortactin Controls Focal Adhesion Kinase Activity and Cell Scattering Induced by Helicobacter pylori. Cell Host Microbe. 2011;9:520–531. doi: 10.1016/j.chom.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Tekletsadik KY, Sonn R, Osman MA. A Conserved Role for IQGAP1 in Regulating TOR Complex 1. J. Cell Sci. 2012;125:2041–2052. doi: 10.1242/jcs.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M. Focal Adhesion Kinase Is a Substrate and Downstream Effector of SHP-2 Complexed with Helicobacter pylori CagA. Mol. Cell. Biol. 2006;26:1–276. doi: 10.1128/MCB.26.1.261-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle B, Morin PJ. Claudins in cancer biology. Curr Top Membr. 2010;13:293–333. [Google Scholar]
- Walther W, Petkov S, Kuvardina ON, Aumann J, Kobelt D, Fichtner I, et al. Novel Clostridium perfringens enterotoxin suicide gene therapy for selective treatment of claudin-3- and-4-overexpressing tumors. Gene Ther. 2012;19:494–503. doi: 10.1038/gt.2011.136. [DOI] [PubMed] [Google Scholar]
- Wang J-B, Sonn R, Tekletsadik YK, Samorodnitsky D, Osman MA. IQGAP1 regulates cell proliferation through a novel CDC42-mTOR pathway. J Cell Sci. 2009;122:2024–2033. doi: 10.1242/jcs.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA: A Cancer Journal for Clinicias. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sato K, Kaibuchi K. Cadherin-mediated Intercellular Adhesion and Signaling Cascades Involving Small GTPases. Cold Spring Harb Perspect Biol. 2009;1:a003020. doi: 10.1101/cshperspect.a003020. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Wessler S, Gimona M, Rieder G. Regulation of the actin cytoskeleton in Helicobacter pylori-induced migration and invasive growth of gastric epithelial cells. Cell Comm and Signal. 2011;9:27. doi: 10.1186/1478-811X-9-27. http://www.biosignaling.com/content/9/1/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiredu EK, Armah HB. Cancer mortality patterns in Ghana: a 10-year review of autopsies and hospital mortality. BMC Public Health. 2006;6:159. doi: 10.1186/1471-2458-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends in Biochemical Sciences. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu. Reb. Physiol. 2003;65:103–131. doi: 10.1146/annurev.physiol.65.072302.114200. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ, Mule J, Dalton WS, Sullivan D. On the Eve of Personalized Medicine in Oncology. Cancer Res. 2008;68:7250–7252. doi: 10.1158/0008-5472.CAN-08-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhong X, Zheng S, Du Q, Xu W. Transformed immortalized gastric epithelial cells by virulence factor CagA of Helicobacter pylori through Erk mitogen-activated protein kinase pathway. Oncogene. 2005;24:3886–3895. doi: 10.1038/sj.onc.1208551. [DOI] [PubMed] [Google Scholar]
- Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends in Cell Biol. 2010;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]