Abstract
Cerebellar long-term depression (LTD) is a type of synaptic plasticity and has been considered as a critical cellular mechanism for motor learning. LTD occurs at excitatory synapses between parallel fibers and a Purkinje cell in the cerebellar cortex, and is expressed as reduced responsiveness to transmitter glutamate. Molecular induction mechanism of LTD has been intensively studied using culture and slice preparations, which has revealed critical roles of Ca2+, protein kinase C and endocytosis of AMPA-type glutamate receptors. Involvement of a large number of additional molecules has also been demonstrated, and their interactions relevant to LTD mechanisms have been studied. In vivo experiments including those on mutant mice, have reported good correlation of LTD and motor learning. However, motor learning could occur with impaired LTD. A possibility that cerebellar synaptic plasticity other than LTD compensates for the defective LTD has been proposed.
Keywords: cerebellum, long-term depression, synaptic plasticity, motor learning, Purkinje cell
Cerebellar circuit and long-term depression (LTD)
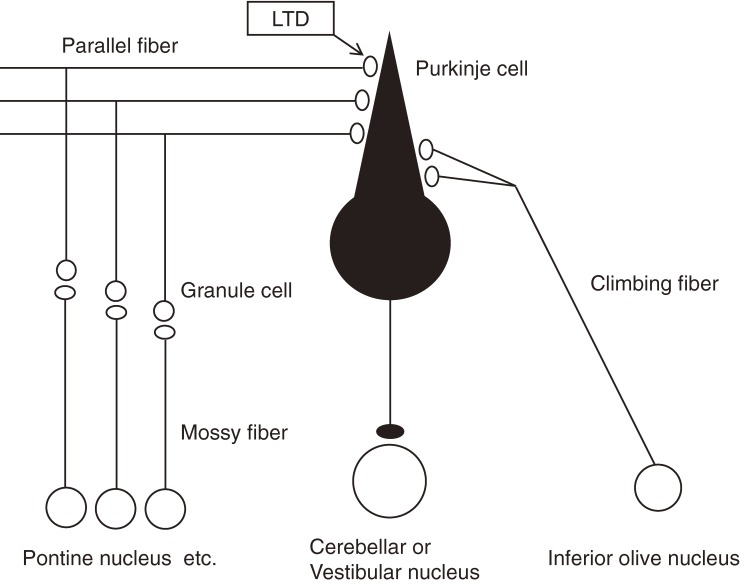
The organization of the cerebellum reflects its involvement in motor control. The cerebellum consists of cortex and nuclei, and major inputs to the cortex come from the spinal cord, pons etc. through mossy fibers. Mossy fibers send excitatory glutamatergic outputs to granule neurons, which in turn transmit excitatory synaptic information to Purkinje cells (Fig. 1). Cerebellar granule cells are most numerous neurons in the whole brain. The cell body of a granule cell is located in the granular layer, and extends an axon to the molecular layer, where it bifurcates to form parallel fibers. Purkinje cell dendrites show extensive branching in the molecular layer and receive >150,000 parallel fiber synaptic inputs. Purkinje cells are the sole output neurons in the cortex. Its axon projects to a cerebellar or vestibular nucleus, and inhibits neurons through secretion of γ-amino butyric acid (GABA) there. A Purkinje cell receives another type of excitatory synaptic inputs from a neuron in an inferior olivary nucleus through a climbing fiber. A single climbing fiber forms >300 synapses on a Purkinje cell, and provides powerful excitatory drive. In contrast, each parallel fiber forms only one or a few synapses on a Purkinje cell, although the number of parallel fibers innervating a Purkinje cell is huge. This synaptic organization is maintained throughout the cerebellar cortex.1,2)
Figure 1.
Main cerebellar cortical circuits. LTD occurs at parallel fiber-Purkinje cell synapses.
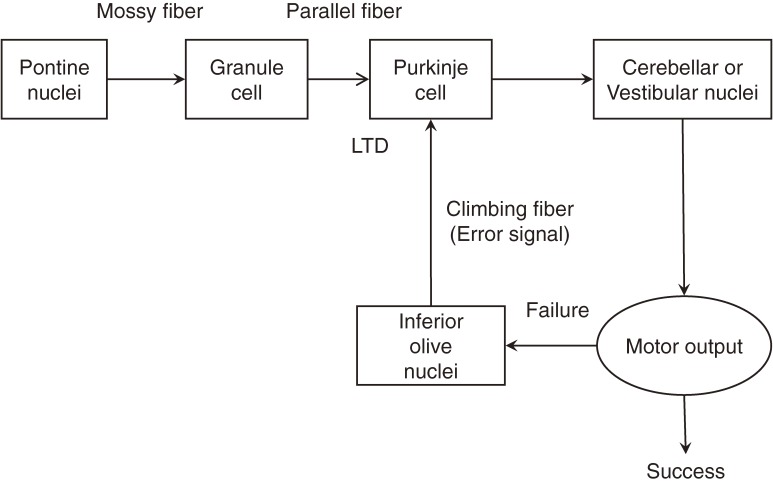
Knowing the above explained characteristic and relatively simple cerebellar neuronal circuit, Brindley, Marr, Ito and Albus proposed models about the functioning mechanism of the cerebellum.1,3–6) According to Albus,6) the major information flow in the cerebellum is mossy fibers > granule cells (parallel fibers) > Purkinje cells > cerebellar and vestibular nuclear neurons. On the other hand, a climbing fiber codes an error signal reflecting the motor performance failure. When a motor performance ends in failure, the signal conveyed by a climbing fiber works to depress the synaptic transmission between parallel fibers and a Purkinje cell, thereby weakening the connection which might have contributed to the failed action (Fig. 2). This model predicts that coupled activation of parallel fibers and a climbing fiber should depress the information flow from the parallel fibers to a Purkinje cell. This prediction was supported experimentally by an in vivo recording of Purkinje cell activities performed by Ito and colleagues,7) and the phenomenon has been called long-term depression (LTD).
Figure 2.
Main information flow in the cerebellar system.
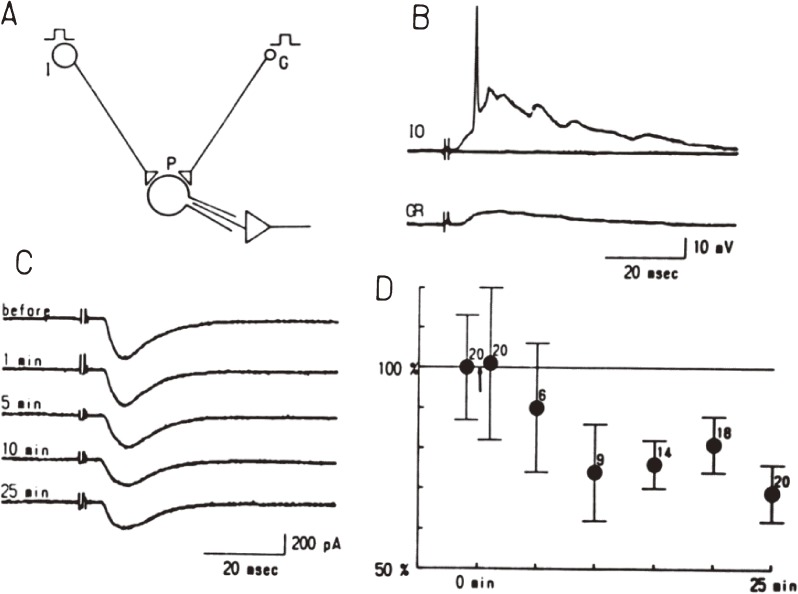
LTD is caused by the decreased transmission efficacy at synapses between parallel fibers and a Purkinje cell. The direct demonstration of this synaptic LTD was first provided in a slice preparation by Sakurai,8) and then in a culture preparation by Hirano,9) who had developed a co-culture preparation of cerebellar neurons and explants of inferior olivary nuclei.10–12) Then, it was demonstrated that coupled repetitive activation of inferior olive neurons and a granule cell induces LTD of excitatory postsynaptic current at synapses between a granule cell and a Purkinje cell (Fig. 3), whereas repetitive activation of only a granule cell induces the long-term potentiation (LTP).9) Potentiation of the transmission was also reported in a slice preparation.8) Following this study, Linden and colleagues developed a simpler and easier method to monitor LTD in the culture.13) These in vitro experimental systems facilitated research on the cellular and molecular mechanism of LTD.
Figure 3.
LTD induction in a co-culture preparation of cerebellar neurons and inferior olive explants.9) A, A scheme of electrophysiological experiments. A granule cell (G) and/or an inferior olive explant (I) were stimulated, and whole-cell recording was obtained from a Purkinje cell (P). B, Voltage responses of a Purkinje cell to the stimulation of either an inferior olive explant (IO) or a granule cell (GR). C, Excitatory postsynaptic currents (EPSCs) recorded from a Purkinje cell before and after the conjunctive stimulation of an inferior olive explant and a granule cell. D, Amplitudes of EPSC in a Purkinje cell before and after the conjunctive stimulation.
On the other hand, implication of LTD in motor learning has also been studied. Two model motor-learning paradigms have been widely used. One is adaptation of vestibulo-ocular reflex,1,14–16) and another is classical conditioning of eye-blink response.17) Studies to examine effects of destruction or inactivation of the cerebellum and also in vivo neuronal activity recording, have been performed.17,18) More recently, motor learning in mutant mice with defects in LTD was examined. Positive correlation between LTD and motor learning has been reported,19–22) although some studies have found normal motor learning with suppressed LTD.23,24) Contribution of cerebellar synaptic plasticity other than LTD to motor learning has also been suggested,25–28) that might account for the discrepancy.
In the following section, I will review and discuss cellular and molecular mechanisms of LTD, implication of LTD in motor learning, and synaptic plasticity other than LTD in the cerebellum.
Molecular mechanism of LTD
Synergistic action of two synaptic inputs.
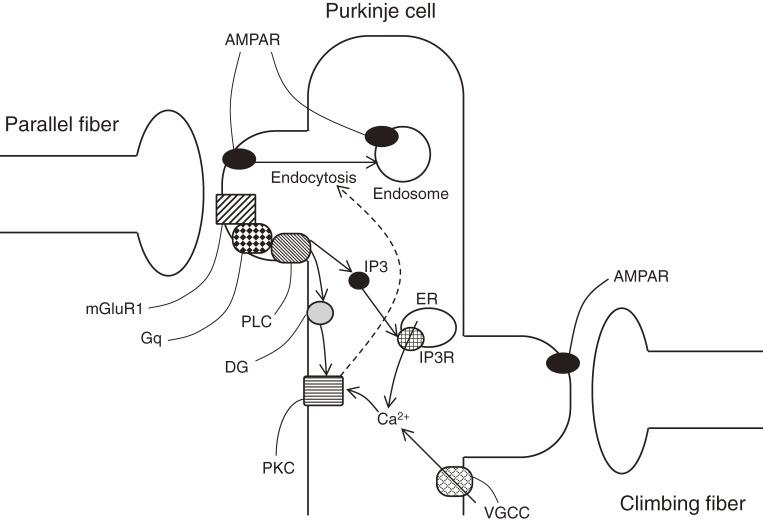
LTD is induced by coupled activation of two glutamatergic synaptic input pathways, parallel fibers and a climbing fiber. How each contributes to LTD and where the two signaling pathways converge in a Purkinje cell, are critical for understanding the induction mechanism of LTD. Activation of a climbing fiber induces a complex spike and large Ca2+ influx.29,30) Subsequent studies have revealed that climbing-fiber activation required for the LTD induction can be replaced by direct depolarization or intracellular Ca2+ increase in a Purkinje cell,31–33) where the increase in intracellular Ca2+ concentration is necessary for the induction of LTD.34) On the other hand, parallel fiber inputs activate not only the ionotropic glutamate receptor but also metabotropic glutamate receptor mGluR1 at the postsynaptic membrane of a Purkinje cell. mGluR1 activation is required for LTD induction.19,35) mGluR1 is coupled to Gq protein and activates phospholipase C, which produces inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 induces Ca2+ release from the intracellular endoplasmic reticulum through IP3 receptor,36) and Ca2+ and diacylglycerol synergistically activate protein kinase C (PKC)37) (Fig. 4).
Figure 4.
Major molecular cascades regulating LTD. AMPAR, AMPA receptor; mGluR1, metabotropic glutamate receptor 1; Gq, Gq protein; PLC, phospholipase C; DG, diacylglycerol; PKC, protein kinase C; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; VGCC, voltage-gated Ca2+ channel; ER, endoplasmic reticulum.
Involvement of PKCα in LTD induction has been demonstrated.38–40) PKCα is a convergent point for the two signaling pathways, as it is synergistically activated by intracellular Ca2+ which is located downstream of a climbing fiber pathway (Fig. 4), and by diacylglycerol located downstream of a parallel fiber pathway. Ca2+ is another convergent point, because both pathways work to increase the cytoplasmic concentration. Activation of a climbing fiber induces Ca2+ influx through voltage-gated Ca2+ channel on the plasma membrane, while activation of a parallel fiber induces the Ca2+ release from intracellular stores through IP3 receptor downstream of mGluR1. mGluR1 also opens transient receptor potential canonical 3 (TRPC3) channel permeable to Ca2+.41) IP3 receptor is also another convergent point of the two pathways,42) as its activity is regulated by the cytoplasmic Ca2+ concentration. Some studies demonstrated that large intracellular Ca2+ increase by itself is sufficient to induce LTD.43,44) In a normal condition however, the intracellular Ca2+ increase caused by activation of a climbing fiber is insufficient to induce LTD, and additional activation of mGluR1 followed by production of IP3 and diacylglycerol seems to be required for LTD induction8,9,13) (Fig. 4).
Expression of LTD as reduced glutamate receptor responsiveness.
LTD is accompanied by the reduced quantal size of excitatory synaptic currents at synapses between a granule cell and a Purkinje cell,45) and by the reduced ionotropic glutamate receptor responsiveness of a Purkinje cell.13) Reduction in the number of AMPA-type ionotropic glutamate receptor (AMPAR) occurs during LTD,46) and enhanced AMPAR endocytosis has been proposed as a main mechanism of the LTD expression.47,48) Unbinding of phosphorylated AMPAR from a postsynaptic protein GRIP (glutamate receptor interacting protein) and binding to another protein PICK1 (protein interacting with C kinase 1) seem to be implicated in the LTD induction.49)
Other molecules involved in LTD.
In addition to the above mentioned molecules, a large number of signaling molecules are implicated in LTD. Involvement of mitogen activated protein kinase (MAPK; also called extracellular signal-regulated kinase 1/2, ERK1/2) was reported.50) Subsequent studies proposed activation of MAPK and PKC together with Raf, MAPK/ERK1/2 kinase (MEK), phospholipase A2 (PLA2) and arachidonic acid (AA), constitute a positive feedback loop that support the LTD induction.51–53) Recently, it was reported that Raf kinase inhibitory protein mediates PKC-dependent MAPK activation.53)
Nitric oxide (NO) is also implicated in LTD through activation of guanylyl cyclase, which produces cyclic guanosine monophosphate (cGMP).54) cGMP activates protein kinase G (PKG), which in turn phosphorylates G substrate. Phosphorylated G substrate suppresses protein phosphatase 2A (PP2A), which counteracts PKC. Thus, suppression of PP2A through the NO and cGMP pathway supports the LTD induction.18,55,56)
Involvement of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in LTD induction was reported more recently. In αCaMKII knockout juvenile mice LTD is impaired, and conversion of LTD to LTP occurs in the adult mutant mice.57) βCaMKII knockout mice also show LTD impairement.58) However, how CaMKII is involved in LTD has been unclear. Kawaguchi and Hirano (2013) recently proposed that CaMKII might contribute to the increase in cytoplasmic cGMP through regulation of phosphodiesterase 1 (PDE1), and that CaMKII activity could also interact with NO pathway.59)
Another interesting molecule involved in LTD is glutamate receptor δ2 subunit (GluD2), an ionotropic glutamate receptor-related molecule which is specifically expressed on the postsynaptic membrane at parallel fiber-Purkinje cell synapses.21,60,61) Suppressing expression or knockout of GluD2 abrogated LTD,62,63) and studies that followed revealed that the intracellular C-terminal of GluD2 plays critical roles.64–66) Interestingly, it was reported that knockout of an intracellular GluD2 binding protein delphilin, facilitated the LTD induction through reduction of the intracellular Ca2+ dependence.22) Recently, interaction of GluD2 with mGluR1 and TRPC3 channel was reported.67)
Late phase of LTD.
How long LTD can last was addressed in a culture preparation by measuring amplitudes of miniature excitatory postsynaptic currents (mEPSCs) after a chemical LTD induction.68) LTD of mEPSC amplitude lasts for >1 day. The late phase of LTD depends on both mRNA and protein synthesis.68,69) Existence of PKC-independent intermediate phase of LTD was also suggested.52)
Conflicts between results in culture and slice.
As described above, molecular and cellular mechanisms of LTD have been studied using slice and dissociated culture preparations. Each has advantages and limitations. The former retains the structural integrity, and the latter is more amenable to experimental manipulations and imaging of neurons and synapses.
NO-dependence of LTD was reported in slices,54–56) but not in a culture preparation.70) However, our recent study in culture showed that NO and CaMKII pathways converge on cGMP, and that NO-dependence of LTD induction becomes weak when CaMKII activity is high.59) These results suggest that previous NO-independent LTD induction in culture might have occurred in a condition where CaMKII activity in a Purkinje cell is elevated.
Another point of conflict has been the duration of PKC activation after the LTD induction. The first theoretical model of molecular signaling cascades inducing LTD postulated long-lasting activation of PKC,51) and immunohistochemistry in a slice preparation showed sustained translocation of PKCα to the plasma membrane.52) Classical PKC including PKCα is fully activated after binding to diacylglycerol in the plasma membrane.37) In contrast, long-term translocation of PKCα was not reported with live-imaging of PKCα fused to GFP (green fluorescent protein) in culture, although transient very clear translocation occurred and PKC activity was necessary for the LTD induction.39) We recently noticed that weak PKCα translocation was sustained in spines of cultured Purkinje cells after the LTD induction,59) suggesting that the different conclusions between the preparations can be ascribed to the differences in detection methods and/or levels of PKCα translocation. Therefore, critical neuronal properties are not likely to be qualitatively different between slice and culture preparations.
Cultured Purkinje cells show specific patterns of synapse formation that preserves the connectivity found in vivo10,12,71) as do cultured hippocampal neurons,72) and recapitulate essential properties of LTD such as Ca2+ and PKC-dependent induction. Culture preparations provide good visibility of synapses and have made it possible to selectively stimulate a single presynaptic terminal.73) Our recent study using a novel culture preparation and total internal reflection fluorescent microscopy, demonstrated subtype-specific exocytosis and transport of AMPAR during hippocampal LTP.74) Thus, together with slice preparations, reduced culture preparations have been facilitating the molecular and cellular level analyses of synaptic plasticity.
Summary and remaining questions.
Molecular mechanism of LTD induction has been extensively studied, and it has been clarified that intracellular Ca2+ increase, PKC activation and endocytosis of AMPAR are critical. A large number of molecules are implicated in the LTD induction, and molecular interactions relevant to LTD have been studied. However, how some critical molecules such as GluD2 are involved in LTD has not been clarified yet.
Functional implication
LTD and motor learning.
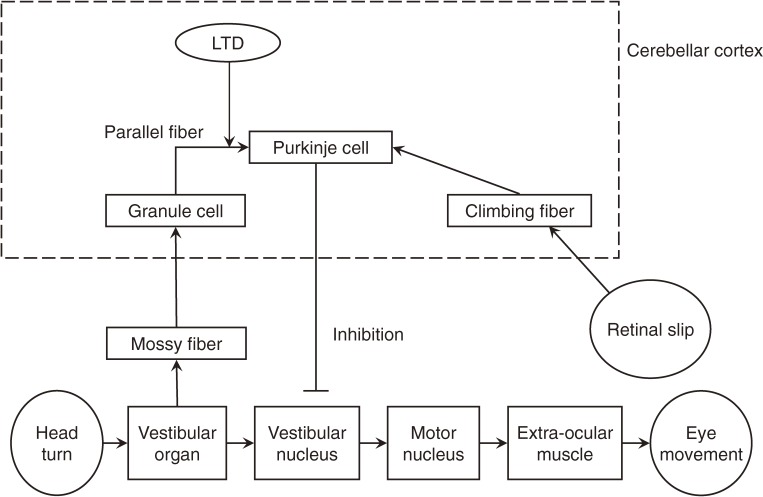
Contribution of LTD to motor learning has been intensively studied in two models, adaptation of vestibulo-ocular reflex (VOR)1,14–16) and classical conditioning of eye blink response.17) VOR is a reflex to stabilize the visual image during head motion. Vestibular organs such as semi-circular canals detect head motion, and drive eye balls to move in the opposite direction of head motion so that the visual image becomes stable. The timing and amplitude of VOR needs to be fine-tuned so that it works adequately in daily life. The adaptation of VOR occurs when the eyeball motion fails to stabilize the visual image. Experimentally this condition is given by rotating a surrounding screen during the rotation of a head-fixed animal. When the screen is rotated in the same direction of an animal, the rotation of eyeballs gets smaller, and when the screen is rotated in the opposite direction of an animal, the rotation of eyeballs becomes larger. Such adaptive change of VOR has been regarded as a type of motor learning. In VOR a climbing fiber conveys the retinal slip information,75) which contributes to the LTD induction. Neuronal activity recording from a Purkinje cell in rabbits and monkeys showed VOR-related activities in the cerebellar flocculus,18,76–78) which constitutes a side pathway of VOR and regulates the timing and amplitude of the reflex (Fig. 5). There have been contradictory ideas about the importance of LTD in the VOR adaptation.15,16)
Figure 5.
Neuronal circuits regulating vestibulo-ocular reflex.
Implication of LTD in classical conditioning has also been studied focusing on the eye-blink response.17,79,80) Here, an unconditioned eye-blinking is induced by applying air-puff to an eye or electrical stimulation around an eye. Coupling air puff stimulation with the preceding conditioning sound presentation leads to the occurrence of conditioned eye-blinking response to the sound stimulation. Involvement of LTD in this conditioned response has been proposed,17,79,80) although other brain area such as the hippocampus also contribute to the eye blink conditioning.17,81,82)
Studies using mutant mice.
Cellular and molecular analyses have revealed molecules that play critical roles in the LTD induction, and have prompted to generate knockout mice defective in LTD. Earlier studies reported deficits of LTD and motor learning in mGluR1 knockout and GluD2 knockout mice.19,63) Purkinje cell-specific expression of PKC inhibitor also abrogates LTD and affects motor learning.20) In mice, besides VOR adaptation, adaptation of optokinetic response (OKR) has also been studied as a model motor learning. OKR is reflexive eye movement following the movement of large visual field, and works to stabilize the visual image during head motion together with VOR. Delphilin knockout mice showed facilitated LTD induction and enhanced adaptation of OKR.22) Collectively, many studies have found good correlation between LTD and motor learning. However, a recent study using mutant mice in which endocytosis of AMPAR is suppressed, has shown normal motor learning with impaired LTD,24) and challenged the idea that LTD is necessary for motor learning. Other synaptic or intrinsic plasticity mechanisms as described below might compensate for the LTD defect.
Summary and remaining questions.
Physiological roles of LTD in a behaving animal have been studied using LTD-deficient mutant mice. Adaptation of reflex eye movements such as VOR and OKR, and classical conditioning of eye blink response have been used as model motor learning paradigms. Several studies reported good correlation between LTD and motor learning, although contradictory results have also been reported. Thus, functional significance of LTD has become enigmatic. To clarify roles of LTD in an animal, examination of the effects of acutely inducing or suppressing LTD at specific synapses on motor control and learning, and conversely demonstration of LTD at specific synapses induced by a motor learning paradigm, will be important.
Cerebellar synaptic plasticity other than LTD
Synaptic plasticity in a Purkinje cell.
Besides LTD, post- and presynaptic LTP take place at synapses between parallel fibers and a Purkinje cell. Presynaptic LTP is induced by repetitive stimulation of parallel fibers at a higher frequency (4–8 Hz) and postsynaptic LTP by that at a lower frequency (1 Hz).8,9,45,83–85) Interestingly, we recently found that GluD2 was involved not only in postsynaptic LTD but also in presynaptic LTP.86) Contribution of LTP to motor learning has also been suggested.87) Theorists have argued that a unidirectional change of synaptic efficacy alone is insufficient for effective learning, and that both LTP and LTD are required. In addition, it was reported that postsynaptic LTD also occurs at climbing fiber-Purkinje cell synapses.88)
Inhibitory synapses on a Purkinje cell also show synaptic plasticity. Activation of a climbing fiber or potent depolarization of a Purkinje cell causing a large intracellular Ca2+ increase, induces the enhancement of GABAergic transmission, which is called rebound potentiation (RP).89,90) RP is accompanied by the enhanced responsiveness to GABA, and works to decrease the excitability of a Purkinje cell similarly to LTD. RP is induced by the conditioning stimulation of a climbing fiber or by depolarization of a Purkinje cell. These stimulations were also used to induce LTD coupled with stimulation of parallel fibers. Thus, RP might work synergistically with LTD. Molecular induction mechanism of RP has been extensively studied,90–96) which revealed that several molecules such as CaMKII, protein phosphatases, PDE1 and mGluR1 are involved in both RP and LTD. Therefore, induction of RP might interact with that of LTD. Recently, we extended a theoretical model of intracellular molecular signaling cascade of LTD51) incorporating RP-related molecules.59) Notably, RP is a cell-wide phenomenon, which is different from synapse-specific induction of LTD. However, there is synapse-specific regulation mechanism for RP.90,91) Recently, we generated RP-deficient transgenic mice,97) and found that VOR adaptation was affected in these mice (unpublished results), suggesting the involvement of RP in motor learning.
At inhibitory synapses on a Purkinje cell, presynaptic plasticity mechanisms have also been reported.98,99) They are depolarization-induced suppression of inhibition (DSI)98) and depolarization-induced potentiation of inhibition (DPI).99) DSI is short-lasting (about a minute) suppression of GABA release mediated by endocannabinoid, and DPI is potentiation of GABA release mediated by glutamate and presynaptic NMDA receptor. DPI enhances the inhibitory synaptic transmission on a Purkinje cell together with RP, although it does not last as long as RP.
In addition to synaptic plasticity, plastic change of intrinsic excitability of a Purkinje cell was reported recently.100) Depolarization or parallel fiber burst stimulation amplified the amplitude of synaptic response and that of passively propagated spike through down-regulation of a type of K+ channel. Such dendritic plasticity might also work with synaptic plasticity to regulate the activity of a Purkinje cell.
Synaptic plasticity on postsynaptic neurons other than a Purkinje cell.
Parallel fiber-molecular layer interneuron synapses are suggested to show bidirectional plasticity in a direction opposite to that found at parallel fiber-Purkinje cell synapses.101,102) Coupled activation of a climbing fiber and parallel fibers induces LTP, whereas stimulation of only parallel fibers induces LTD at parallel fiber-interneuron synapses. As molecular layer interneurons inhibit a Purkinje cell, the plasticity can synergistically work with LTD and LTP at parallel fiber-Purkinje cell synapses. Although direct synapse formation of climbing fibers on molecular layer interneurons has not been demonstrated, it has been reported that climbing fibers influence molecular layer interneurons through spillover of glutamate.103)
Synaptic plasticity has also been reported in mossy fiber-granule cell synapses in the granular layer and in the cerebellar nuclei.104,105) In cerebellar nuclei, mossy fiber-nuclear neuron synapses show plasticity depending on the activity of inputs from Purkinje cells.105) Some studies suggested that the memory transfer from the cortex to the nuclei occurs in the later phase of motor learning.106) Purkinje cell output-dependent nuclear synaptic plasticity might contribute to such memory transfer. However, it is to be noted that the number of neurons and synapses are much larger in the cortex. Thus, the memory transfer to nuclear synapses could lose information contents.
Conclusion and perspective
Cerebellar LTD is a long-lasting decrease in the transmission efficacy at parallel fiber-Purkinje cell synapses, which is induced by co-activation of parallel fibers and a climbing fiber. Intensive research efforts have revealed that Ca2+, protein kinases and endocytosis of AMPA receptors are involved in LTD. LTD has been regarded as an essential cellular mechanism for motor learning, although contradictory results have also been published. Implication of cerebellar synaptic plasticity other than LTD in motor learning has been proposed. Comprehensive understanding of molecular regulation mechanism of LTD, and elucidation of specific functional roles of LTD and other synaptic plasticity in the cerebellum, are important issues to be addressed in future.
Acknowledgements
The author thanks Drs. S. Kawaguchi, Y. Tagawa, Y. Goda and M. Yamashita for comments on the manuscript, and Ms. Y. Tanaka for preparing figures.
Abbreviations
- LTD
long-term depression
- GABA
γ-amino butyric acid
- IP3
inositol 1,4,5-trisphosphate
- TRPC3
transient receptor potential canonical 3
- AMPA
α-amino-3-hydroxy-5-methyl-4-isozaxole propionic acid
- PKC
protein kinase C
- GRIP
glutamate receptor interacting protein
- PICK1
protein interacting with C kinase 1
- MAPK
mitogen activated protein kinase
- ERK1/2
extracellular signal-regulated kinase 1/2
- MEK
MAPK/ERK1/2 kinase
- PLA2
phospholipase A2
- AA
arachidonic acid
- NO
nitric oxide
- cGMP
cyclic guanosine monophosphate
- PKG
protein kinase G
- PP2A
protein phosphatase 2A
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- PDE1
phosphodiesterase 1
- GluD2
glutamate receptor δ2 subunit
- mEPSC
miniature excitatory postsynaptic current
- GFP
green fluorescent protein
- VOR
vestibulo-ocular reflex
- OKR
optokinetic response
- LTP
long-term potentiation
- RP
rebound potentiation
- DSI
depolarization-induced suppression of inhibition
- DPI
depolarization-induced potentiation of inhibition
Profile
Tomoo Hirano was born in 1955. He graduated from Department of Zoology, Faculty of Science, University of Tokyo in 1979, and obtained the degree of Doctor of Medical Science from University of Tokyo in 1985. He was appointed as an assistant Professor in Faculty of Medicine, University of Tokyo in 1985, and started electrophysiological studies on cultured rat cerebellar neurons. He worked in Medical School, University of California, Los Angeles from 1986 to 1988. In 1988 he became a lecturer of Faculty of Medicine, Gunma University. He reported the induction of long-term depression in the cerebellar culture in 1990. The cerebellar long-term depression has been thought to be a cellular basis of motor learning. This work was the first demonstration of synaptic plasticity between identified cultured neurons prepared from mammalian central nervous system. In 1991 he was appointed as an associate professor, in Faculty of Medicine, Kyoto University, and started co-works on molecular mechanisms of cerebellar long-term depression. In 1997 he became a professor in Department of Biophysics, Graduate School of Science, Kyoto University. There, he studied not only the cellular and molecular mechanisms of synaptic plasticity but also functional roles of cerebellar synaptic plasticity, working on adaptation of reflex eye movements using mutant mice. He also performed analytical studies aiming for comprehensive understanding of complicated molecular regulation mechanisms of synaptic plasticity, combining cellular physiology and system biological computer simulations. Recently, he developed a novel visualization method of receptor trafficking around the postsynaptic membrane, which would contribute to clarify how synaptic plasticity is expressed.
References
- 1).Ito, M. (1984) The Cerebellum and Neural Control. Raven, New York, pp. 1–580. [Google Scholar]
- 2).Llinás, R.R., Walton, K.D. and Lang, E.J. (2004) Cerebellum. In The Synaptic Organization of the Brain (ed. Shepherd, G.M.) Oxford University Press, New York, pp. 271–309. [Google Scholar]
- 3).Brindley G.S. (1964) The use made by the cerebellum of the information that it receives from sense organs. Int. Brain Res. Org. Bulletin 3, 80 [Google Scholar]
- 4).Marr D. (1969) A theory of cerebellar cortex. J. Physiol. 202, 437–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Ito M. (1970) Neurophysiological aspects of the cerebellar motor control system. Int. J. Neurol. 7, 162–176 [PubMed] [Google Scholar]
- 6).Albus J. (1971) A theory of cerebellar function. Math. Biosci. 10, 25–61 [Google Scholar]
- 7).Ito M., Sakurai M., Tongroach P. (1982) Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. 324, 113–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Sakurai M. (1987) Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J. Physiol. 394, 463–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Hirano T. (1990) Depression and potentiation of the synaptic transmission between a granule cell and a Purkinje cell in rat cerebellar culture. Neurosci. Lett. 119, 141–144 [DOI] [PubMed] [Google Scholar]
- 10).Hirano T., Kubo Y., Wu M.M. (1986) Cerebellar granule cells in culture: monosynaptic connections with Purkinje cells and ionic currents. Proc. Natl. Acad. Sci. U.S.A. 83, 4957–4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Hirano T., Hagiwara S. (1988) Synaptic transmission between rat cerebellar granule and Purkinje cells in dissociated cell culture: effects of excitatory-amino acid transmitter antagonists. Proc. Natl. Acad. Sci. U.S.A. 85, 934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hirano T. (1990) Synaptic transmission between rat inferior olivary neurons and cerebellar Purkinje cells in culture. J. Neurophysiol. 63, 181–189 [DOI] [PubMed] [Google Scholar]
- 13).Linden D.J., Dickinson M.H., Smeyne M., Connor J.A. (1991) A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron 7, 81–89 [DOI] [PubMed] [Google Scholar]
- 14).Robinson D.A. (1981) The use of control systems analysis in the neurophysiology of eye movements. Annu. Rev. Neurosci. 4, 463–503 [DOI] [PubMed] [Google Scholar]
- 15).Ito M. (1982) Cerebellar control of the vestibulo-ocular reflex—around the flocculus hypothesis. Annu. Rev. Neurosci. 5, 275–296 [DOI] [PubMed] [Google Scholar]
- 16).du Lac S., Raymond J.L., Sejnowski T.J., Lisberger S.G. (1995) Learning and memory in the vestibulo-ocular reflex. Annu. Rev. Neurosci. 18, 409–441 [DOI] [PubMed] [Google Scholar]
- 17).Thompson R.F. (2005) In search of memory traces. Annu. Rev. Psychol. 56, 1–23 [DOI] [PubMed] [Google Scholar]
- 18).Ito, M. (2011) The Cerebellum: Brain for an Implicit Self. FT Press, New Jersey, pp. 1–285. [Google Scholar]
- 19).Aiba A., Kano M., Chen C., Stanton M.E., Fox G.D., Herrup K., Zwingman T.A., Tonegawa S. (1994) Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 7, 377–388 [PubMed] [Google Scholar]
- 20).De Zeeuw C.I., Hansel C., Bian F., Koekkoek S.K., van Alphen A.M., Linden D.J., Oberdick J. (1998) Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20, 495–508 [DOI] [PubMed] [Google Scholar]
- 21).Hirano T. (2006) Cerebellar regulation mechanisms learned from studies on GluRδ2. Mol. Neurobiol. 33, 1–16 [DOI] [PubMed] [Google Scholar]
- 22).Takeuchi T., Ohtsuki G., Yoshida T., Fukaya M., Wainai T., Yamashita M., Yamazaki Y., Mori H., Sakimura K., Kawamoto S., Watanabe M., Hirano T., Mishina M. (2008) Enhancement of both long-term depression induction and optokinetic response adaptation in mice lacking delphilin. PLoS ONE 3, e2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Welsh J.P., Yamaguchi H., Zeng X.H., Kojo M., Nakada Y., Takagi A., Sugimori M., Llinás R.R. (2005) Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc. Natl. Acad. Sci. U.S.A. 102, 17166–17171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Schonewille M., Gao Z., Boele H.J., Veloz M.F., Amerika W.E., Simek A.A., De Jeu M.T., Steinberg J.P., Takamiya K., Hoebeek F.E., Linden D.J., Huganir R.L., De Zeeuw C.I. (2011) Reevaluating the role of LTD in cerebellar motor learning. Neuron 70, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Hansel C., Linden D.J., D'Angelo E. (2001) Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 4, 467–475 [DOI] [PubMed] [Google Scholar]
- 26).Jörntell H., Hansel C. (2006) Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52, 227–238 [DOI] [PubMed] [Google Scholar]
- 27).Dean P., Porrill J., Ekerot C.F., Jörntell H. (2010) The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat. Rev. Neurosci. 11, 30–43 [DOI] [PubMed] [Google Scholar]
- 28).Gao Z., van Beugen B.J., De Zeeuw C.I. (2012) Distributed synergistic plasticity and cerebellar learning. Nat. Rev. Neurosci. 13, 619–635 [DOI] [PubMed] [Google Scholar]
- 29).Llinás R., Sugimori M. (1980) Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J. Physiol. (London) 305, 171–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Miyakawa H., Lev-Ram V., Lasser-Ross N., Ross W.N. (1992) Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. J. Neurophysiol. 68, 1178–1189 [DOI] [PubMed] [Google Scholar]
- 31).Hirano T. (1990) Effects of postsynaptic depolarization in the induction of synaptic depression between a granule cell and a Purkinje cell in rat cerebellar culture. Neurosci. Lett. 119, 145–147 [DOI] [PubMed] [Google Scholar]
- 32).Crepel F., Jaillard D. (1991) Pairing of pre- and postsynaptic activities in cerebellar Purkinje cells induces long-term changes in synaptic efficacy in vitro. J. Physiol. 432, 123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Kasono K., Hirano T. (1994) Critical role of postsynaptic calcium in the cerebellar long-term depression. Neuroreport 6, 17–20 [DOI] [PubMed] [Google Scholar]
- 34).Sakurai M. (1990) Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc. Natl. Acad. Sci. U.S.A. 87, 3383–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Shigemoto R., Abe T., Nomura S., Nakanishi S., Hirano T. (1994) Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron 12, 1245–1255 [DOI] [PubMed] [Google Scholar]
- 36).Berridge M.J. (2009) Inositol trisphosphate and calcium signaling mechanisms. Biochim. Biophys. Acta 1793, 933–940 [DOI] [PubMed] [Google Scholar]
- 37).Nishizuka Y. (2003) Discovery and prospect of protein kinase C research: epilogue. J. Biochem. 133, 155–158 [DOI] [PubMed] [Google Scholar]
- 38).Hirono M., Sugiyama T., Kishimoto Y., Sakai I., Miyazawa T., Kishio M., Inoue H., Nakao K., Ikeda M., Kawahara S., Kirino Y., Katsuki M., Horie H., Ishikawa Y., Yoshioka T. (2001) Phospholipase Cβ4 and protein kinase Cα and/or protein kinase Cβ are involved in the induction of long term depression in cerebellar Purkinje cells. J. Biol. Chem. 276, 45236–45242 [DOI] [PubMed] [Google Scholar]
- 39).Tsuruno S., Hirano T. (2007) Persistent activation of protein kinase Cα is not necessary for expression of cerebellar long-term depression. Mol. Cell. Neurosci. 35, 38–48 [DOI] [PubMed] [Google Scholar]
- 40).Leitges M., Kovac J., Plomann M., Linden D.J. (2004) A unique PDZ ligand in PKCα confers induction of cerebellar long-term synaptic depression. Neuron 44, 585–594 [DOI] [PubMed] [Google Scholar]
- 41).Chae H.G., Ahn S.J., Hong Y.H., Chang W.S., Kim J., Kim S.J. (2012) Transient receptor potential canonical channels regulate the induction of cerebellar long-term depression. J. Neurosci. 32, 12909–12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Doi T., Kuroda S., Michikawa T., Kawato M. (2005) Inositol 1,4,5-trisphosphate-dependent Ca2+ threshold dynamics detect spike timing in cerebellar Purkinje cells. J. Neurosci. 25, 950–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Hartell N. (1996) Strong activation of parallel fibers produces localized calcium transients and a form of LTD that spreads to distant synapses. Neuron 16, 601–610 [DOI] [PubMed] [Google Scholar]
- 44).Tanaka K., Khiroug L., Santamaria F., Doi T., Ogasawara H., Ellis-Davies G., Kawato M., Augustine G.J. (2007) Ca2+ requirements for cerebellar long-term synaptic depression: role for a postsynaptic leaky integrator. Neuron 54, 787–800 [DOI] [PubMed] [Google Scholar]
- 45).Hirano T. (1991) Differential pre- and postsynaptic mechanisms for synaptic potentiation and depression between a granule cell and a Purkinje cell in rat cerebellar culture. Synapse 7, 321–323 [DOI] [PubMed] [Google Scholar]
- 46).Linden D.J. (2001) The expression of cerebellar LTD in culture is not associated with changes in AMPA-receptor kinetics, agonist affinity, or unitary conductance. Proc. Natl. Acad. Sci. U.S.A. 98, 14066–14071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Wang Y.T., Linden D.J. (2000) Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25, 635–647 [DOI] [PubMed] [Google Scholar]
- 48).Matsuda S., Launey T., Mikawa S., Hirai H. (2000) Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 19, 2765–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Xia J., Chung H.J., Wihler C., Huganir R.L., Linden D.J. (2000) Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron 28, 499–510 [DOI] [PubMed] [Google Scholar]
- 50).Kawasaki H., Fujii H., Gotoh Y., Morooka T., Shimohama S., Nishida E., Hirano T. (1999) Requirement for mitogen-activated protein kinase in cerebellar long-term depression. J. Biol. Chem. 274, 13498–13502 [DOI] [PubMed] [Google Scholar]
- 51).Kuroda S., Schweighofer N., Kawato M. (2001) Exploration of signal transduction pathways in cerebellar long-term depression by kinetic simulation. J. Neurosci. 21, 5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Tanaka K., Augustine G.J. (2008) A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron 59, 608–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Yamamoto Y., Lee D., Kim Y., Lee B., Seo C., Kawasaki H., Kuroda S., Tanaka-Yamamoto K. (2012) Raf kinase inhibitory protein is required for cerebellar long-term synaptic depression mediating PKC-dependent MAPK activation. J. Neurosci. 32, 14254–14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Shibuki K., Okada D. (1991) Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature 349, 326–328 [DOI] [PubMed] [Google Scholar]
- 55).Boxall A., Garthwaite J. (1996) Long-term depression in rat cerebellum requires both NO synthase and NO-sensitive guanylyl cyclase. Eur. J. Neurosci. 8, 2209–2212 [DOI] [PubMed] [Google Scholar]
- 56).Lev-Ram V., Jiang T., Wood J., Lawrence D.S., Tsien R.Y. (1997) Synergies and coincidence requirements between NO, cGMP, and Ca2+ in the induction of cerebellar long-term depression. Neuron 18, 1025–1038 [DOI] [PubMed] [Google Scholar]
- 57).Hansel C., de Jeu M., Belmeguenai A., Houtman S.H., Buitendijk G.H., Andreev D., De Zeeuw C.I., Elgersma Y. (2006) αCaMKII is essential for cerebellar LTD and motor learning. Neuron 51, 835–843 [DOI] [PubMed] [Google Scholar]
- 58).van Woerden G.M., Hoebeek F.E., Gao Z., Nagaraja R.Y., Hoogenraad C.C., Kushner S.A., Hansel C., De Zeeuw C.I., Elgersma Y. (2009) βCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat. Neurosci. 12, 823–825 [DOI] [PubMed] [Google Scholar]
- 59).Kawaguchi S., Hirano T. (2013) Gating of long-term depression by CaMKII through enhanced cGMP signaling in cerebellar Purkinje cells. J. Physiol. 591, 1707–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Araki K., Meguro H., Kushiya E., Takayama C., Inoue Y., Mishina M. (1993) Selective expression of the glutamate receptor channel δ2 subunit in cerebellar Purkinje cells. Biochem. Biophys. Res. Commun. 197, 1267–1276 [DOI] [PubMed] [Google Scholar]
- 61).Lomeli H., Sprengel R., Laurie D.J., Kohr G., Herb A., Seeburg P.H., Wisden W. (1993) The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 315, 318–322 [DOI] [PubMed] [Google Scholar]
- 62).Hirano T., Kasono K., Araki K., Shinozuka K., Mishina M. (1994) Involvement of the glutamate receptor δ2 subunit in the long-term depression of glutamate responsiveness in cultured rat Purkinje cells. Neurosci. Lett. 182, 172–176 [DOI] [PubMed] [Google Scholar]
- 63).Kashiwabuchi N., Ikeda K., Araki K., Hirano T., Shibuki K., Takayama C., Inoue Y., Kutsuwada T., Yagi T., Kang Y., Aizawa S., Mishina M. (1995) Disturbed motor coordination, Purkinje cell synapse formation and cerebellar long-term depression of mice defective in the δ2 subunit of the glutamate receptor channel. Cell 81, 245–252 [DOI] [PubMed] [Google Scholar]
- 64).Yawata S., Tsuchida H., Kengaku M., Hirano T. (2006) Membrane-proximal region of GluRδ2 is critical for LTD and interaction with PICK1 in a cerebellar Purkinje neuron. J. Neurosci. 26, 3626–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Kohda K., Kakegawa W., Matsuda S., Nakagami R., Kakiya N., Yuzaki M. (2007) The extreme C-terminus of GluRδ2 is essential for induction of long-term depression in cerebellar slices. Eur. J. Neurosci. 25, 1357–1362 [DOI] [PubMed] [Google Scholar]
- 66).Uemura T., Kakizawa S., Yamasaki M., Sakimura K., Watanabe M., Iino M., Mishina M. (2007) Regulation of long-term depression and climbing fiber territory by glutamate receptor δ2 at parallel fiber synapses through its C-terminal domain in cerebellar Purkinje cells. J. Neurosci. 27, 12096–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Kato A.S., Knierman M.D., Siuda E.R., Isaac J.T.R., Nisenbaum E.S., Bredt D.S. (2012) Glutamate receptor δ2 associates with metabotropic glutamate receptor 1 (mGluR1), protein kinase Cγ, and canonical transient receptor potential 3 and regulates mGluR1-mediated synaptic transmission in cerebellar Purkinje neurons. J. Neurosci. 32, 15296–15308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Murashima M., Hirano T. (1999) Entire course and distinct phases of day-lasting depression of mEPSC amplitudes in cultured Purkinje neurons. J. Neurosci. 19, 7317–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Linden D.J. (1996) A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron 17, 483–490 [DOI] [PubMed] [Google Scholar]
- 70).Linden D.J., Connor J.A. (1992) Long-term depression of glutamate currents in cultured cerebellar Purkinje neurons does not require nitric oxide signaling. Eur. J. Neurosci. 4, 10–15 [DOI] [PubMed] [Google Scholar]
- 71).Hirano T., Kasono K. (1993) Spatial distribution of excitatory and inhibitory synapses on a Purkinje cell in a rat cerebellar culture. J. Neurophysiol. 70, 1316–1325 [DOI] [PubMed] [Google Scholar]
- 72).Williams M.E., Wilke S.A., Daggett A., Davis E., Otto S., Ravi D., Ripley B., Bushong E.A., Ellisman M.H., Klein G., Ghosh A. (2011) Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron 71, 640–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Miyawaki H., Hirano T. (2011) Different correlations among physiological and morphological properties at single glutamatergic synapses in the rat hippocampus and the cerebellum. Synapse 65, 412–423 [DOI] [PubMed] [Google Scholar]
- 74).Tanaka H., Hirano T. (2012) Visualization of subunit-specific delivery of glutamate receptors to postsynaptic membrane during hippocampal long-term potentiation. Cell Rep. 1, 291–298 [DOI] [PubMed] [Google Scholar]
- 75).Maekawa K., Simpson J.I. (1973) Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual pathway. J. Neurophysiol. 36, 649–666 [DOI] [PubMed] [Google Scholar]
- 76).Nagao S. (1989) Behavior of floccular Purkinje cells correlated with adaptation of vestibulo-ocular reflex in pigmented rabbits. Exp. Brain Res. 77, 531–540 [DOI] [PubMed] [Google Scholar]
- 77).Lisberger S.G., Pavelko T.A., Bronte-Stewart H.M., Stone L.S. (1994) Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J. Neurophysiol. 72, 954–973 [DOI] [PubMed] [Google Scholar]
- 78).Hirata Y., Highstein S. (2001) Acute adaptation of the vestibuloocular reflex: signal processing by floccular and ventral parafloccular Purkinje cells. J. Neurophysiol. 85, 2267–2288 [DOI] [PubMed] [Google Scholar]
- 79).Mauk M.D., Donegan N.H. (1997) A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn. Mem. 4, 130–158 [DOI] [PubMed] [Google Scholar]
- 80).Freeman J.H., Steinmetz A.B. (2011) Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn. Mem. 18, 666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Moyer J.R., Jr., Deyo R.A., Disterhoft J.F. (1990) Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 104, 243–252 [DOI] [PubMed] [Google Scholar]
- 82).Takatsuki K., Kawahara S., Kotani S., Fukunaga S., Mori H., Mishina M., Kirino Y. (2003) The hippocampus plays an important role in eyeblink conditioning with a short trace interval in glutamate receptor subunit δ2 mutant mice. J. Neurosci. 23, 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Salin P., Malenka R., Nicoll R. (1996) Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 16, 797–803 [DOI] [PubMed] [Google Scholar]
- 84).Lev-Ram V., Wong S., Storm D., Tsien R. (2002) A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc. Natl. Acad. Sci. U.S.A. 99, 8389–8393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Coesmans M., Weber J., De Zeeuw C., Hansel C. (2004) Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44, 691–700 [DOI] [PubMed] [Google Scholar]
- 86).Yamashita, M., Kawaguchi, S. and Hirano, T. (2013) Contribution of postsynaptic GluD2 to presynaptic R-type Ca2+ channel function, glutamate release and long-term potentiation at parallel fiber to Purkinje cell synapses. Cerebellum (online). [DOI] [PubMed] [Google Scholar]
- 87).Schonewille M., Belmeguenai A., Koekkoek S.K., Houtman S.H., Boele H.J., van Beugen B.J., Gao Z., Badura A., Ohtsuki G., Amerika W.E., Hosy E., Hoebeek F.E., Elgersma Y., Hansel C., De Zeeuw C.I. (2010) Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 67, 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Hansel C., Linden D.J. (2000) Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron 26, 473–482 [DOI] [PubMed] [Google Scholar]
- 89).Kano M., Rexhausen U., Dreessen J., Konnerth A. (1992) Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature 356, 601–604 [DOI] [PubMed] [Google Scholar]
- 90).Kawaguchi S., Hirano T. (2000) Suppression of inhibitory synaptic potentiation by presynaptic activity through postsynaptic GABAB receptors in a Purkinje neuron. Neuron 27, 339–347 [DOI] [PubMed] [Google Scholar]
- 91).Kawaguchi S., Hirano T. (2002) Signaling cascade regulating long-term potentiation of GABAA receptor responsiveness in cerebellar Purkinje neurons. J. Neurosci. 22, 3969–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Kawaguchi S., Hirano T. (2006) Integrin α3β1 suppresses long-term potentiation at inhibitory synapses on the cerebellar Purkinje neuron. Mol. Cell. Neurosci. 31, 416–426 [DOI] [PubMed] [Google Scholar]
- 93).Kawaguchi S., Hirano T. (2007) Sustained structural change of GABAA receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J. Neurosci. 27, 6788–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Sugiyama Y., Kawaguchi S., Hirano T. (2008) mGluR1-mediated facilitation of long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. Eur. J. Neurosci. 27, 884–896 [DOI] [PubMed] [Google Scholar]
- 95).Kitagawa Y., Hirano T., Kawaguchi S. (2009) Prediction and validation of a mechanism to control the threshold for inhibitory synaptic plasticity. Mol. Syst. Biol. 5, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Kawaguchi S., Nagasaki N., Hirano T. (2011) Dynamic impact of temporal context of Ca2+ signals on inhibitory synaptic plasticity. Sci. Rep. 1, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Tanaka, S., Kawaguchi, S. and Hirano, T. (2012) Generation of transgenic mice deficient in cerebellar inhibitory synaptic plasticity. Abstract, The 35th annual meeting of the Japan neuroscience society. [Google Scholar]
- 98).Yoshida T., Hashimoto K., Zimmer A., Maejima T., Araishi K., Kano M. (2002) The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J. Neurosci. 22, 1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Duguid I.C., Smart T.G. (2004) Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat. Neurosci. 7, 525–533 [DOI] [PubMed] [Google Scholar]
- 100).Ohtsuki G., Piochon C., Adelman J.P., Hansel C. (2012) SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron 75, 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Jörntell H., Ekerot C.F. (2002) Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron 34, 797–806 [DOI] [PubMed] [Google Scholar]
- 102).Jörntell H., Ekerot C.F. (2003) Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J. Neurosci. 23, 9620–9631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103).Szapiro G., Barbour B. (2007) Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat. Neurosci. 10, 735–742 [DOI] [PubMed] [Google Scholar]
- 104).D'Angelo E., Rossi P., Gall D., Prestori F., Nieus T., Maffei A., Sola E. (2005) Long-term potentiation of synaptic transmission at the mossy fiber-granule cell relay of cerebellum. Prog. Brain Res. 148, 69–80 [DOI] [PubMed] [Google Scholar]
- 105).Pugh J., Raman I. (2006) Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 51, 113–123 [DOI] [PubMed] [Google Scholar]
- 106).Okamoto T., Endo S., Shirao T., Nagao S. (2011) Role of cerebellar cortical protein synthesis in transfer of memory trace of cerebellum-dependent motor learning. J. Neurosci. 31, 8958–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]