Abstract
This guideline presents recommendations for the evaluation and management of patients with gastroparesis. Gastroparesis is identified in clinical practice through the recognition of the clinical symptoms and documentation of delayed gastric emptying. Symptoms from gastroparesis include nausea, vomiting, early satiety, postprandial fullness, bloating, and upper abdominal pain. Management of gastroparesis should include assessment and correction of nutritional state, relief of symptoms, improvement of gastric emptying and, in diabetics, glycemic control. Patient nutritional state should be managed by oral dietary modifications. If oral intake is not adequate, then enteral nutrition via jejunostomy tube needs to be considered. Parenteral nutrition is rarely required when hydration and nutritional state cannot be maintained. Medical treatment entails use of prokinetic and antiemetic therapies. Current approved treatment options, including metoclopramide and gastric electrical stimulation (GES, approved on a humanitarian device exemption), do not adequately address clinical need. Antiemetics have not been specifically tested in gastroparesis, but they may relieve nausea and vomiting. Other medications aimed at symptom relief include unapproved medications or off-label indications, and include domperidone, erythromycin (primarily over a short term), and centrally acting antidepressants used as symptom modulators. GES may relieve symptoms, including weekly vomiting frequency, and the need for nutritional supplementation, based on open-label studies. Second-line approaches include venting gastrostomy or feeding jejunostomy; intrapyloric botulinum toxin injection was not effective in randomized controlled trials. Most of these treatments are based on open-label treatment trials and small numbers. Partial gastrectomy and pyloroplasty should be used rarely, only in carefully selected patients. Attention should be given to the development of new effective therapies for symptomatic control.
This clinical guideline addresses the definition, diagnosis, differential diagnosis, and treatment of gastroparesis, including nutritional supplementation, glycemic control, pharmacological, endoscopic, device, and surgical therapy.
Each section of this document will present the key recommendations related to the section topic and a subsequent summary of the evidence supporting those recommendations. An overall summary will be presented in the first table. A search of OVID Medline, Pubmed, and ISI Web of Science was conducted for the years from 1960 to 2011 using the following major search terms and subheadings including “gastroparesis,” “electrical stimulation,” “botulinum toxin,” “drug therapy,” “glycemic control,” “dietary therapy,” and “alternative therapy”. We used systematic reviews and meta-analyses for each topic when available, followed by a review of clinical trials.
The GRADE system was used to evaluate the strength of the recommendations and the overall quality of evidence (1) (Table 1). The strength of a recommendation was graded as “strong” when the desirable effects of an intervention clearly outweigh the undesirable effects and as “conditional” when there is uncertainty about the trade-offs. The quality of evidence could range from “high” (implying that further research was unlikely to change the authors ‘ confidence in the estimate of the effect) to “moderate” (further research would be likely to have an impact on the confidence in the estimate of effect) or “low” (further research would be expected to have an important impact on the confidence in the estimate of the effect and would be likely to change the estimate).
Table 1.
Criteria for assigning grade of evidence
| Type of evidence |
| Randomized trial=high |
| Observational study=low |
| Any other evidence=very low |
| Decrease grade if: |
| Serious (−1) or very serious (−2) limitation to study quality |
| Important inconsistency (−1) |
| Some (−1) or major (−2) uncertainty about directness |
| Imprecise or sparse data (−1) |
| High probability of reporting bias (−1) |
| Increase grade if: |
| Strong evidence of association— significant relative risk of > 2 (< 0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1) |
| Very strong evidence of association — significant relative risk of > 5 (< 0.2) based on direct evidence with no major threats to validity (+ 2) |
| Evidence of a dose response gradient (+ 1) |
| All plausible confounders would have reduced the effect (+ 1) |
| Definitions of grades of evidence |
| High = Further research is unlikely to change our confidence in the estimate of effect |
| Moderate =Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| Low =Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low =Any estimate of effect is very uncertain |
DEFINITION OF GASTROPARESIS SYNDROME AND GASTROPARESIS SYMPTOMS
Summary of Evidence
Gastroparesis is defined as a syndrome of objectively delayed gastric emptying in the absence of mechanical obstruction and cardinal symptoms including early satiety, postprandial fullness, nausea, vomiting, bloating, and upper abdominal pain (2); the same constellation of complaints may be seen with other etiologies, including gastritis secondary to Heli-cobacter pylori infection, peptic ulcer, and functional dyspepsia. Symptoms have not been well correlated with gastric emptying. Nausea, vomiting, early satiety, and postprandial fullness correlate better with delayed gastric emptying than upper abdominal pain and bloating (3,4). The epidemiology and impact of gastroparesis are reviewed elsewhere (2). In summary, although a high prevalence of gastroparesis has been reported in type 1 diabetics (40 %) and type 2 diabetics (10–20 %), these studies were from tertiary academic medical centers where the prevalence is expected to be higher than the general population; the community prevalence was estimated to be ∼ 5 % among type 1 diabetics, 1 % among type 2 diabetics, and 0.2 % of controls in Olmsted County, Minnesota (5). More community-based data are required to confirm or enhance the published figures. Gastroparesis significantly impacts quality of life (6,7), increases direct health-care costs through hospitalizations, emergency room, or doctor visits, and is associated with morbidity and mortality (8,9).
The symptoms are often the same with the different etiologies of gastroparesis: nausea, vomiting, early satiety, and postprandial fullness (10). In 416 patients from the NIH Gastroparesis Registry, symptoms prompting evaluation more often included vomiting for diabetic gastroparesis (DG) and abdominal pain for idiopathic gastroparesis (IG). Patients with IG have more early satiety and abdominal pain compared with patients with DG who have more severe retching; all the patients included in these multicenter studies had documentation of delayed gastric emptying in their medical record (11,12).
Abdominal pain is an often under-appreciated symptom in gastro paresis. In a multicenter study from an NIH consortium on gastroparesis, 72 % of patients with gastroparesis had abdominal pain, but was the dominant symptom in only 18 % (13), reflecting the heterogeneous patient population in this cohort. A tertiary referral study showed that abdominal pain was reported in 90 % of 68 patients with delayed gastric emptying (18 DG and 50 IG). Pain was induced by eating (72 %), was nocturnal (74 %), and interfered with sleep (66 %). Severity ranking of abdominal pain was in the same range as other symptoms (e.g., fullness, bloating, and nausea) and was not correlated with gastric emptying rate, but was associated with impaired quality of life. The preponderance of the idiopathic group and large proportion of daily (43 %) or even constant pain (38 %) in this cohort of patients may reflect the type of referred patients often seen in tertiary academic centers (12). The presence of anxiety or depression has been associated with more severe symptoms (14,15).
The combination of symptoms and delayed gastric emptying is required to establish the diagnosis of gastroparesis as the epidemiology, natural history, pathophysiology, and treatment of gastroparesis (which are reviewed in detail elsewhere (2)) are typically based on combined criteria. Diabetes with evidence of gastroparesis on objective testing has been associated with increased health-care costs, including increased clinic visits, emergency room visits, hospitalizations, overall morbidity and mortality (8,9).
Since accelerated gastric emptying and functional dyspepsia can also present with symptoms similar to gastroparesis, documentation of delayed gastric emptying (3,16) is necessary before selecting therapy with prokinetics agents or GES.
IDENTIFYING THE CAUSE OF GASTROPARESIS
Summary of Evidence
Diabetic (29%), postsurgical (13%), and idiopathic (36 %) etiologies comprise the majority of cases in tertiary referral setting (8). Diabetes mellitus is the most commonly recognized systemic disease associated with gastroparesis. In the NIH consortium cohort, delayed gastric emptying was more pronounced in patients with type 1 DG (10). The 10-year incidence of gastroparesis has been reported to be 5.2 % in type 1 diabetes, 1 % in type 2 diabetes, and 0.2 % in non-diabetic controls in a US community (5).
Idiopathic gastroparesis refers to a symptomatic patient from delayed gastric empting with no detectable primary underlying abnormality for the delayed gastric emptying. This may represent the most common form of gastroparesis (10,17). Most patients with IG are women; typically young or middle aged. Symptoms of IG overlap with those of functional dyspepsia; it may be difficult to provide a definitive distinction between the two based on symptoms, and many regard IG and functional dyspepsia with delayed gastric emptying as the same condition. Abdominal pain/discomfort typically is the predominant symptom in functional dyspepsia, whereas nausea, vomiting, early satiety, and bloating predominate in IG. Therefore, measurement of gastric emptying is important, as therapies differ if gastric emptying is delayed, normal, or rapid.
A subset of patients with gastroparesis report sudden onset of symptoms after a viral prodrome, suggesting a potential viral etiology for their symptoms, and the diagnosis of postviral gastroparesis (18,19). Previously, healthy subjects have developed the sudden onset of nausea, vomiting, diarrhea, fever, and cramps suggestive of a systemic viral infection. However, instead of experiencing resolution of symptoms, these individuals note persistent nausea, vomiting, and early satiety. Over a period of about a year, the gastroparesis often improves. In general, this course is typical of postviral gastroparesis that is not associated with autonomic neuropathy. On the other hand, a minority of patients with infections due to viruses such as cytomegalovirus, Epstein – Barr virus, and varicella zoster may develop a form of autonomic neuropathy (generalized or selective cholinergic dysautonomia) that includes gastroparesis. These patients with autonomic dysfunction may have slower resolution of their symptoms that may take several years and the prognosis is worse than in postviral gastroparesis without autonomic disorders (20,21).
Postsurgical gastroparesis (PSG), often with vagotomy or vagus nerve injury, represents the third most common etiology of gastroparesis. In the past, most cases resulted from vagotomy performed in combination with gastric drainage to correct medically refractory or complicated peptic ulcer disease. Since the advent of laparoscopic techniques for the treatment of GERD, gastroparesis has become a recognized complication of fundoplication (possibly from vagal injury during the surgery) or bariatric surgery that involves gastroplasty or bypass procedures. The combination of vagotomy, distal gastric resection, and Roux-en-Y gastrojejunostomy predisposes to slow emptying from the gastric remnant and delayed transit in the denervated Roux efferent limb. The Roux-en-Y stasis syndrome — characterized by postprandial abdominal pain, bloating, nausea, and vomiting — is particularly difficult to manage, and its severity may be proportional to the length of the Roux limb (generally, 25 cm is ideal to avoid stasis).
The precise role of the antireflux surgery itself is not clearly demonstrated in the published literature. Thus, while symptoms suggesting gastric stasis are extremely common in the first 3 months after fundoplication, they persist in a minority of patients at 1 year post surgery. In a series of 615 patients who underwent laparoscopic Nissen fundoplication, all had symptoms during the first 3 postoperative months (e.g., early satiety in 88 % and bloating/flatulence in 64 %); however, by 1 year these symptoms suggestive of gastroparesis like bloating/flatulence had resolved in > 90 % of patients (22). Moreover, among 81 patients with antireflux operations followed for > 1 year, the finding of postoperative symptoms suggesting delayed gastric emptying was usually associated with delayed gastric emptying pre-operatively (23). The precise role of fundoplication is therefore difficult to determine unless the patient undergoes testing for abdominal vagal dysfunction, such as the plasma pancreatic polypeptide response to modified sham feeding; such tests are described elsewhere (24).
In patients with refractory symptoms of GERD, investigation for delayed gastric emptying should be considered, since delayed gastric emptying can be associated with GERD and possibly aggravate symptoms of heartburn, regurgitation, and other symptoms associated with GERD.
Known causes of iatrogenic gastroparesis include surgical vagal disruption, which may be due to vagal nerve injury (e.g., after fundoplication for GERD), or intentional vagotomy as part of peptic ulcer surgery. The second major category of iatrogenic gastroparesis is induced by pharmacological agents as may occur with narcotic opiate analgesics, anticholinergic agents, and some diabetic medications. Administration of µ-opiate receptor agonists results in delayed gastric emptying and also may cause nausea and vomiting. These include agents such as morphine (25), as well as oxycodone and tapentadol (26), but less with tramadol (27). Therefore, patients receiving such agents should first undergo withdrawal of the agent before assuming a diagnosis of gastroparesis. GLP-1 analogs, such as exenatide, used for treatment of type 2 diabetes mellitus (28) can delay gastric emptying. In contrast to GLP-1 analogs, which substantially increase plasma GLP-1 concentrations, dipeptidyl peptidase IV inhibitors, which increase plasma GLP-1 concentrations by inhibiting metabolism of GLP-1, do not delay gastric emptying (29). Nausea (43.5 %) was the most commonly reported adverse event with exenatide treatment, and vomiting was also quite commonly encountered (12.8% (30)). The antirejection drug, cyclosporine, can delay gastric emptying. Thus, in patients with prior pancreatic transplantation treated with antirejection treatment with cyclosporine, there may be delay in gastric emptying (31). This does not apply to another calcineurin inhibitor, tacrolimus, which is derived from a macrolide molecule and retains prokinetic properties (32).
Other rarer causes of gastroparesis include diseases affecting the extrinsic neural control (such as Parkinsonism, amyloidosis, and paraneoplastic disease) or disorders that result in infiltration or degeneration of the muscle layer of the stomach (such as scleroderma). Mesenteric ischemia should also be considered as a rare cause of gastroparesis that is potentially reversible.
DIAGNOSIS OF GASTROPARESIS
Summary of evidence
There are three tests to objectively demonstrate delayed gastric emptying: scintigraphy, wireless motility capsule (WMC), and breath testing.
For any type of gastric emptying test, patients should discontinue medications that may affect gastric emptying. For most medications, this will be 48 – 72 h. These include medications that can delay gastric emptying, such as narcotic opioid analgesics and anticholinergic agents. These agents may give a falsely delayed result. Medications that accelerate gastric emptying, such as metoclopramide, domperidone, and erythromycin, may give a falsely normal result. Hyperglycemia (glucose level >200 mg/dl) delays gastric emptying in diabetic patients. It is recommended to defer gastric emptying testing until relative euglycemia (blood glucose < 275 mg/dl) is achieved in diabetics to obtain a reliable determination of emptying parameters in the absence of acute metabolic derangement.
The conventional test for measurement of gastric emptying is scintigraphy (33,34). Gastric emptying scintigraphy of a solid-phase meal is considered as the standard for diagnosis of gastroparesis, as it quantifies the emptying of a physiologic caloric meal. For solid-phase testing, most centers use a 99m Tc sulfur colloidlabeled egg sandwich as the test meal, with standard imaging at 0,1,2, and 4h. A4-h gastric emptying scintigraphy test using radiolabeled EggBeaters (ConAgra Foods Inc., Omaha, NE, USA) meal with jam, toast, and water is advocated by the Society of Nuclear Medicine and The American Neurogastroenterology and Motility Society (34). Assessment of gastric emptying over 4 h is necessary (35). Shorter duration solid emptying or sole liquid emptying by scintigraphy is associated with lower diagnostic sensitivity. Measurement of liquid gastric emptying, simultaneously or in addition to solid emptying, has been advocated as a means of increasing sensitivity (by an estimated 25 – 36 % in non-diabetics) to detect the presence of gastroparesis in patients with upper gastrointestinal (GI) symptoms (36,37). On the other hand, the clinical significance of selectively delayed gastric emptying of liquids has not been assessed, for example, in terms of its value in predicting response of symptoms to treatment. There is evidence that the effect of hyperglycemia on gastric emptying in diabetics is more clearly demonstrated in the retardation of the gastric emptying of liquids (38).
The most reliable parameter to report gastric emptying is the gastric retention at 4 h. Gastric emptying T1/2 is also acceptable if imaging has been performed for 4 h or at least to 50 % emptying, as extrapolation to measure t1/2 may be erroneous. However, it is also important to assess emptying at least 1 and 2 h after radiolabeled meal ingestion, since prolongation of the early phases of emptying may also be associated with symptoms of gastroparesis, even though the gastric retention at 4 h is normal or mildly delayed. Gastric emptying T1/2 can be quite easily inferred from the linear interpolation of the data points at 1, 2, and 4 h, since the emptying phase of solids is generally linear after the initial lag phase and gastroparesis due to neuropathic or myopathic motility disorders retards gastric emptying T1/2 (39–41).
A WMC that measures pH, pressure, and temperature can assess gastric emptying by the acidic gastric residence time of the capsule. Gastric emptying is determined when there is a rapid increase in the pH recorded indicating emptying from the acidic stomach to the alkaline duodenum. The gastric residence time of the WMC (e.g., SmartPill, Given Imaging, Yoqneam, Israel) had a high correlation 85 % with the T-90 % of gastric emptying scintigraphy (that is the time when there was only 10 % of the meal remaining in the stomach), suggesting that the gastric residence time of the WMC represents a time near the end of the emptying of a solid meal (42). The overall correlation between gastric emptying time of the WMC and gastric emptying at 4 h by scintigraphy was 0.73. A 5-h gastric residence time of the WMC was best to differentiate subjects with delayed or normal gastric emptying based on scintigraphy conducted simultaneously with sensitivity of 83 % and specificity of 83%.
Breath testing has been used in both clinical and clinical research studies for determining gastric emptying (43). These breath tests using 13 C-octanoate or -spirulina (44) provide reproducible results that correlate with results on gastric emptying scintigraphy, including responsiveness to pharmacological therapy. The optimization of mathematical models for measurement of gastric emptying derived from breath excretion profiles has been thoroughly examined in the literature (45). Both WMC and breath testing require further validation before they can be considered as alternates to scintigraphy for diagnosis of gastroparesis.
EXCLUSION CRITERIA AND DIFFERENTIAL DIAGNOSIS
Summary of Evidence
The vomiting symptom of a patient can be difficult to differentiate from the regurgitation seen in GERD or the regurgitation seen in rumination syndrome. Rumination syndrome is a condition characterized by the repetitive, effortless regurgitation of recently ingested food into the mouth followed by re-chewing and re-swallowing or expectorating of food. Although initially described in infants and the developmentally disabled, rumination syndrome is now widely recognized at all ages and cognitive abilities; the condition is more frequent in females, but it is recognized in adolescent and adult males (46,47). Rumination can become a habit, often initiated by a belch, a swallow, or by stimulation of the palate with the tongue. Abdominal muscle contraction with lower esophageal sphincter relaxation in the early postprandial period is responsible for regurgitation. Typically, the effortless repetitive regurgitation occurs within 15 min of starting a meal, in contrast to vomiting from gastroparesis, which occurs later in the postprandial period.
Eating disorders, such as anorexia and bulimia, can present with similar presentations. Anorexia nervosa is a psychiatric disorder occurring primarily in adolescent and young adult women characterized by distorted body image and fear of obesity with compulsive dieting and self-imposed starvation to maintain a profoundly low body weight. GI symptoms are common and include lack of appetite, early satiety, epigastric fullness, abdominal bloating, nausea, and vomiting. The loss of body weight seen in eating disorders can cause a compensatory delay in gastric emptying. Interestingly, re-alimentation and maintenance of normal body weight improve gastric emptying and GI symptoms, but do not totally normalize them (reviewed in ref. (48)).
Bulimia nervosa is characterized by recurrent episodes of binge eating with a feeling of lack of control over the eating behavior during the binges, often followed by self-induced vomiting, the use of laxatives or diuretics, strict dieting or fasting, or vigorous exercise to prevent weight gain. Gastric emptying studies in bulimia have yielded conflicting results (49–51).
CVS or episodic vomiting episodes are becoming more frequently diagnosed in adults (52). CVS refers to recurrent episodes of intense nausea and vomiting lasting hours to days separated by symptom-free periods of variable lengths. Typically, each episode is similar. Vomiting often starts abruptly, although a prodrome of nausea and abdominal pain can occur. In adults, as compared to children with CVS, the vomiting episodes are longer (3 – 5 days), less frequent (every 3 – 4 months), and triggering events are less evident; there is usually a long delay in diagnosis. Gastric emptying has been reported to be rapid in the symptom-free period. When the episodes of vomiting become closer together, differentiation of “coalescent” CVS from the more typical daily symptoms of gastroparesis in an adult can be challenging. New data on the prevalence of gastric stasis in migraine offer the potential for a better understanding of the mechanisms of CVS. Typically, gastric emptying in CVS is normal or rapid; however, 14 % of a large series of patients had delayed gastric emptying (53).
MANAGEMENT OF GASTROPARESIS
Summary of Evidence
Diet and Nutritional Support
Gastroparesis can lead to poor oral intake, a calorie-deficient diet, and deficiencies in vitamins and minerals (54,55). The choice of nutritional support depends on the severity of disease. In mild disease, maintaining oral nutrition is the goal of therapy. In severe gastroparesis, enteral or parenteral nutrition may be needed. For oral intake, dietary recommendations rely on measures that optimize gastric emptying such as incorporating a diet consisting of small meals that are low in fat and fiber. Since gastric emptying of liquids is often preserved in gastroparesis, blenderized solids or nutrient liquids may empty normally. The rationale of this approach is not validated by controlled studies, but mainly derived from an empirical approach.
Oral Nutrition
Meals with low-fat content and with low residue should be recommended for gastroparesis patients, since both fat and fiber tend to delay gastric emptying. Small meal size is advisable because the stomach may only empty an ∼ 1 – 2 kcal/ min. Therefore, small, low-fat, low-fiber meals, 4 – 5 times a day, are appropriate for patients with gastroparesis. Increasing the liquid nutrient component of a meal should be advocated, as gastric emptying of liquids is often normal in patients with delayed emptying for solids (56,57). Poor tolerance of a liquid diet is predictive of poor outcome with oral nutrition (57). High calorie liquids in small volumes can deliver energy and nutrients without exacerbating symptoms. The caloric requirement of a patient can be calculated by multiplying 25 kcal by their current body weight in kilograms (58).
In some patients, carbonated beverages, with release of carbon dioxide, can aggravate gastric distension; their intake should be minimized (56). Alcohol and tobacco smoking should be avoided because both can modify gastric emptying (59–61). In diabetics, near normal glycemic control with diet and hypoglycemic drugs should be aimed for, as improvement of hyperglycemia can accelerate gastric emptying.
Enteral Nutrition
For patients with gastroparesis who are unable to maintain nutrition with oral intake, a feeding jejunostomy tube, which bypasses the affected stomach, can improve symptoms and reduce hospitalizations (62). Placement of a jejunal feeding tube, if needed for alimentation, should be preceded by a successful trial of nasojejunal feeding. Occasionally, small bowel dysfunction may occur in patients with gastroparesis leading to intolerance to jejunal feeding.
Usefulness and disadvantages of different forms of intubation are summarized in Table 2 . In appropriate patients with normal small bowel function, jejunal feeding maintains nutrition, relieves symptoms, and reduces the frequency of hospital admissions for acute exacerbation of symptoms (64). Small intestinal motility/transit can be assessed before placement of jejunostomy tube with antroduodenojejunal manometry, WMC, and small intestinal transit scintigraphy. Given the large coefficient of variation of small bowel transit time, and the difficulty in interpretation of orocecal transit measurements in the setting of gastroparesis, a practical way to assess small bowel function is by a trial of nasojejunal feeding. Nutrient feeds are started with diluted infusions and advanced gradually to iso-osmolar preparations at relatively low infusion rates (e.g., 20 ml/h) increasing to the target infusion rate to support nutrition and hydration typically to at least 60 ml/h over 12 – 15 h/day. Regulated enteral nutrition may improve glycemic control in diabetic patients with recurrent vomiting and unpredictable oral intake. Complications include infection, tube migration, and dislodgement (65). Such nutritional support may also be effective in patients with systemic sclerosis with significant malnutrition, and lead to restoration of adequate nutritional status, improved quality of life, and few metabolic or technical complications over a period of 12 – 86 months (66). There is a theoretical risk of increased pulmonary aspiration in patients with weak lower esophageal sphincter; hence, it is advisable that the feeding tube should be placed well beyond the angle of Treitz in such patients.
Table 2.
Intubations for decompression and feeding in patients with gastroparesis
| Type of access | Usefulness | Disadvantages |
|---|---|---|
| Nasogastric tube | Gastric decompression in acute management | Not meant for long-term use Large tube size often causes is comfort Is a poor choice for feeding due to delayed gastric emp tying as significant gastroesophageal reflux can occur |
| Nasoduodenal/ nasojejunal tube | Used to give trial feedings to determine if jejunal feedings are tolerated. May be acceptable if there are no other options |
Not for long-term use Vomiting may expel the tube into the stomach |
| Gastrostomy tubes | May be used for venting of secretions to decrease vomiting and fullness |
Poor choice for feeding due to delayed gastric emptying May prevent proper electrode placement for gastric electrical stimulation |
| PEG-J or Jet-PEG |
Allows the patient to vent gastric secretions to decrease / prevent persistent emesis Provides jejunal feedings New PEG-Js have distal feeding ports to reduce duodeno- gastric reflux |
Migration of the J-tube extension into stomach Pyloric obstruction from J-tube May prevent proper electrode placement for gastric electrical stimulation |
| Jejunostomy (surgical, endoscopic, radiographic) | Stable access for reliable jejunal nutrient delivery Avoids gastric penetration that would interfere with proper electrode placement for gastric electrical stimulation |
Cannot vent stomach |
| Dual gastrostomy and jejunostomy | Two sites — one for venting and one for enteral nutrition | Increased risk of leakage, infection Cosmetic issues |
PEG, percutaneous endoscopic gastrostomy; PEG-J, percutaneous endoscopic gastrostomy with jejunal extension tube. Table created from text of ref. (63).
Enteral feeding should always be preferred over parenteral nutrition for a wide range of practical reasons, such as costs, potential for complications, and ease of delivery.
GLYCEMIC CONTROL IN DG
Summary of Evidence
The evidence that hyperglycemia is clinically relevant in delaying gastric emptying or in causing symptoms is controversial and is summarized in Table 3. Acute hyperglycemia induced in experimental clinical studies has been shown to worsen gastric emptying or inhibit antral contractility, though the relationship to symptoms is unclear. The efficacy of long-term improvement in glycemic control on normalization of gastric emptying and relief of symptoms in diabetic patients is controversial. Nevertheless, short- and long-term glycemic control is indicated for improved long-term outcome of diabetes. Attempts to normalize glycemic control using amylin analogs (e.g., pramlintide) or GLP-1 analogs (e.g., exenatide) may result in delayed gastric emptying (75,76). In contrast, dipeptidyl peptidase IV inhibitors (e.g., sitagliptin and vildagliptin (29)) do not delay gastric emptying.
Table 3.
Relationship of glycemic control and gastrointestinal symptoms or gastric emptying
| Reference # | Nature of evidence | Assessment of glycemic control | Outcome |
|---|---|---|---|
| (67) | Epidemiological | Patient report or HbA1c | Higher prevalence of upper GI symptoms |
| (68–70) | Experimental | Acute hyperglycemic clamp | Delayed GE or inhibition of antral motility index |
| (71) | Case series | Poor glucose control | Poor glycemic control in 36 % of patients hospitalized with acute exacerbation of gastroparesis |
| (72) | Case series | HbA1c | Does not predict abnormal vs. normal GE |
| (73) | Case series | Long-term glucose control | No association with delayed GE in T2DM |
| (74) | Case series | Renal and pancreas transplant with normalized blood glucose |
Positive impact on GE and associated GI symptoms |
GE, gastric emptying; GI, gastrointestinal; T2DM, type 2 diabetics.
PHARMACOLOGIC THERAPY
Summary of Evidence
The evidence for use of current prokinetics is based on trials performed two or three decades ago. Therefore, the level of evidence is not based on the currently suggested rigorous, large trials with validated patient response outcomes measured on a daily basis. Current trials include the daily diary Gastroparesis Cardinal Symptom Index (77) and a validated instrument to assess quality of life specific for upper GI disorders, the Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life (78)); however, there are no full manuscripts published using such instruments and the recent institution of patient reported outcome requirements at the FDA may result in modification of this and other diaries.
Metoclopramide , a dopamine D2-receptor antagonist, is the only US FDA-approved medication for the treatment of gastroparesis for no longer than a 12-week period (79), unless patients have therapeutic benefit that outweighs the potential for risk. Metoclopramide is available in several formulations including oral dissolution tablet, oral tablet, liquid formulation, and parenteral formulation. The latter may be administered IV, by intramuscular injection, or subcutaneously (80). The FDA placed a black-box warning on metoclopramide because of the risk of side effects, including tardive dyskinesia. The most common adverse extrapyramidal side effects of metoclopramide are acute dystonias (incidence of 0.2% (81)). The incidence of acute dystonias in a UK series was higher in females, patients receiving higher doses, in children, and young adults. Whereas prolonged reactions were more common in elderly patients. About 95 % of metoclopramide-induced involuntary movements reported over 15 years were dystonias, 4 % parkinsonism type movements, and 1 % tardive dyskinesia (82). Involuntary movements may be more likely with parenteral administration (83). The dystonic reactions may be reversed with antihistamines (e.g., diphenhydramine 25 – 50 mg IV administered over 2 min), benzodiazepines (e.g., diazepam 5 – 10 mg IV) or centrally acting anticholinergic agents (e.g., benztropine 1 – 4 mg IV up to 6 mg/day). Metoclopramide can also be associated with corrected QT interval prolongation.
The efficacy of metoclopramide in the treatment of DG has been assessed in four placebo-controlled trials, two active comparator-controlled and open-label studies that are summarized in Table 4 . In summary, symptoms improved in five studies in which the primary objective was clinical; gastric emptying was accelerated in all studies in which it was appraised. None of the trials was conducted for > 4 weeks, and longer term efficacy is unproven and limited to open-label experience in small numbers of patients (92). Recommendations on when and how to use metoclopramide for the treatment of gastroparesis in clinical practice have been published (93) and include careful monitoring of the patient for earliest signs of tardive dyskinesia (which may be reversible with early recognition and cessation of therapy), use of the lowest effective dose for each patient, starting at 5 mg t.i.d. before meals, use of the liquid formulation to improve absorption and facilitate dose titration to a maximum dose of 40 mg/day and use of “drug holidays” or dose reductions (e.g., 5 mg, before two main meals of the day) whenever clinically possible. Drug – drug interactions may occur with concomitant administration of drugs that alter cytochrome P450-2D6 (CYP2D6) function (94).
Table 4.
Trials of metoclopramide for gastroparesis
| Reference # | Design | #, Etiology | Dose | Duration | Results |
|---|---|---|---|---|---|
| (84) | DB, PC, XO, RCT | 10 DG | 10 mg q.i.d. | 3 weeks/arm | Improved symptoms and vomiting; ∼ 60 % acceleration in GE liquid 150 kcal meal |
| (85) | DB, PC, PG, RCT | 28: 5 DM, 4 PSG, 19 IG | 10 mg q.i.d. | 3 weeks | Improved symptoms by 29 % |
| (86) | PC, RCT | 18 DG | 10 mg q.i.d. | 3 weeks | Improved symptom score by 29 %, improved GE by 25 % |
| (87) | DB, PC, XO, RCT | 13 DG with GE acceler ated by metoclopramide |
10 mg q.i.d. | 3 weeks/arm | Improved symptoms with mean reduction of 52.6 % |
| (88) | DB, RCT, domperidone- controlled, multicenter |
45 DG | 10 mg q.i.d. | 4 weeks | Improved symptoms by 39 %; similar efficacy with domperidone which had less AEs |
| (89) | DB, XO, erythromycin- controlled RCT |
13 DG | 10 mg t.i.d. | 3 weeks/arm | Both treatments accelerated GE compared with baseline and improved symptoms score |
| (90) | Open | 1 DG | 15 mg q.i.d. | 6 months | Improved symptoms, GE liquids, antral contraction frequency |
| (91) | Open | 10 GI symptomatic (N, V) T1DM; 6 asymptomatic T1DM, 18 controls |
10 mg once | Acute | Improved GE solids |
AEs, adverse effects; DB, double blind; DG, diabetic gastroparesis; DM, diabetic; GE, gastric emptying; GI, gastrointestinal; IG, idiopathic gastroparesis; N, nausea; PC, placebo controlled; PG, parallel group; PSG, postsurgical gastroparesis; RCT, randomized-controlled trial; T1DM, type 1 diabetics; T2DM, type 2 diabetics; V, vomiting; XO, crossover.
Domperidone is a type II dopamine antagonist similar to metoclopramide, and is equally efficacious but with lower central side effects. It is available for use under a special program administered by the FDA and via other pathways. Table 5 summarizes the full articles of clinical trials with domperidone; this drug is generally as effective as metoclopramide with main efficacy on nausea and vomiting and lower risk of adverse effects than with metoclopramide. The starting dose is 10 mg t.i.d. increasing to 20 mg t.i.d. and at bedtime. Given the propensity of domperidone to prolong corrected QT interval on electrocardiogram and to rarely cause cardiac arrhythmias, a baseline electrocardiogram is recommended and treatment with this agent should be withheld if the corrected QT is > 470 ms in male and over 450 ms in female patients. Follow-up electrocardiogram on domperidone is also advised to check for prolongation of the corrected QT interval. Domperidone may also cause increased prolactin levels and result in lactation; drug – drug interactions may occur with concomitant administration of drugs that alter CYP2D6 function (106). Drugs that influence CYP2D6 include antiemetics and antidepressants that are frequently co-administered in patients with gastroparesis.
Table 5.
Trials of domperidone in gastroparesis
| Reference # | Type of study | N Etiology | Duration | Symptom improvement vs baseline (OPEN) or vs. placebo (RCT) |
Δ Gastric emptying | Adverse effects |
|---|---|---|---|---|---|---|
| (95) | Open, 10 mg q.i.d. | 3 DG | 1 week | Yes, not quantified | Improved, not quantified |
NA |
| (96) | Open | 12 IG; 3 DG, 2 PSG |
48 months | 68.3 % (P < 0.05) | 34.5 % (P < 0.05) | ↑ Prolactin (100 %), symptoms (17.6 %) |
| (97) | Retrospective | 57 DM | 377 days | 70 % Patients improved | NA | 16 % |
| (98) | Open | 6 DG | 6 months | 79.2 % (P < 0.01) | 26.9 % (NS) | NA |
| (99) | Open | 12 DG | Single oral dose |
Chronic oral administra tion (35 – 51 days) reduced symptoms |
↑ Solid and liquid emptying (P < 0.005) |
NA |
| (100) | RCT, PG, withdrawal study |
208 DG: 105 DOM, 103 PLA |
4 weeks | 53.8 % Lower overall score with DOM (P =0.025) |
NA | 2 – 3 % ↑ Prolactin, similar to PLA |
| (101) | RCT, PC, XO | 13 DG | 8 weeks | ↓ In symptom frequency and intensity vs. PLA (P < 0.03) |
NA | NA |
| (102) | RCT, PC, XO | 6 DG | Single IV 10 mg |
NA | ↑ Homogenized solid emptying |
NA |
| (103) | RCT, PC, XO | 8 IG; 3 DG | 4 weeks | No overall benefit over PLA; two of three DM improved |
NA | Gas pains, skin rash |
| (104) | RCT, PG vs. cisapride, 14 per group |
Total 31 pediatric DG; 3 excluded for poor compliance |
8 weeks | DOM improved vs. baseline (P < 0.001); DOM vs. cisapride (P < 0.01) |
DOM significantly more effective than PLA in reducing the gastric emptying time measured by ultrasound |
None recorded |
| (105) | RCT, PG, vs. metoclopramide |
95 DG | 4 weeks | 41.19 % Improved vs. baseline (NA); NS vs. metoclopramide |
ND | CNS effects more severe and common with metoclopramide: somnolence, mental acuity (49 % M vs. 29 % D) |
CNS, central nervous system; DM, diabetic; DG, diabetic gastroparesis; IG, idiopathic gastroparesis; IV, intravenous; PSG, postsurgical gastroparesis; NA, not assessed; NS, not statistically; DOM, domperidone; PLA, placebo.
Erythromycin lactobionate is effective when given IV at a dose of 3 mg/kg every 8 h (by IV infusion over 45 min to avoid sclerosing veins), as was shown in hospitalized diabetics with gastroparesis (107). Many motilin agonists, including erythromycin, when given orally may also improve gastric emptying and symptoms for several weeks, but over longer periods are often associated with tachyphylaxis due to downregulation of the motilin receptor. Clinical responsiveness drops after 4 weeks of oral erythromycin s (108); however, some patients may continue to experience benefit. Erythromycin is also subject to drug interactions with agents that alter or are metabolized by CYP3A4. Administration of erythromycin can also be associated with the development of corrected QT prolongation.
Metoclopramide and erythromycin are available in liquid form. In healthy volunteers, an orally disintegrating tablet was bioequivalent to a conventional tablet. In healthy volunteers, single administration of 10-mg metoclopramide orally disintegrating tablet (ODT) was well tolerated and bioequivalent to single administration of a conventional 10-mg metoclopramide tablet (109). It is possible that their pharmacokinetic profiles will be enhanced relative to tablet formulation in patients with gastroparesis; however, this has not been demonstrated in trials in patients. In patients with gastroparesis, liquid formulation is less likely to accumulate in the stomach in contrast to tablets, which may require more effective gastric motility to empty from the stomach; such erratic emptying may conceivably lead to several retained tablets being emptied together and lead to high plasma levels after absorption, potentially causing adverse events. Another potential advantage of the liquid formulation is that it allows for easier dose titration. For these reasons, a recent review recommended use of the liquid formula of metoclopramide in patients with severe gastroparesis (93).
Symptomatic Treatment of Nausea, Vomiting, and Pain in Gastroparesis Syndrome
Other than prokinetics, the symptomatic treatment of these symptoms remains empirical and off -label use of these drugs from the indications for non-specific nausea and vomiting, or chemotherapy-induced emesis and palliative care. The most commonly prescribed antiemetic drugs are the phenothiazines (including prochlorperazine and thiethylperazine) or antihistamine agents (including promethazine). Several US medical centers have recently placed several additional restrictions on promethazine, related to concerns about sedation, possible cardiac toxicity (corrected QT prolongation (110)), damage to peripheral veins, and lack of availability of the drug (111). There are no studies that compare efficacy of phenothiazines with newer antiemetics (such as serotonin 5-HT 3 -receptor antagonists) for gastroparesis. There is no evidence that ondansetron is superior to metoclopramide and promethazine in reducing nausea in adults attending an emergency department (112). 5-HT3 -receptor antagonists are reasonable second-line medications; the neurokinin receptor-1 antagonist, aprepitant, was effective in treatment of severe vomiting and repeated episodes of ketoacidosis in a patient with diabetes (113).
The synthetic cannabinoid, dronabinol, is also used in practice, but there is risk of hyperemesis on withdrawal (114), and optimum treatment strategies are unclear. Transdermal scopolamine, which is effective for nausea associated with motion sickness, is used for nausea and vomiting of gastroparesis, albeit without peer-reviewed publications to support this practice. Among alternative medicine therapies, acupuncture is the method most studied in treatment of nausea and vomiting; one study reported impressive relief in 94 % of patients (115) (see section on alternative medicine).
TCA can be considered for refractory nausea and vomiting in gastroparesis (116,117). The management of pain remains a challenge, which has not been addressed in clinical trials of patients with gastroparesis. Agents used in practice are not based on evidence of efficacy for pain. TCA and selective serotonin reuptake inhibitors are effective for depression in diabetes, and this is associated with improved glycemic control and physical symptoms (118,119). Open-label treatment studies have reported that TCA in low doses may decrease symptoms of nausea, vomiting, and abdominal pain in DG and IG (116,117). However, some tricyclic agents, such as amitriptyline, have anticholinergic effects and should be avoided in patients with gastroparesis, as they delay gastric emptying. Nortriptyline has lower incidence of anticholinergic side effects than amitriptyline. The 5-HT 2 receptor antagonist, mirtazapine, has been reported efficacious in a single report in gastroparesis (120).
For patients taking narcotic opiate analgesics, these narcotics should be stopped, if possible, as these agents worsen gastric emptying and may themselves induce symptoms of nausea and vomiting. In addition, chronic use may be associated with increasing abdominal pain. Tramadol, tapentadol, gabapentin, pregabalin, and nortriptyline may be alternatives for pain; however, their effect on gastric emptying is still unclear. The µ -opioid receptor agonist, tramadol (which also releases serotonin and inhibits the reuptake of norepinephrine), is also used. In one study (27), it did not delay gastric emptying, though it significantly delayed colonic transit in healthy volunteers. No data are available in patients with gastroparesis. Both the related compound, tapentadol, and the more selective µ -opioid receptor agonist, oxycodone, are reported to retard gastric emptying in healthy subjects (26).
INTRAPYLORIC BOTULINUM TOXIN INJECTION
Summary of Evidence
Manometric studies of patients with DG show prolonged periods of increased pyloric tone and phasic contractions, a phenomenon termed as “pylorospasm.” Botulinum toxin is a potent inhibitor of neuromuscular transmission. Several open-label studies in small numbers of patients with DG and IG observed mild improvements in gastric emptying and modest in symptoms for several months (see Table 6). Two double-blind, placebo-controlled studies have shown some improvement in gastric emptying, but no improvement in symptoms compared with placebo (131,135). Thus, botulinum toxin injection into the pylorus is not recommended as a treatment for gastroparesis (134), although there is a need for further study in patients with documented “pylorospasm.”
Table 6.
Systematic review of studies on botulinum toxin injection into the pylori sphincter for treatment of gastroparesis
| Reference # | Type of study | Dose of botulinum toxin |
NEtiology | Duration of follow-up |
Outcome/Result | Δ Gastric emptying |
|---|---|---|---|---|---|---|
| (121) | Open label | 80 U | 1 DG | 4 months | Symptoms improved | GE improved by 33 % |
| (122) | Open label | 200 U | 3 DG | 4 – 10 weeks | Symptoms improved | |
| (123) | Open label | 200 U | 1 DG | 4.5 months | Symptoms improved | GE improved by 43 % |
| (124) | Open label | 100 U | 6 DG | 6 weeks | Symptoms decreased by 55 % at 2 and 6 weeks |
GE improved by 43 % at 2 and 6 weeks |
| (125) | Open label | 200 U | 8 DG | 12 weeks | Symptoms improved by 58 % | GE improved in 50 % of patients |
| (126) | Open label | 80 – 100 U | 10 IG | 4 weeks | Symptoms improved by 38 % at 1 month |
GE improved by 48 % at 1 month |
| (127) | Open label | 100 U | 20 Total: 3 DG, 17 IG |
1 month | Symptoms improved by 29 % at 1 month |
GE of solids improved by 35 % at 1 month |
| (128) | Open label | 200 U | 8 DG | 12 weeks | Symptoms improved in 50 % of patients |
|
| (129) | Open label | 100 – 200 U | 63 Total: 26 DG, 35 IG |
Mean 9.3 weeks | Symptoms improved in 42.9 % of patients |
|
| (130) | Open label | 100 U | 20 Total: 3 DG, 17 IG |
4 weeks | Symptoms improved | GE of solids improved |
| (131) | Randomized, double-blind, placebo-controlled |
100 U | 23 Total: 2 DG, 19 IG |
4 weeks | Symptoms improved similar to placebo response |
GE improved |
| (132) | Randomized, double-blind, placebo-controlled |
200 U | 32 Total: 18 DG, 13 IG |
4 weeks | Symptoms improved similar to placebo response |
GE improved |
| (133) | Open label | 100 – 200 U | 179 Total: 81 DG, 82 IG, 16 PSG |
1 – 4 months | Symptoms improved in 51.4 % of patients; responses depended on the dose |
GASTRIC ELECTRICAL STIMULATION
Summary of evidence
GES delivers high frequency (Table 7) (several fold higher than the intrinsic gastric electrical frequency), lower energy electrical stimulation to the stomach. The device was approved by the FDA as a humanitarian device exemption in patients with refractory symptoms of gastroparesis of diabetic or idiopathic etiology in 2000 based on two studies (158). The first, an open-labeled study showed improvement in both specific and global gastroparesis symptoms and gastric emptying (137). The second, a double-blind, randomized, crossover study reported improvement of weekly vomiting frequency (WVF) and quality of life in DG and in the whole patient cohort, but not in the IG subgroup. The study sample size enrolled only about 50 % of what had originally been planned and was underpowered (159). Most subsequent reports have been open-label studies, including long-term efficacy reports of several hundred patients, suggesting that GES enhances symptom control and quality of life and improves oral tolerance of feeding (155). An initial meta-analysis (157) suggested substantial benefits for gastroparesis, but identified that, among 13 included studies, 12 lacked controls and only 1 was blinded and randomized. A more recent meta-analysis on GES showed similar results and identified DG patients as the most responsive to GES, both subjectively and objectively, while the IG and PSG subgroups were less responsive (160). Both meta-analyses and review of the literature indicate that further controlled studies are required to confirm the clinical benefits of highfrequency GES.
Table 7.
Summary of trials of gastric electrical stimulation in patients with gastroparesis
| Reference # | Type of study | NEtiology | Duration of follow-up |
Outcome/ Result | Δ Gastric emptying | Main adverse effects |
|---|---|---|---|---|---|---|
| (136) | Open label | 25 Total: DM 19, idiopathic 3, PS 3 |
12 months | Severity and frequency of nausea and vomiting improved signifi- cantly at 3 months and sustained for 12 months |
GE faster at 3 months, not at 6 or 12 months |
Three devices removed; one death unrelated to device |
| (137) | Open label | Total 38 | Median 12 (range 2.9 – 15.6) months |
97 % Experienced > 80 % reduce tion in vomiting and nausea; average weight gain 5.5 %; 9/ 14 stopped enteral or parenteral nutrition support |
GE improved in most patients at 12 months |
Magnetic inactivation of earlier device; removal of device because of local infec tion; two underwent total gastrectomy |
| (138) | RCT crossover, and open prospective (10 months) |
33 Total: DM 17, idiopathic 16 |
1 month; each crossover |
ON vs. OFF period: self-reported vomiting frequency significantly reduced; efficacy in DM not in idi opathic group; open phase of trial: vomiting, other symptoms, and QOL improved |
Improved GE at 12 months in open- label phase in DM |
Five devices explanted or revised because of infection |
| (139) | Open label | 48 DM | 12 months | All 6 upper GI symptoms, total symptom score, physical and men- tal composite score on HRQOL significantly improved at 6 and 12 months; 8/ 13 stopped nutrition support and 9/ 9 stopped TPN; HbA1c improved by average 1 % |
No effect on GE overall; 5/ 48 had normalization of GE |
Four device-pocket infections required removal of device; one immediate postsurgery death from pulmonary embolism; eight other deaths unrelated |
| (140) | Open label | 9 Total, 7 studied with GES off/ on |
Not stated | Reduced total symptoms score ∼ 40 % |
ND | None |
| (141) | Open label | 17 DM | 12 months | Weekly vomiting and nausea frequencies decreased signify cantly at 6 and 12 months; HbA1c reduced in all, average 2.3 % |
ND | None |
| (142) | Open label | 29 Total: DM 24 idiopathic 5 |
Median 20 months | Symptom control excellent to good in 19/ 27; stopped nutrition sup port in 19/ 19; BMI mean increase of 2.2 kg/ m 2; poor outcome in 3 |
GE in 15/ 27: ∼ twice as fast | Additional procedures in four patients; postoperative morbidity in four |
| (143) | Open label | 9 GES vs. 9 medical Rx |
36 months | GI symptoms improved on GES relative to baseline and to medical Rx; lower health-care use lower at 12, 24, and 36 months; no differ ence in hospitalizations |
ND | ND |
| (144) | Open label | 16 PS | 12 months | All 6 upper GI symptoms, total symptom score, physical and mental composite score on HRQOL significantly improved at 6 and 12 months; 4/ 7 stopped nutri ation support; reduced hospitaliza tions compared with prior year |
GE normalized in three |
One device removed; one re-implantation |
| (145) | Open label | 16 Total: DM 7, idiopathic 7, 1 PS, 1 brain injury | 10 with > 6 months f/ up | improvement in QOL (RAND 36 Health Survey); decreased pyrosis, early satiety and pain; 6/ 10 stopped prokinetics; 75 % jejunal feeding tubes removed |
Normalized GE in 6/ 8 patients |
Two repositioning of device for pain or skin erosion |
| (146) | Open label | 15 Total: DM 5, idiopathic 6, PS 4 |
6 months | GI QOL Index and nausea/ vomiting scores improved overall; bloating, regurgitations, abdo pain and appetite improved in eight with baseline delayed GE |
4/ 8 With delayed GE normalized |
Two epigastric pain |
| (147) | Open label | 156 Total | Median 48 months | Reduced GI symptoms, and improved HRQOL; 90 % had response in at least one of three main symptoms |
Improved | Pocket infections, later re-implanted success fully; no deaths directly related to the device |
| (148) | Open label | 42 Total: DM 24, idiopathic 17, PS 1 |
Median 12 (range 1 – 42) months |
Relative to preoperative, 11/ 42 no response; others improved dyspepsia, and bowel dysfunction of GSRS and 2 domains on SF-36 |
ND | 2 % Immediate post operative morbidity rate and 7 % long-term morbidity rate (device extrusion) |
| (149) | Open label | 28 Total: DM 12, idiopathic 16 |
Mean 5 months | 14 Improved, 8 unchanged, and 6 worsened; on GCSI, improvements in N/ V and postprandial subscores |
ND | 9 Hospitalized; 4 jeju- nostomy placed; 2 GES removed; 2 sepsis; 2 unrelated deaths |
| (150) | Open label | 9 Pediatric | 8 – 42 months | Improved combined symptoms score, nausea, vomiting; 7/ 9 patients sustained improvement in symptoms and QOL |
No change | 1/ 9 Required repeated surgery, jejunal tube placement, local infec tion, skin erosion |
| (151) | Open label | 13 Total: 3 postlung trans plant; 5 DM, 5 idiopathic |
Mean 12 months | 100 % Reported improved QOL; all groups similar improvements in nausea, vomiting, and retching and postprandial symptoms |
ND | Not reported |
| (152) | First open label;, second, crossover RCT; final open trial |
55 DM | 6-week open la bel; two 3-month crossover |
Open phase: 57 % reduction in weekly vomiting frequency; no difference in frequency or sever ity of individual or TSS between OFF and ON periods of crossover study; individual and TSS, and QOL all improved in 1-year open phase |
Accelerated GE in 1-year open phase |
94 % Patient-related AEs (such as hospi talizations for gastro paresis symptoms); 6 % device related AEs (e.g. lead or device migration, infection) with minority requiring surgery |
| (153) | Temporary Mucosal GES DB, PC, crossover RCT |
Total 58: 13 DM, 38 idiopathic, 7 PS |
crossover, two 3-day sessions with 1 day washout |
Improved vomiting in both ON and OFF Rx arms; significantly better vomiting scores with stimula tion on day 3 (particularly in DM group); overall treatment effect not significant |
No effect on GE | Lead dislodgement of orally placed electrodes |
| (154) | Temporary, percutaneous GES; open label in 14; crossover RCT in 13 |
Total 27: 11 idiopathic (gas troparesis or CIP); 9 dyspepsia; 2 PS; 2 DM; 3 other |
Crossover (n =13; ON for 12 – 14 days, OFF for 12 – 14 days) |
22 of 27 Evaluable patients had a favorable symptom reduction; 6 had symptom reduction during ON compared with OFF |
Baseline GE not predictive of out come |
Abdominal cramp ing, local infection with temporary GES; myocardial infarction unrelated to perma nent GES |
| (155) | Open label | Total 221: 142 DM; 48 idiopathic; 31 PS |
Follow-up 1 – 10 years |
TSS, hospitalization, use of medical Rx all reduced; weight increased; 89 % jejunal tubes removed; in 37 DG patients, HbA1c reduced 0.7 % |
ND | 7 % Devices removed because of infection at device site 1 – 43 months after implant |
| (156) | Open label | 20/ 31 Available at follow-up |
5 years | QOL 27 % improvement; 15/ 20 had 50 % improvement with global satisfaction |
Baseline GE not predictive of outcome |
6/ 31 Device explanted; 1 death;12/ 20 epigas tric pain; 1 device- related infection |
AEs, adverse effects; BMI, body mass index; CIP, chronic intestinal pseudo-obstruction; DG, diabetic gastroparesis; DM, diabetic; GCSI, gastroparesis cardinal symptom index; GE, gastric emptying; GES, gastric electrical stimulation; GI, gastrointestinal; GSRS, gastrointestinal symptom rating scale; HRQOL, health-related quality of life; N, nausea; ND, not done; PS, postsurgical; QOL, quality of life; TPN, total parenteral nutrition; TSS, total symptom score; V, vomiting. Reproduced in part from ref. (157). N reports patients recruited into each study; outcomes were often available on fewer patients.
A multicenter, randomized, controlled study involving 55 patients with DG (mean age 38 years, 66 % female, average 5.9 years of gastroparesis), in which all patients had the devices on for several weeks before the randomization occurred, showed no significant diff erence in WVF between on vs. off periods during the subsequent crossover period (161). However, at 1 year post implant, when all patients had the device switched on, the WVF remained lower than baseline (median reduction of WVF of 67.8 % , P< 0.001), reflecting the previously reported open-label experience. Similar reports have been recorded in IG (162).
More recent data (153) have shown effects of GES on GI symptoms in as little as 72 h of stimulation, suggesting rapid effect of GES on gastric motor activity. In this study, after a temporary endoscopic lead was implanted for a trial of high-frequency/lowenergy GES using an external device, patients were randomized to either on/off or off/on at baseline. Although temporary endoscopic placement of stimulation leads in the stomach may predict response to the permanent device (153), this proposal needs further studies to support this practice. In summary, the data presented in Table 7 show that open-label treatment is associated with symptomatic improvement, particularly WVF, and a propensity to cessation of special methods to provide nutrition (such as enteral or parenteral nutrition). Improvement in gastric emptying has been variable. Complications from the device such as local infection or lead migration, as well as complications related to the surgery may occur in up to 10 % of patients implanted. In general, efficacy for symptomatic improvement appears to be greater for DG than for IG. There is no consensus or societal guideline on the selection of patients (e.g., failed therapeutic trials, or level of nutritional compromise) for the use of GES as compassionate treatment.
SURGICAL TREATMENTS: VENTING GASTROSTOMY, GASTROJEUNOSTOMY, PYLOROPLASTY, AND GASTRECTOMY
Summary of Evidence
In patients with signifi cant upper GI motility disorders, surgically placed venting gastrostomy, with or without a venting enterostomy, reduced hospitalization rate by a factor of 5 during the year after placement (163,164). Results of endoscopic venting (percutaneous endoscopic gastrostomy and direct percutaneous endoscopic jejunostomy) on nutritional outcomes and gastroparesis symptoms have not been formally studied and remain unclear. In an open-label study, patients experienced marked symptomatic improvement, weight was maintained, and total symptom score was reduced up to 3 years post venting gastrostomy (165). It is assumed that the same beneficial outcome occurs with percutaneous endoscopic gastrostomy, though this is not proven.
Several types of surgical interventions have been tried for treatment of gastroparesis: gastrojejunostomy, pyloromyotomy, and completion or subtotal gastrectomy. A recent study reported on a series of 28 patients with gastroparesis in whom pyloroplasty resulted in symptom improvement, with significant improvement in gastric emptying and reduction in the need for prokinetic therapy when followed at 3 months post surgery (166). It is unclear whether the efficacy of pyloroplasty depends on the residual antral motor function; thus, in the few diabetics included in the series, there was no significant improvement in gastric emptying (166), and further studies with longer follow-up are needed to determine overall efficacy and optimal candidates for pyloroplasty to treat gastroparesis. Completion or subtotal gastrectomy was applied most often for gastroparesis that followed gastric surgery for peptic ulcer disease (167,168); experience from tertiary referral centers suggests that, in carefully selected patients, major gastric surgery can relieve distressing vomiting from severe gastroparesis and improve quality of life (169,170) in seriously affected patients where risk of subsequent renal failure is high and where life expectancy is poor. The risk of malnutrition and weight loss following gastrectomy has to be weighed relative to the symptom relief. The use of completion or subtotal gastrectomy in patients with intact gastroparetic stomachs has not been favorable. Pyloroplasty may relieve symptoms in gastroparesis and is often combined with operative jejunal tube placement to support nutrition (166,171). Subtotal gastrectomy with Roux-Y reconstruction may be needed for gastric atony secondary to PSG (167). In patients undergoing surgical treatment for gastroparesis, a full-thickness gastric biopsy may be helpful to assess the pathologic basis associated with the patient’s gastroparesis (172–175).
COMPLEMENTARY AND ALTERNATIVE MEDICINES
Summary of evidence
As with many chronic conditions that are poorly understood, patients may search for alternative therapies. These can include: dietary manipulations, physical retraining modalities (autogenic retraining such as that developed by NASA for space motion sickness), and therapies such as acupuncture. Dietary manipulations have been discussed above. The use of autonomic retraining in the one series using NASA technology showed that patients with more intact autonomic nervous system activity responded better than patients whose autonomic function was more impaired (176).
Other therapies, such as acupuncture, have been tried in a more systematic way than other alternative therapies of gastroparesis. Several recent studies, including one single-blinded, randomized pilot study with sham treatment control, have demonstrated that acupuncture may be of benefit in gastroparesis (177). This study of 19 patients with type 2 diabetics was conducted for 2 weeks with 2 week follow-up: symptom severity (Gastroparesis Cardinal Symptom Index) and, particularly, the postprandial fullness and early satiety and bloating subscales were reduced at end of treatment and end of follow-up. Gastric emptying of solids was shortened with active electroacupuncture relative to baseline; however, gastric emptying times in the active and sham-controlled arms were not well matched at baseline (177). Further studies are needed to assess clinical benefit of acupuncture and other complementary and alternative treatments in patients with gastroparesis.
Summary of recommendations
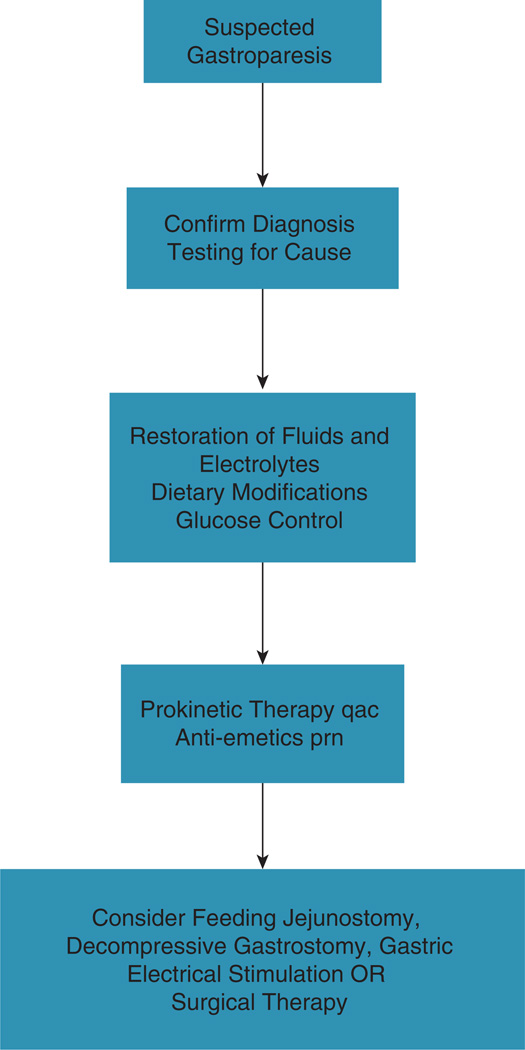
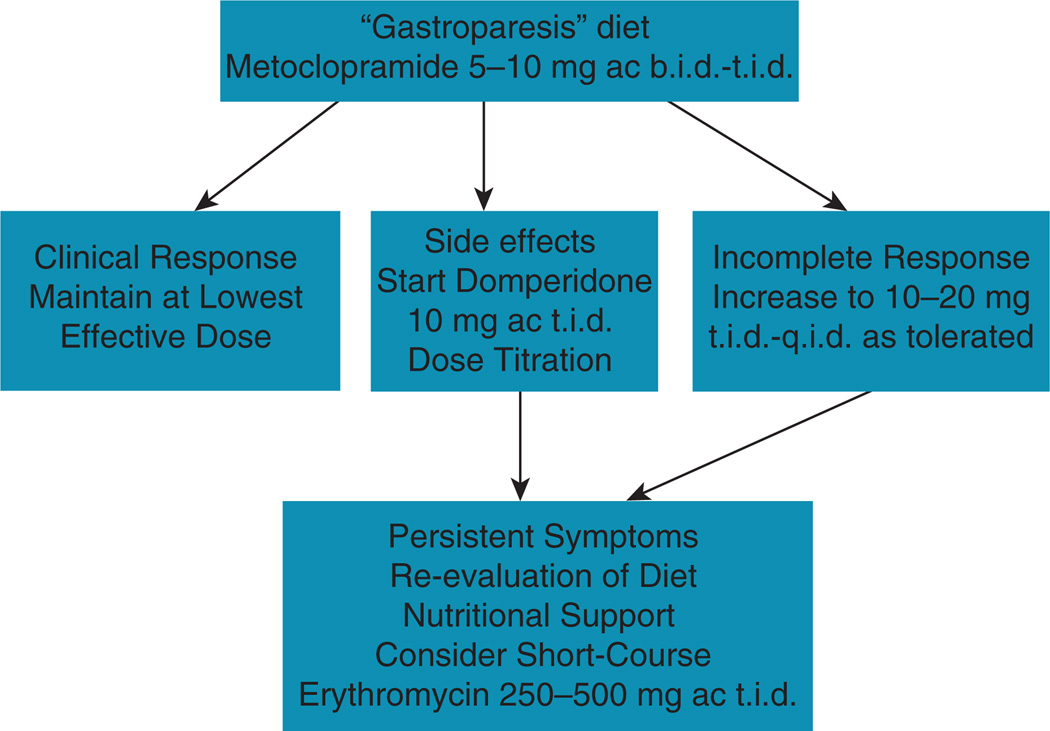
Figure 1 demonstrates a stepwise approach to management of gastroparesis based on these recommendations. An algorithm for management is shown in Figure 2, and suggestions for prokinetic dosing are outlined in Figure 3. Clearly, there are specific refinements to this approach based on individual differences: Degree of nutritional deficiency or weight loss, degree of impairment of gastric emptying (or gastric retention at 4 h), and response to earlier “steps” in the management.
Figure 1.
Stepwise algorithm for gastroparesis diagnosis and management.
Figure 2.
Treatment algorithm for gastroparesis.
Figure 3.
Algorithm for prokinetic therapy in gastroparesis.
SUMMARY OF RECOMMENDATIONS
Recommendations.
The diagnosis of gastroparesis is based on the combination of symptoms of gastroparesis, absence of gastric outlet obstruction or ulceration, and delay in gastric emptying. (Strong recommendation, high level of evidence)
Accelerated gastric emptying and functional dyspepsia can present with symptoms similar to those of gastroparesis; therefore, documentation of delayed gastric emptying is recommended before selecting therapy with prokinetics agents or gastric electrical stimulation (GES). (Strong recommendation, moderate level of evidence)
Recommendations.
Patients with gastroparesis should be screened for the presence of diabetes mellitus, thyroid dysfunction, neurological disease, prior gastric or bariatric surgery, and autoimmune disorders. Patients should undergo biochemical screen for diabetes and hypothyroidism; other tests are as indicated clinically. (Strong recommendation, high level of evidence)
A prodrome suggesting a viral illness may lead to gastroparesis (postviral gastroparesis). This condition may improve over time in some patients. Clinicians should inquire about the presence of a prior acute illness suggestive of a viral infection. (Conditional recommendation, low level of evidence)
Markedly uncontrolled (> 200 mg/dl) glucose levels may aggravate symptoms of gastroparesis and delay gastric emptying. (Strong recommendation, high level of evidence.) Optimization of glycemic control should be a target for therapy; this may improve symptoms and the delayed gastric emptying. (Moderate recommendation, moderate level of evidence)
Medication-induced delay in gastric emptying, particularly from narcotic and anticholinergic agents and glucagon like peptide-1 (GLP–1) and amylin analogs among diabetics, should be considered in patients before assigning an etiological diagnosis. Narcotics and other medications that can delay gastric emptying should be stopped to establish the diagnosis with a gastric emptying test. (Strong recommendation, high level of evidence)
Gastroparesis can be associated with and may aggravate gastroesophageal reflux disease (GERD). Evaluation for the presence of gastroparesis should be considered in patients with GERD that is refractory to acid-suppressive treatment. (Conditional recommendation, moderate level of evidence)
Recommendations.
Documented delay in gastric emptying is required for the diagnosis of gastroparesis. Scintigraphic gastric emptying of solids is the standard for the evaluation of gastric emptying and the diagnosis of gastroparesis. The most reliable method and parameter for diagnosis of gastroparesis is gastric retention of solids at 4 h measured by scintigraphy. Studies of shorter duration or based on a liquid challenge result in decreased sensitivity in the diagnosis of gastroparesis. (Strong recommendation, high level of evidence)
Alternative approaches for assessment of gastric emptying include wireless capsule motility testing and 13C breath testing using octanoate or spirulina incorporated into a solid meal; they require further validation before they can be considered as alternates to scintigraphy for the diagnosis of gastroparesis. (Conditional recommendation, moderate level of evidence)
Medications that affect gastric emptying should be stopped at least 48 h before diagnostic testing; depending on the pharmacokinetics of the medication, the drug may need to be stopped > 48 h before testing. (Strong recommendation, high level of evidence)
Patients with diabetes should have blood glucose measured before starting the gastric emptying test, and hyperglycemia treated with test started after blood glucose is < 275 mg/ dl. (Strong recommendation, moderate-high level of evidence)
Recommendations.
The presence of rumination syndrome and/ or eating disorders (including anorexia nervosa and bulimia) should be considered when evaluating a patient for gastroparesis. These disorders may be associated with delayed gastric emptying, and identification of these disorders may alter management. (Strong recommendation, moderate-high level of evidence)
Cyclic vomiting syndrome (CVS) defined as recurrent episodic episodes of nausea and vomiting, should also be considered during the patient history. These patients may require alternative therapy. (Conditional recommendation, moderate level of evidence)
Chronic usage of cannabinoid agents may cause a syndrome similar to CVS. Patients presenting with symptoms of gastroparesis should be advised to stop using these agents. (Conditional recommendation, low level of evidence)
Recommendations.
The first line of management for gastroparesis patients should include restoration of fluids and electrolytes, nutritional support and in diabetics, optimization of glycemic control. (Strong recommendation, moderate level of evidence.)
Oral intake is preferable for nutrition and hydration. Patients should receive counseling from a dietician regarding consumption of frequent small volume nutrient meals that are low in fat and soluble fiber. If unable to tolerate solid food, then use of homogenized or liquid nutrient meals is recommended. (Conditional recommendation, low level of evidence)
Oral intake is the preferable route for nutrition and hydration. If oral intake is insufficient, then enteral alimentation by jejunostomy tube feeding should be pursued (after a trial of nasoenteric tube feeding). Indications for enteral nutrition include unintentional loss of 10 % or more of the usual body weight during a period of 3 – 6 months, and/ or repeated hospitalizations for refractory symptoms. (Strong recommendation, moderate level of evidence)
For enteral alimentation, postpyloric feeding is preferable to gastric feeding because gastric delivery can be associated with erratic nutritional support. (Conditional recommendation, low level of evidence)
Enteral feeding is preferable to parenteral nutrition. (Conditional recommendation, low level of evidence)
Recommendations.
Good glycemic control should be the goal. Since acute hyperglycemia inhibits gastric emptying, it is assumed that improved glycemic control may improve gastric emptying and reduce symptoms. (Conditional recommendation, moderate level of evidence)
Pramlintide and GLP-1 analogs may delay gastric emptying in diabetics. Cessation of these treatments and use of alternative approaches should be considered before initiation of therapy for gastroparesis. (Conditional recommendation, low level of evidence)
Recommendations.
In addition to dietary therapy, prokinetic therapy should be considered to improve gastric emptying and gastroparesis symptoms, taking into account benefits and risks of treatment. (Strong recommendation, moderate level of evidence)
Metoclopramide is the first line of prokinetic therapy and should be administered at the lowest effective dose in a liquid formation to facilitate absorption. The risk of tardive dyskinesia has been estimated to be < 1%. Patients should be instructed to discontinue therapy if they develop side effects including involuntary movements. (Moderate recommendation, moderate level of evidence)
For patients unable to use metoclopramide, domperidone can be prescribed with investigational new drug clearance from the Food and Drug Administration (FDA) and has been shown to be as effective as metoclopramide in reducing symptoms without the propensity for causing central nervous system side effects; given the propensity of domperidone to prolong corrected QT interval on electrocardiogram, a baseline electrocardiogram is recommended and treatment withheld if the corrected QT is > 470 ms in male and 450 ms in female patients. Follow-up electrocardiogram on treatment with domperidone is also advised. (Moderate recommendation, moderate level of evidence)
Erythromycin improves gastric emptying and symptoms from delayed gastric emptying. Administration of intravenous (IV) erythromycin should be considered when IV prokinetic therapy is needed in hospitalized patients. Oral treatment with erythromycin improves gastric emptying also. However, the long-term effectiveness of oral therapy is limited by tachyphylaxis. (Strong recommendation, moderate level of evidence)
Treatment with antiemetic agents should occur for improvement of associated nausea and vomiting but will not result in improved gastric emptying. (Conditional recommendation, moderate level of evidence)
Tricyclic antidepressants (TCA) can be considered for refractory nausea and vomiting in gastroparesis but will not result in improved gastric emptying and may potentially retard gastric emptying. (Conditional recommendation, low level of evidence)
Recommendations.
Intrapyloric injection of botulinum toxin is not recommended for patients with gastroparesis based on randomized controlled trials. (Strong recommendation, high level of evidence.)
Recommendations.
GES may be considered for compassionate treatment in patients with refractory symptoms, particularly nausea and vomiting. Symptom severity and gastric emptying have been shown to improve in patients with DG, but not in patients with IG or PSG. (Conditional recommendation, moderate level of evidence.)
Recommendations.
Gastrostomy for venting and/or jejunostomy for feeding may be performed for symptom relief. (Conditional recommendation, low level of evidence)
Completion gastrectomy could be considered in patients with PSG who remain markedly symptomatic and fail medical therapy. (Conditional recommendation, low level of evidence)
Surgical pyloroplasty or gastrojejunosotomy has been performed for treatment for refractory gastroparesis. However, further studies are needed before advocating this treatment. Partial gastrectomy and pyloroplasty should be used rarely, only in carefully selected patients. (Conditional recommendation, low level of evidence)
Recommendations.
Acupuncture can be considered as an alternative therapy. This has been associated with improved rates of gastric emptying and reduction of symptoms. (Conditional recommendation, low level of evidence)
The diagnosis of gastroparesis is based on the combination of symptoms of gastroparesis, absence of gastric outlet obstruction or ulceration, and delay in gastric emptying. (Strong recommendation, high level of evidence)
Accelerated gastric emptying and functional dyspepsia can present with symptoms similar to those of gastroparesis; therefore, documentation of delayed gastric emptying is recommended before selecting therapy with prokinetics agents or GES. (Strong recommendation, moderate level of evidence)
Patients with gastroparesis should be screened for the presence of diabetes mellitus, thyroid dysfunction, neuro logical disease, prior gastric or bariatric surgery, and autoimmune disorders. Patients should undergo biochemical screen for diabetes and hypothyroidism; other tests are as indicated clinically. (Strong recommendation, high level of evidence)
A prodrome suggesting a viral illness may lead to gastroparesis (postviral gastroparesis). This condition may improve over time in some patients. Clinicians should inquire about the presence of a prior acute illness suggestive of a viral infection. (Conditional recommendation, low level of evidence)
Markedly uncontrolled (> 200 mg/dl) glucose levels may aggravate symptoms of gastroparesis and delay gastric emptying. (Strong recommendation, high level of evidence.) Optimization of glycemic control should be a target for therapy; this may improve symptoms and the delayed gastric emptying. (Moderate recommendation, moderate level of evidence)
Medication-induced delay in gastric emptying, particularly from narcotic and anticholinergic agents and GLP-1 and amylin analogs among diabetics, should be considered in patients before assigning an etiological diagnosis. Narcotics and other medications that can delay gastric emptying should be stopped to establish the diagnosis with a gastric emptying test. (Strong recommendation, high level of evidence)
Gastroparesis can be associated with and may aggravate GERD. Evaluation for the presence of gastroparesis should be considered in patients with GERD that is refractory to acid-suppressive treatment. (Conditional recommendation, moderate level of evidence)
Documented delay in gastric emptying is required for the diagnosis of gastroparesis. Scintigraphic gastric emptying of solids is the standard for the evaluation of gastric emptying and the diagnosis of gastroparesis. The most reliable method and parameter for diagnosis of gastroparesis is gastric retention of solids at 4 h measured by scintigraphy. Studies of shorter duration or based on a liquid challenge result in decreased sensitivity in the diagnosis of gastroparesis. (Strong recommendation, high level of evidence)
Alternative approaches for assessment of gastric emptying include wireless capsule motility testing and 13C breath testing using octanoate or spirulina incorporated into a solid meal; they require further validation before they can be considered as alternates to scintigraphy for diagnosis of gastroparesis. (Conditional recommendation, moderate level of evidence)
Medications that affect gastric emptying should be stopped at least 48 h before diagnostic testing; depending on the pharmacokinetics of the medication, the drug may need to be stopped > 48h before testing. (Strong recommendation, high level of evidence)
Patients with diabetes should have blood glucose measured before starting the gastric emptying test, and hyperglycemia treated with test started after blood glucose is < 275 mg/dl. (Strong recommendation, moderate-high level of evidence)
The presence of rumination syndrome and/or eating disorders (including anorexia nervosa and bulimia) should be considered when evaluating a patient for gastroparesis. These disorders may be associated with delayed gastric emptying, and identification of these disorders may alter management. (Strong recommendation, moderate-high level of evidence)
CVS defined as recurrent episodic episodes of nausea and vomiting should also be considered during the patient history. These patients may require alternative therapy. (Conditional recommendation, moderate level of evidence)
Chronic usage of cannabinoid agents may cause a syndrome similar to CVS. Patients presenting with symptoms of gastroparesis should be advised to stop usage of these agents. (Conditional recommendation, low level of evidence)
The first line of management for gastroparesis patients should include restoration of fluids and electrolytes, nutritional support and in diabetics, optimization of glycemic control. (Strong recommendation, moderate level of evidence)
Oral intake is preferable for nutrition and hydration. Patients should receive counseling from a dietician regarding consumption of frequent small volume nutrient meals that are low in fat and soluble fiber. If unable to tolerate solid food, then use of homogenized or liquid nutrient meals is recommended. (Conditional recommendation, low level of evidence)
Oral intake is the preferable route for nutrition and hydration. If oral intake is insufficient, then enteral alimentation by jejunostomy tube feeding should be pursued (after a trial of nasoenteric tube feeding). Indications for enteral nutrition include unintentional loss of 10 % or more of the usual body weight during a period of 3 – 6 months, and/or repeated hospitalizations for refractory symptoms. (Strong recommendation, moderate level of evidence)
For enteral alimentation, postpyloric feeding is preferable to gastric feeding because gastric delivery can be associated with erratic nutritional support. (Conditional recommendation, low level of evidence)
Enteral feeding is preferable to parenteral nutrition. (Conditional recommendation, low level of evidence)
Good glycemic control should be the goal. Since acute hyperglycemia inhibits gastric emptying, it is assumed that improved glycemic control may improve gastric emptying and reduce symptoms. (Conditional recommendation, moderate level of evidence)
Pramlintide and GLP-1 analogs may delay gastric emptying in diabetics. Cessation of these treatments and use of alternative approaches should be considered before initiation of therapy for gastroparesis. (Conditional recommendation, low level of evidence)
In addition to dietary therapy, prokinetic therapy should be considered to improve gastric emptying and gastroparesis symptoms, taking into account benefits and risks of treatment. (Strong recommendation, moderate level of evidence)
Metoclopramide is the first line of prokinetic therapy and should be administered at the lowest effective dose. The risk of tardive dyskinesia has been estimated to be < 1 %. Patients should be instructed to discontinue therapy if they develop side effects including involuntary movements. (Moderate recommendation, moderate level of evidence)
For patients unable to use metoclopramide, domperidone can be prescribed with investigational new drug clearance from the FDA and has been shown to be as effective as metoclopramide in reducing symptoms without the propensity for causing central nervous system side effects; given propensity of domperidone to prolong corrected QT interval on electrocardiogram, a baseline electrocardiogram is recommended and treatment withheld if the corrected QT is > 470ms in male and 450 ms in female patients. Follow-up electrocardiogram on treatment with domperidone is also advised. (Moderate recommendation, moderate level of evidence)
Erythromycin improves gastric emptying and symptoms from delayed gastric emptying. Administration of IV erythromycin should be considered when IV prokinetic therapy is needed in hospitalized patients. Oral treatment with erythromycin improves gastric emptying also. However, the long-term ef ectiveness of oral therapy is limited by tachyphylaxis. (Strong recommendation, moderate level of evidence)
Treatment with antiemetic agents should occur for improvement of associated nausea and vomiting but will not result in improved gastric emptying. (Conditional recommendation, moderate level of evidence)
TCA can be considered for refractory nausea and vomiting in gastroparesis but will not result in improved gastric emptying and may potentially retard gastric emptying. (Conditional recommendation, low level of evidence)
Intrapyloric injection of botulinum toxin is not recommended for patients with gastroparesis based on randomized controlled trials. (Strong recommendation, high level of evidence)
GES may be considered for compassionate treatment in patients with refractory symptoms, particularly nausea and vomiting. Symptom severity and gastric emptying have been shown to improve in patients with DG, but not in patients with IG or PSG. (Conditional recommendation, moderate level of evidence)
Gastrostomy for venting and/or jejunostomy for feeding may be performed for symptom relief. (Conditional recommendation, low level of evidence)
Completion gastrectomy could be considered in patients with PSG who remain markedly symptomatic and fail medical therapy. (Conditional recommendation, low level of evidence)
Surgical pyloroplasty or gastrojejunosotomy has been performed for treatment for refractory gastroparesis. However, further studies are needed before advocating this treatment. Partial gastrectomy and pyloroplasty should be used rarely, only in carefully selected patients.
Acupuncture can be considered as an alternative therapy. This has been associated with improved rates of gastric emptying and reduction of symptoms. (Conditional recommendation, low level of evidence)
Acknowledgments
Financial support: The authors are supported by National Institutes of Health PO1 DK68055-04 and DK67071 (M.C.), NIH 1 U01 DK073975-06 (H.P.P.), and U01 DK074007 (T.L.A.).
Footnotes
Guarantor of the article: Michael Camilleri, MD.
Specific author contributions: All authors were involved in writing the manuscript and providing critical revision of the manuscript for important intellectual content.
Potential competing interests: Dr Camilleri has received support from Shire (prucalopride), Theravance (velusetrag), Rhythm (RM-131, research grant), and Tranzyme (TZP-101, 102). Dr Parkman has received support from SmartPill, Tranzyme, GSK, Evoke, and Rhythm. Dr Abell NIH GPCRC is an investigator, consultant, and licensor for Medtronic; is a consultant and investigator for Rhythm.
Dr Shafi has received support from Salix Pharmaceuticals.
Dr Gerson is a consultant for Takeda, Santarus, and IntroMedic.
REFERENCES
- 1.Grades of Recommendation, Assessment, Development, Evaluation (GRADE) Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–1494. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239–1255. doi: 10.1053/j.gastro.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783–788. doi: 10.1111/j.1572-0241.2003.07389.x. [DOI] [PubMed] [Google Scholar]
- 5.Choung RS, Locke GR, III, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–88. doi: 10.1038/ajg.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talley NJ, Young L, Bytzer P, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–76. doi: 10.1111/j.1572-0241.2001.03350.x. [DOI] [PubMed] [Google Scholar]
- 7.Punkkinen J, Färkkil M, Mätzke S, et al. Upper abdominal symptoms in patients with Type 1 diabetes:unrelated to impairment in gastric emptying caused by autonomic neuropathy. Diabet Med. 2008;25:570–577. doi: 10.1111/j.1464-5491.2008.02428.x. [DOI] [PubMed] [Google Scholar]
- 8.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. 2009;137:445–452. doi: 10.1053/j.gastro.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 9.Jung HK, Choung RS, Locke GR, III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:1056–1064. doi: 10.1016/j.cgh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherian D, Parkman HP. Nausea and vomiting in diabetic and idiopathic gastroparesis. Neurogastroenterol Motil. 2012;24:217. doi: 10.1111/j.1365-2982.2011.01828.x. e103. [DOI] [PubMed] [Google Scholar]
- 12.Cherian D, Sachdeva P, Fisher RS, et al. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676–681. doi: 10.1016/j.cgh.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Hasler WL, Wilson L, Parkman HP, et al. Importance of abdominal pain as a symptom in gastroparesis:relation to clinical factors, disease severity, quality of life,gastric retention, and medication use. Gastroenterology. 2010;138(Suppl 1) S-461. [Google Scholar]
- 14.Hasler WL, Parkman HP, Wilson LA, et al. Psychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesis. Am J Gastroenterol. 2010;105:2357–2367. doi: 10.1038/ajg.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maleki D, Locke GR, III, Camilleri M, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808–2816. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 16.Bredenoord AJ, Chial HJ, Camilleri M, et al. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1:264–272. [PubMed] [Google Scholar]
- 17.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex,body mass,symptom onset,delay in gastric emptying,and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soykan I, Sivri B, Sarosie I, et al. Demography, clinical characteristics, psychological profiles, treatment and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 19.Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis—clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501–1506. [PubMed] [Google Scholar]
- 20.Vassallo M, Camilleri M, Caron BL, et al. Gastrointestinal motor dysfunction in acquired selective cholinergic dysautonomia associated with infectious mononucleosis. Gastroenterology. 1991;100:252–258. doi: 10.1016/0016-5085(91)90609-o. [DOI] [PubMed] [Google Scholar]
- 21.Debinski HS, Kamm MA, Talbot IC, et al. DNA viruses in the pathogenesis of sporadic chronic idiopathic intestinal pseudo-obstruction. Gut. 1997;41:100–106. doi: 10.1136/gut.41.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frantzides CT, Carlson MA, Zografakis JG, et al. Postoperative gastrointestinal complaints after laparoscopic Nissen fundoplication. JSLS. 2006;10:39–42. [PMC free article] [PubMed] [Google Scholar]
- 23.Lundell LR, Myers JC, Jamieson GG. Delayed gastric emptying and its relationship to symptoms of “gas bloat” after antireflux surgery. Eur J Surg. 1994;160:161–166. [PubMed] [Google Scholar]
- 24.Camilleri M, Balm RK, Low PA. Autonomic dysfunction in patients with chronic intestinal pseudo-obstruction. Clin Auton Res. 1993;3:95–100. doi: 10.1007/BF01818993. [DOI] [PubMed] [Google Scholar]
- 25.Mittal RK, Frank EB, Lange RC, et al. Effects of morphine and naloxone on esophageal motility and gastric emptying in man. Dig Dis Sci. 1986;31:936–942. doi: 10.1007/BF01303214. [DOI] [PubMed] [Google Scholar]
- 26.Jeong I-D, Camilleri M, Shin A, et al. A randomized,placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans. Aliment Pharmacol Ther. 2012;35:1088–1096. doi: 10.1111/j.1365-2036.2012.05040.x. [DOI] [PubMed] [Google Scholar]
- 27.Maurer AH, Krevsky B, Knight LC, et al. Opioid and opioid-like drug effects on whole-gut transit measured by scintigraphy. J Nucl Med. 1996;37:818–822. [PubMed] [Google Scholar]
- 28.Salehi M, Aulinger BA, D’Alessio DA. Targeting beta-cell mass in type 2 diabetes:promise and limitations of new drugs based on incretins. Endocr Rev. 2008;29:367–379. doi: 10.1210/er.2007-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vella A, Bock G, Giesler PD, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function,meal appearance,and glucose metabolism in type 2 diabetes. Diabetes. 2007;56:1475–1480. doi: 10.2337/db07-0136. [DOI] [PubMed] [Google Scholar]
- 30.Iltz JL, Baker DE, Setter SM, et al. Exenatide:an incretin mimetic for the treatment of type 2 diabetes mellitus. Clin Ther. 2006;28:652–665. doi: 10.1016/j.clinthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Maes BD, Vanwalleghem J, Kuypers D, et al. Differences in gastric motor activity in renal transplant recipients treated with FK-506 versus cyclosporine. Transplantation. 1999;68:1482–1485. doi: 10.1097/00007890-199911270-00009. [DOI] [PubMed] [Google Scholar]
- 32.Park JM, Lake KD, Cibrik DM. Impact of changing from cyclosporine to tacrolimus on pharmacokinetics of mycophenolic acid in renal transplant recipients with diabetes. Ther Drug Monit. 2008;30:591–596. doi: 10.1097/FTD.0b013e3181858169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camilleri M. Diabetic gastroparesis. N Engl J Med. 2007;356:820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 34.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy. A Joint Report of the Society of Nuclear Medicine and the American Neurogastroenterology and Motility Society. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 35.Pathikonda M, Sach deva P, Malhotra N, et al. Gastric emptying scintigraphy:is four hours necessary? J Clin Gastroenterol. 2012;46:209–215. doi: 10.1097/MCG.0b013e31822f3ad2. [DOI] [PubMed] [Google Scholar]
- 36.Ziessman HA, Chander A, Clarke JO, et al. The added diagnostic value of liquid gastric emptying compared with solid emptying alone. J Nucl Med. 2009;50:726–731. doi: 10.2967/jnumed.108.059790. [DOI] [PubMed] [Google Scholar]
- 37.Sachdeva P, Malhotra N, Pathikonda M, et al. Gastric emptying of solids and liquids for evaluation for gastroparesis. Dig Dis Sci. 2011;56:1138–1146. doi: 10.1007/s10620-011-1635-9. [DOI] [PubMed] [Google Scholar]
- 38.Jones KL, Horowitz M, Wishart MJ, et al. Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. J Nucl Med. 1995;36:2220–2228. [PubMed] [Google Scholar]
- 39.Camilleri M, Malagelada JR, Brown ML, et al. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985;249:G580–G585. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 40.Siegel JA, Urbain JL, Adler LP, et al. Biphasic nature of gastric emptying. Gut. 1988;29:85–89. doi: 10.1136/gut.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greydanus MP, Camilleri M, Colemont LJ, et al. Ileocolonic transfer of solid chyme in small intestinal neuropathies and myopathies. Gastroenterology. 1990;99:158–164. doi: 10.1016/0016-5085(90)91243-y. [DOI] [PubMed] [Google Scholar]
- 42.Kuo B, McCallum RW, Koch K, et al. Comparison of gastric emptying of a non-digestible capsule to a radiolabeled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–1647. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 44.Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6:635–643. doi: 10.1016/j.cgh.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odunsi ST, Camilleri M, Szarka LA, et al. Optimizing analysis of stable isotope breath tests to estimate gastric emptying of solids. Neurogastroenterol Motil. 2009;21:706. doi: 10.1111/j.1365-2982.2009.01283.x. e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien MD, Bruce BK, Camilleri M. Rumination syndrome:clinical features rather than manometric diagnosis. Gastroenterology. 1995;108:1024–1029. doi: 10.1016/0016-5085(95)90199-x. [DOI] [PubMed] [Google Scholar]
- 47.Chial HJ, Camilleri M, Williams DE, et al. Rumination syndrome in children and adolescents: diagnosis, treatment and prognosis. Pediatrics. 2003;111:158–162. doi: 10.1542/peds.111.1.158. [DOI] [PubMed] [Google Scholar]
- 48.Chial HJ, McAlpine DE, Camilleri M. Anorexianervosa: manifestations and management for the gastroenterologist. Am J Gastroenterol. 2002;97:255–269. doi: 10.1111/j.1572-0241.2002.05452.x. [DOI] [PubMed] [Google Scholar]
- 49.Geliebter A, Melton PM, McCray RS, et al. Gastric capacity,gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. 1992;56:656–661. doi: 10.1093/ajcn/56.4.656. [DOI] [PubMed] [Google Scholar]
- 50.Devlin MJ, Walsh BT, Guss JO, et al. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr. 1997;56:114–120. doi: 10.1093/ajcn/65.1.114. [DOI] [PubMed] [Google Scholar]
- 51.Devlin MJ, Kissileff HR, Zimmerli EJ, et al. Gastric emptying and symptoms of bulimia nervosa: effect of a prokinetic agent. Physiol Behav. 2012;106:238–242. doi: 10.1016/j.physbeh.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abell TL, Adams KA, Boles RG, et al. Cyclic vomiting syndrome in adults. Neurogastroenterol Motil. 2008;20:269–284. doi: 10.1111/j.1365-2982.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- 53.Hejazi RA, Lavenbarg TH, McCallum RW. Spectrum of gastric emptying patterns in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motil. 2010;22:1298–1302. doi: 10.1111/j.1365-2982.2010.01584.x. [DOI] [PubMed] [Google Scholar]
- 54.Ogorek CP, Davidson L, Fisher RS, et al. Idiopathic gastroparesis is associated with a multiplicity of severe dietary deficiencies. Am J Gastroenterol. 1991;86:423–428. [PubMed] [Google Scholar]
- 55.Parkman HP, Yates KP, Hasler WL, et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology. 2011;141:486–498. doi: 10.1053/j.gastro.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camilleri M. Appraisal of medium-and long-term treatment of gastro paresis and chronic intestinal dysmotility. Am J Gastroenterol. 1994;89:1769–1774. [PubMed] [Google Scholar]
- 57.Abell TL, Bernstein VK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 58.Bouras EP, Scolapio JS. Gastric motility disorders:management that optimizes nutritional status. J Clin Gastroenterol. 2004;38:549–557. doi: 10.1097/00004836-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Pfeiffer A, Holgl B, Kaess H. Effect of ethanol and commonly ingested alcoholic beverages on gastric emptying and gastrointestinal transit. Clin Investig. 1992;70:487–491. doi: 10.1007/BF00210229. [DOI] [PubMed] [Google Scholar]
- 60.Sanaka M, Anjiki H, Tsutsumi H, et al. Effect of cigarette smoking on gastric emptying of solids in Japanese smokers:a crossover study using the 13C–octanoic acid breath test. J Gastroenterol. 2005;40:578–582. doi: 10.1007/s00535-005-1591-2. [DOI] [PubMed] [Google Scholar]
- 61.Scott AM, Kellow JE, Eckersley GM, et al. Cigarette smoking and nicotine delay postprandial mouth-cecum transit time. Dig Dis Sci. 1992;37:1544–1547. doi: 10.1007/BF01296500. [DOI] [PubMed] [Google Scholar]
- 62.Fontana RJ, Barnett JL. Jejunostomy tube placement in refractory diabetic gastroparesis:a retrospective review. Am J Gastroenterol. 1996;91:2174–2178. [PubMed] [Google Scholar]
- 63.Maple JT, Petersen BT, Baron TH, et al. Direct percutaneous endoscopic jejunostomy:outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100:2681–2688. doi: 10.1111/j.1572-0241.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 64.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 65.Karamanolis G, Tack J. Nutrition and motility disorders. Best Pract Res Clin Gastroenterol. 2006;20:485–505. doi: 10.1016/j.bpg.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Grabowski G, Grant JP. Nutritional support in patients with systemic scleroderma. JPEN J Parenter Enteral Nutr. 1989;13:147–151. doi: 10.1177/0148607189013002147. [DOI] [PubMed] [Google Scholar]
- 67.Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–611. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 68.Fraser RJ, Horowitz M, Maddox AF, et al. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675–680. doi: 10.1007/BF00400569. [DOI] [PubMed] [Google Scholar]
- 69.Schvarcz E, Palmer M, Aman J, et al. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60–66. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]
- 70.Hasler WL, Soudah HC, Dulai G, et al. Mediation of hyperglycemiaevoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology. 1995;108:727–736. doi: 10.1016/0016-5085(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 71.Uppalapati SS, Ramzan Z, Fisher RS, et al. Factors contributing to hospitalization for gastroparesis exacerbations. Dig Dis Sci. 2009;54:2404–2409. doi: 10.1007/s10620-009-0975-1. [DOI] [PubMed] [Google Scholar]
- 72.Bharucha AE, Camilleri M, Forstrom LA, et al. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–420. doi: 10.1111/j.1365-2265.2008.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holzäpfel A, Festa A, Stacher-Janotta G, et al. Gastric emptying in Type II (non-insulin-dependent) diabetes mellitus before and after therapy readjustment:no influence of actual blood glucose concentration. Diabetologia. 1999;42:1410–1412. doi: 10.1007/s001250051311. [DOI] [PubMed] [Google Scholar]
- 74.Gaber AO, Oxley D, Karas J, et al. Changes in gastric emptying in recipients of successful combined pancreas-kidney transplants. Dig Dis. 1991;9:437–443. doi: 10.1159/000171334. [DOI] [PubMed] [Google Scholar]
- 75.Vella A, Lee JS, Camilleri M, et al. Effects of pramlintide, an amylin analogue, on gastric emptying in type 1 and 2 diabetes mellitus. Neurogastroenterol Motil. 2002;14:123–131. doi: 10.1046/j.1365-2982.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 76.Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62:173–181. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 77.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a Gastroparesis Cardinal Symptom Index daily diary. Aliment Pharmacol Ther. 2009;30:670–680. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 78.De La Loge C, Trudeau E, Marquis P, et al. Responsiveness and interpretation of a quality of life questionnaire specific to upper gastrointestinal disorders. Clin Gastroenterol Hepatol. 2004;2:778–786. doi: 10.1016/s1542-3565(04)00349-0. [DOI] [PubMed] [Google Scholar]
- 79. [Accessed 6 May, 2012]; http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm149533.htm.
- 80.McCallum RW, Valenzuela G, Polepalle S, et al. Subcutaneous metoclopramide in the treatment of symptomatic gastroparesis:clinical efficacy and pharmacokinetics. J Pharmacol Exp Ther. 1991;258:136–142. [PubMed] [Google Scholar]
- 81.Bateman DN, Rawlins MD, Simpson JM. Extrapyramidal reactions with metoclopramide. BMJ. 1985;291:930–932. doi: 10.1136/bmj.291.6500.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heeley E, Riley J, Layton D, et al. Prescription-event monitoring and report- ing of adverse drug reactions. Lancet. 2001;358:1872–1873. doi: 10.1016/S0140-6736(01)06898-2. [DOI] [PubMed] [Google Scholar]
- 83.Miller LG, Jankovic J. Metoclopramide-induced movement disorders. Arch Intern Med. 1989;149:2486–2492. doi: 10.1001/archinte.149.11.2486. [DOI] [PubMed] [Google Scholar]
- 84.Snape## WJ, Jr, Battle WM, Schwartz SS, et al. Metoclopramide to treat gastroparesis due to diabetes mellitus:a double-blind, controlled trial. Ann Intern Med. 1982;96:444–446. doi: 10.7326/0003-4819-96-4-444. [DOI] [PubMed] [Google Scholar]
- 85.Perkel MS, Moore C, Hersh T, et al. Metoclopramide therapy in patients with delayed gastric emptying:a randomized, double-blind study. Dig Dis Sci. 1979;24:662–666. doi: 10.1007/BF01314461. [DOI] [PubMed] [Google Scholar]
- 86.McCallum RW, Ricci DA, Rakatansky H, et al. A multicenter placebocontrolled clinical trial of oral metoclopramide in diabetic gastroparesis. Diabetes Care. 1983;6:463–467. doi: 10.2337/diacare.6.5.463. [DOI] [PubMed] [Google Scholar]
- 87.Ricci DA, Saltzman MB, Meyer C, et al. Effect of metoclopramide in diabetic gastroparesis. J Clin Gastroenterol. 1985;7:25–32. doi: 10.1097/00004836-198502000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230–1234. doi: 10.1111/j.1572-0241.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 89.Erbas T, Varoglu E, Erbas B, et al. Comparison of metoclopramide and erythromycin in the treatment of diabetic gastroparesis. Diabetes Care. 1993;16:1511–1514. doi: 10.2337/diacare.16.11.1511. [DOI] [PubMed] [Google Scholar]
- 90.Longstreth GF, Malagelada JR, Kelly KA. Metoclo pramide stimulation of gastric motility and emptying in diabetic gastroparesis. Ann Intern Med. 1977;86:195–196. doi: 10.7326/0003-4819-86-2-195. [DOI] [PubMed] [Google Scholar]
- 91.Loo FD, Palmer DW, Soergel KH, et al. Gastric emptying in patients with diabetes mellitus. Gastroenterology. 1984;86:485–494. [PubMed] [Google Scholar]
- 92.Lata PF, Pigarelli DL. Chronic metoclopramide therapy for diabetic gastroparesis. Ann Pharmacother. 2003;37:122–126. doi: 10.1345/aph.1C118. [DOI] [PubMed] [Google Scholar]
- 93.Rao AS, Camilleri M. Review article:metoclopramide tardive dyskinesia. Aliment Phar macol Ther. 2010;31:11–19. doi: 10.1111/j.1365-2036.2009.04189.x. [DOI] [PubMed] [Google Scholar]
- 94.Parkman HP, Misra A, Jacobs M, et al. Clinical response and side effects of metoclopramide:associations with clinical, demographic, and pharmacogenetic parameters. JClin Gastroenterol. 2012;46:494–503. doi: 10.1097/MCG.0b013e3182522624. [DOI] [PubMed] [Google Scholar]
- 95.Watts GF, Armitage M, Sinclair J, et al. Treatment of diabetic gastroparesis with oral domperidone. Diabet Med. 1985;2:491–492. doi: 10.1111/j.1464-5491.1985.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 96.Soykan I, Sarosie k I, McCallum RW. The effect of chronic oral domperidone therapy on gastrointestinal symptoms, gastric emptying, and quality of life in patients with gastroparesis. Am J Gastroenterol. 1997;92:976–980. [PubMed] [Google Scholar]
- 97.Kozarek R. Domperidone for symptomatic management of diabetic gastroparesis in metoclopramide treatment failures. Adv Ter. 1990;7:61–68. [Google Scholar]
- 98.Koch KL, Stern RM, Stewart WR, et al. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis:effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84:1069–1075. [PubMed] [Google Scholar]
- 99.Horowitz M, Harding PE, Chatterton BE, et al. Acute and chronic effects of domperidone on gastric emptying in diabetic autonomic neuropathy. Dig Dis Sci. 1985;30:1–9. doi: 10.1007/BF01318363. [DOI] [PubMed] [Google Scholar]
- 100.Silvers M, Kipnes V, Broadstone A, et al. Domperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. Clin Ther. 1998;20:438–453. doi: 10.1016/s0149-2918(98)80054-4. [DOI] [PubMed] [Google Scholar]
- 101.Braun AP. Domperidone in the treatment of symptoms of delayed gastric emptying in diabetic patients. Adv Ther. 1989;6:51–62. [Google Scholar]
- 102.Heer M, Müller-Duysing W, Benes I, et al. Diabetic gastroparesis:treatment with domperidone—a double-blind, placebo-controlled trial. Digestion. 1983;27:214–217. doi: 10.1159/000198955. [DOI] [PubMed] [Google Scholar]
- 103.Nagler J, Miskovitz P. Clinical evaluation of domperidone in the treatment of chronic postprandial idiopathic upper gastrointestinal distress. Am J Gastroenterol. 1981;76:495–499. [PubMed] [Google Scholar]
- 104.Franzese A, Borrelli O, Corrado G, et al. Domperidone is more effective than cisapride in children with diabetic gastroparesis. Aliment Pharmacol Ther. 2002;16:951–957. doi: 10.1046/j.1365-2036.2002.01240.x. [DOI] [PubMed] [Google Scholar]
- 105.Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230–1234. doi: 10.1111/j.1572-0241.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 106.Parkman HP, Jacobs MR, Mishra A, et al. Domperidone treatment for gastroparesis:demographic and pharmacogenetic characterization of clinical efficacy and side-effects. Dig Dis Sci. 2011;56:115–124. doi: 10.1007/s10620-010-1472-2. [DOI] [PubMed] [Google Scholar]
- 107.Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990;322:1028–1031. doi: 10.1056/NEJM199004123221502. [DOI] [PubMed] [Google Scholar]
- 108.Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol. 1993;88:203–207. [PubMed] [Google Scholar]
- 109.Fass R, Pieniaszek HJ, Thompson JR. Pharmacokinetic comparison of orally-disintegrating metoclopramide with conventional metoclopramide tablet formulation in healthy volunteers. Aliment Pharmacol Ther. 2009;30:301–306. doi: 10.1111/j.1365-2036.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- 110.Owczuk R, Twardowski P, Dylczyk-Sommer A, et al. Influence of promethazine on cardiac repolarisation:a double-blind, midazolam-controlled study. Anaesthesia. 2009;64:609–614. doi: 10.1111/j.1365-2044.2009.05890.x. [DOI] [PubMed] [Google Scholar]
- 111.Patanwala AE, Amini R, Hays DP, et al. Antiemetic therapy for nausea and vomiting in the emergency department. J Emerg Med. 2010;39:330–336. doi: 10.1016/j.jemermed.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 112.Barrett TW, DiPersio DM, Jenkins CA, et al. A randomized, placebo-controlled trial of ondansetron, metoclopramide, and promethazine in adults. Am J Emerg Med. 2011;29:247–255. doi: 10.1016/j.ajem.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 113.Chong K, Dhatariya K. A case of severe, refractory diabetic gastroparesis managed by prolonged use of aprepitant. Nat Rev Endocrinol. 2009;5:285–288. doi: 10.1038/nrendo.2009.50. [DOI] [PubMed] [Google Scholar]
- 114.Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566–1570. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang L. Clinical observation on acupuncture treatment in 35 cases of diabetic gastroparesis. J Tradit Chin Med. 2004;24:163–165. [PubMed] [Google Scholar]
- 116.Prakash C, Lustman PJ, Freedland KE, et al. Tricyclic antidepressants for functional nausea and vomiting:clinical outcome in 37 patients. Dig Dis Sci. 1998;43:1951–1956. doi: 10.1023/a:1018878324327. [DOI] [PubMed] [Google Scholar]
- 117.Sawhney MS, Prakash C, Lustman PJ, et al. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418–424. doi: 10.1007/s10620-006-9378-8. [DOI] [PubMed] [Google Scholar]
- 118.Lustman PJ, Freedland KE, Griffith LS, et al. Fluoxetine for depression in diabetes:a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 119.Lustman PJ, Griffith LS, Clouse RE, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59:241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 120.Kim SW, Shin IS, Kim JM, et al. Mirtazapine for severe gastroparesis unresponsive to conventional prokinetic treatment. Psychosomatics. 2006;47:440–442. doi: 10.1176/appi.psy.47.5.440. [DOI] [PubMed] [Google Scholar]
- 121.Sharma VK, Glassman SB, Howden CW, et al. Pyloric intrasphincteric botulinum toxin (Botox) improved symptoms and gastric emptying in a patient with diabetic gastroparesis (abstr) Am J Gastroenterol. 1998;93:456. [Google Scholar]
- 122.Lacy BE, Schettler-Duncan VA, Crowell MD. The treatment of diabetic gastroparesis with botulinum toxin (abstr) Am J Gastroenterol. 2000;95:2455–2456. [Google Scholar]
- 123.Muddasani P, Ismail-Beigi F. Diabetic gastroparesis a possible new indication for botulinum toxin injection (abstr) Am J Gastroenterol. 2001;97:S255. [Google Scholar]
- 124.Ezzeddine D, Jit R, Katz N, et al. Pyloric injection of botulinum toxin for treatment of diabetic gastroparesis. Gastrointest Endosc. 2002;55:920–923. doi: 10.1067/mge.2002.124739. [DOI] [PubMed] [Google Scholar]
- 125.Lacy BE, Zayat EN, Crowell MD, et al. Botulinum toxin for the treatment of gastroparesis:a preliminary report. Am J Gastroenterol. 2002;97:1548–1552. doi: 10.1111/j.1572-0241.2002.05741.x. [DOI] [PubMed] [Google Scholar]
- 126.Miller LS, Szych GA, Kantor SB, et al. Treatment of idiopathic gastroparesis with injection of botulinum toxin into the pyloric sphincter muscle. Am J Gastroenterol. 2002;97:1653–1660. doi: 10.1111/j.1572-0241.2002.05823.x. [DOI] [PubMed] [Google Scholar]
- 127.Arts J, Van Gool S, Caenepeel P, et al. Effect of intrapyloric injection of Botulinum toxin on gastric emptying and meal-related symptoms in gastroparesis (abstr) Gastroenterology. 2003;124:A53. doi: 10.1111/j.1365-2036.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 128.Lacy BE, Crowell MD, Schettler-Duncan A, et al. The treatment of diabetic gastroparesis with botulinum toxin injection of the pylorus. Diabetes Care. 2004;27:2341–2347. doi: 10.2337/diacare.27.10.2341. [DOI] [PubMed] [Google Scholar]
- 129.Bromer MQ, Friedenberg F, Miller LS, et al. Endoscopic pyloric injection of botulinum toxin A for the treatment of refractory gastroparesis. Gastrointest Endosc. 2005;61:833–839. doi: 10.1016/s0016-5107(05)00328-7. [DOI] [PubMed] [Google Scholar]
- 130.Arts J, van Gool S, Caenepeel P, et al. Influence of intrapyloric botulinum toxin injection on gastric emptying and meal-related symptoms in gas troparesis patients. Aliment Pharmacol Ther. 2006;24:661–667. doi: 10.1111/j.1365-2036.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 131.Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomizedcon trolled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251–1258. doi: 10.1111/j.1365-2036.2007.03467.x. [DOI] [PubMed] [Google Scholar]
- 132.Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416–423. doi: 10.1111/j.1572-0241.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 133.Coleski R, Anderson MA, Hasler WL. Factors associated with symptom response to pyloric injection of botulinum toxin in a large series of gas troparesis patients. Dig Dis Sci. 2009;54:2634–2642. doi: 10.1007/s10620-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 134.Bai Y, Xu MJ, Yang X, et al. A systematic review of intrapyloric botulinum toxin injection for gastroparesis. Digestion. 2010;81:27–34. doi: 10.1159/000235917. [DOI] [PubMed] [Google Scholar]
- 135.Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416–423. doi: 10.1111/j.1572-0241.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 136.Forster J, Sarosiek I, Delcore R, et al. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg. 2001;182:676–681. doi: 10.1016/s0002-9610(01)00802-9. [DOI] [PubMed] [Google Scholar]
- 137.Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–212. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]
- 138.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 139.Lin Z, Forster J, Sarosiek I, et al. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071–1076. doi: 10.2337/diacare.27.5.1071. [DOI] [PubMed] [Google Scholar]
- 140.Luo J, Al Juburi A, Rashed H, et al. Gastric electrical stimulation is associated with improvement in pancreatic exocrine function in humans. Pancreas. 2004;29:e41–e44. doi: 10.1097/00006676-200408000-00017. [DOI] [PubMed] [Google Scholar]
- 141.van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38–42. doi: 10.1055/s-2004-830525. [DOI] [PubMed] [Google Scholar]
- 142.Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: an alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841–848. doi: 10.1001/archsurg.140.9.841. [DOI] [PubMed] [Google Scholar]
- 143.Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term healthcare benefits? Neurogastro enterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 144.Mc Callum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49–54. doi: 10.1016/s1542-3565(04)00605-6. [DOI] [PubMed] [Google Scholar]
- 145.de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis: gastric motility restored. Surg Endosc. 2006;20:302–306. doi: 10.1007/s00464-005-0119-4. [DOI] [PubMed] [Google Scholar]
- 146.Gourcerol G, Leblanc I, Leroi AM, et al. Gastric electrical stimulation in medically refractory nausea and vomiting. Eur J Gastroenterol Hepatol. 2007;19:29–35. doi: 10.1097/01.meg.0000250584.15490.b4. [DOI] [PubMed] [Google Scholar]
- 147.Anand C, Al-Juburi A, Familoni B, et al. Gastric electrical stimulation is safe and effective: a long-term study in patients with drug-refractory gastroparesis in three regional centers. Digestion. 2007;75:83–89. doi: 10.1159/000102961. [DOI] [PubMed] [Google Scholar]
- 148.Velanovich V. Quality of life and symptomatic response to gastric neuro stimulation for gastroparesis. J Gastrointest Surg. 2008;12:1656–1663. doi: 10.1007/s11605-008-0655-z. [DOI] [PubMed] [Google Scholar]
- 149.Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078. doi: 10.1007/s10620-007-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Islam S, Vick L, Gosche J, et al. Gastric Electrical Stimulation for adolescents with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437–442. doi: 10.1016/j.jpedsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 151.Filichia LA, Cendan JC. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90–93. doi: 10.1016/j.jss.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 152.McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947–954. doi: 10.1016/j.cgh.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 153.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496–503. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Andersson S, Ringström G, Elfvin A, et al. Temporary percutaneous gastric electrical stimulation: a novel technique tested in patients with non-established indications for gastric electrical stimulation. Digestion. 2011;83:3–12. doi: 10.1159/000291905. [DOI] [PubMed] [Google Scholar]
- 155.McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314–319. doi: 10.1016/j.cgh.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 156.Gourcerol G, Huet E, Vandaele N, et al. Long term efficacy of gastric electrical stimulation in intractable nausea and vomiting. Dig Liver Dis. 2012;44:563–568. doi: 10.1016/j.dld.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 157.O’Grady G, Egbuji JU, Du P, et al. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 2009;33:1693–1701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Humanitarian device exemption for Enterra device. Federal Registry. 2000;65:78495–78496. [Google Scholar]
- 159.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 160.Chu H, Lin Z, Zhong L, et al. A meta-analysis: the treatment of high-frequency gastric electrical stimulation for gastroparesis. J Gastroenterol Hepatol. 2012;27:1017–1026. doi: 10.1111/j.1440-1746.2011.06999.x. [DOI] [PubMed] [Google Scholar]
- 161.McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947–954. doi: 10.1016/j.cgh.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 162.McCallum R, Snape WJ, Jr., Wo JM, et al. Enterra® gastric electrical stimulation for idiopathic gastroparesis: results from a multicenter randomized study. Gastroenterology. 2010;138:1065. (abstract) [Google Scholar]
- 163.Pitt HA, Mann LL, Berquist WE, et al. DenBesten L Chronic intestinal pseudo-obstruction. Management with total parenteral nutrition and a venting enterostomy. Arch Surg. 1985;120:614–618. doi: 10.1001/archsurg.1985.01390290090015. [DOI] [PubMed] [Google Scholar]
- 164.Murr MM, Sarr MG, Camilleri M. The surgeon’s role in the treatment of chronic intestinal pseudoobstruction. Am J Gastroenterol. 1995;90:2147–2151. [PubMed] [Google Scholar]
- 165.Kim CH, Nelson DK. Venting percutaneous gastrostomy in the treatment of refractory idiopathic gastroparesis. Gastrointest Endosc. 1998;47:67–70. doi: 10.1016/s0016-5107(98)70301-3. [DOI] [PubMed] [Google Scholar]
- 166.Hibbard ML, Dunst CM, Swanström LL. Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg. 2011;15:1513–1519. doi: 10.1007/s11605-011-1607-6. [DOI] [PubMed] [Google Scholar]
- 167.Karlstrom L, Kelly KA. Roux-Y gastrectomy for chronic gastric atony. Am J Surg. 1989;157:44–49. doi: 10.1016/0002-9610(89)90418-2. [DOI] [PubMed] [Google Scholar]
- 168.Forstner-Barthell AW, Murr MM, Nitecki S, et al. Near-total completion gastrectomy for severe postvagotomy gastric stasis: analysis of early and long-term results in 62 patients. J Gastrointest Surg. 1999;3:15–21. doi: 10.1016/s1091-255x(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 169.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, et al. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–495. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 170.Watkins PJ, Buxton -Tomas MS, H oward ER. Long-term outcome after gastrectomy for intractable diabetic gastroparesis. Diabet Med. 2003;20:58–63. doi: 10.1046/j.1464-5491.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- 171.Sodhi SS, Guo JP, Maurer AH, et al. Gastroparesis after combined heart and lung transplantation. J Clin Gastroenterol. 2002;34:34–39. doi: 10.1097/00004836-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 172.Abell TL, Familoni B, Voeller G, et al. Electrophysiologic, morphologic, and serologic features of chronic unexplained nausea and vomiting: lessons learned from 121 consecutive patients. Surgery. 2009;145:476–485. doi: 10.1016/j.surg.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 173.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grover M, Bernard CE, Pasricha PJ, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531–539. doi: 10.1111/j.1365-2982.2012.01894.x. e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Faussone-Pellegrini MS, Grover M, Pasricha P, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rashed H, Cutts T, Abell TL, et al. Predictors of response to a behavioral treatment in patients with chronic gastric motility disorders. Dig Dis Sci. 2002;47:1020–1026. doi: 10.1023/a:1015029805498. [DOI] [PubMed] [Google Scholar]
- 177.Wang CP, Kao CH, Chen WK, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Altern Complement Med. 2008;14:833–839. doi: 10.1089/acm.2008.0107. [DOI] [PubMed] [Google Scholar]