Abstract
Background
Ventilator-associated pneumonia is associated with increased morbidity and mortality.
Objective
To examine the effects of mechanical (toothbrushing), pharmacological (topical oral chlorhexidine), and combination (toothbrushing plus chlorhexidine) oral care on the development of ventilator-associated pneumonia in critically ill patients receiving mechanical ventilation.
Methods
Critically ill adults in 3 intensive care units were enrolled within 24 hours of intubation in a randomized controlled clinical trial with a 2 × 2 factorial design. Patients with a clinical diagnosis of pneumonia at the time of intubation and edentulous patients were excluded. Patients (n = 547) were randomly assigned to 1 of 4 treatments: 0.12% solution chlorhexidine oral swab twice daily, toothbrushing thrice daily, both toothbrushing and chlorhexidine, or control (usual care). Ventilator-associated pneumonia was determined by using the Clinical Pulmonary Infection Score (CPIS).
Results
The 4 groups did not differ significantly in clinical characteristics. At day 3 analysis, 249 patients remained in the study. Among patients without pneumonia at baseline, pneumonia developed in 24% (CPIS ≥6) by day 3 in those treated with chlorhexidine. When data on all patients were analyzed together, mixed models analysis indicated no effect of either chlorhexidine (P = .29) or toothbrushing (P = .95). However, chlorhexidine significantly reduced the incidence of pneumonia on day 3 (CPIS ≥6) among patients who had CPIS <6 at baseline (P = .006). Toothbrushing had no effect on CPIS and did not enhance the effect of chlorhexidine.
Conclusions
Chlorhexidine, but not toothbrushing, reduced early ventilator-associated pneumonia in patients without pneumonia at baseline.
Ventilator-associated pneumonia (VAP) is defined as pneumonia in patients receiving mechanical ventilation that was neither present nor developing at the time of intubation. VAP increases mortality,1 hospital length of stay,2,3 and health care costs.2,4,5 Oral health can be compromised by critical illness and by mechanical ventilation and is influenced by nursing care.6,7 The effect of oral care interventions on the development of VAP has been of interest to clinicians; however, data from well-controlled experimental research with adequate sample sizes have not been published.
Many risk factors for VAP have been identified.8 Major ones include inadequate hand washing by staff, ventilatory circuit management practices, supine positioning of patients without backrest elevation, previous antibiotic therapy, presence of a nasogastric tube, and gastric alkalinization.9,10 Interventions included in the Institute for Healthcare Improvement’s ventilator bundle11 to reduce risk of complications in patients treated with mechanical ventilation include elevating the head of the bed to 30° or more, prophylaxis for peptic ulcer disease and deep vein thrombosis, daily interruption of sedation (sedation vacation), and assessment of readiness to extubate.
Another risk factor for VAP is colonization of the oropharynx by potential pathogens such as Staphylococcus aureus, Streptococcus pneumoniae, or gram-negative rods.12–15 Several factors contribute to the association between oral health and development of VAP. Within 48 hours of admission to the intensive care unit (ICU), oral flora of critically ill patients undergoes a change to predominantly gram-negative flora that includes more virulent organisms.16,17 Dental plaque can provide a habitat for microorganisms responsible for VAP, and dental plaque of patients in the ICU can be colonized by potential respiratory pathogens such as methicillin-resistant S aureus and Pseudomonas aeruginosa.18
Because contamination of the oral cavity by pathogenic bacteria is associated with VAP, interventions to reduce bacteria in the oral cavity have been investigated. Several studies19–21 have indicated that application of topical oral chlorhexidine, initiated before intubation, reduces nosocomial infections in patients having elective cardiac surgery. Importantly, however, cardiac surgery patients are generally extubated within 48 hours and thus have a low risk for VAP. However, in a recent meta-analysis, Pineda et al22 found that chlorhexidine did not reduce nosocomial pneumonia or mortality rate. A recommendation for use of chlorhexidine in patients other than those having elective cardiac surgery is not included in national ventilator bundles or in recommendations from the Centers for Disease Control and Prevention because no evidence of the effectiveness of chlorhexidine in general critical care patients is available. Controlled studies of the effects of toothbrushing on VAP have not been reported.
Dental plaque can provide a habitat for microorganisms responsible for ventilator-associated pneumonia.
Oral care in critically ill adults is now emerging as an important issue but has not been well studied in patients other than those having elective cardiac surgery. We conducted a randomized, controlled clinical trial to test the effects of toothbrushing and/or chlorhexidine in reducing the risk for VAP in adult ICU patients receiving mechanical ventilation. We hypothesized that oral interventions would reduce the incidence of VAP.
Methods
Design and Sample
A randomized controlled 2 × 2 factorial experimental design was used, and staff who performed interventions had no knowledge of patients′ Clinical Pulmonary Infection Score (CPIS). The study was approved by the Office of Research Subjects Protection of Virginia Commonwealth University, Richmond, Virginia, and prospective informed consent for participation was obtained from each patient′s legally authorized representative. Calculations of sample sizes were based on the use of a 2 × 2 factorial experiment. This design permitted the testing of hypotheses about the effect of each of the individual interventions (chlorhexidine alone and toothbrushing alone) and also provided information about any interaction between chlorhexidine and toothbrushing. The sample size required to detect an interaction (ie, the effect of chlorhexidine and toothbrushing in combination) was larger than that required to detect main effects (ie, chlorhexidine alone or toothbrushing alone) for a test at a given level of significance. The study was designed to detect an interactive effect resulting in a 0.755 difference in mean CPIS score at a power of 80% and a significance level of .05. An interim analysis was done and a Bonferroni adjustment was used to avoid inflation in the overall significance level related to interim analyses; for this reason, the level of significance for final analysis was .025.
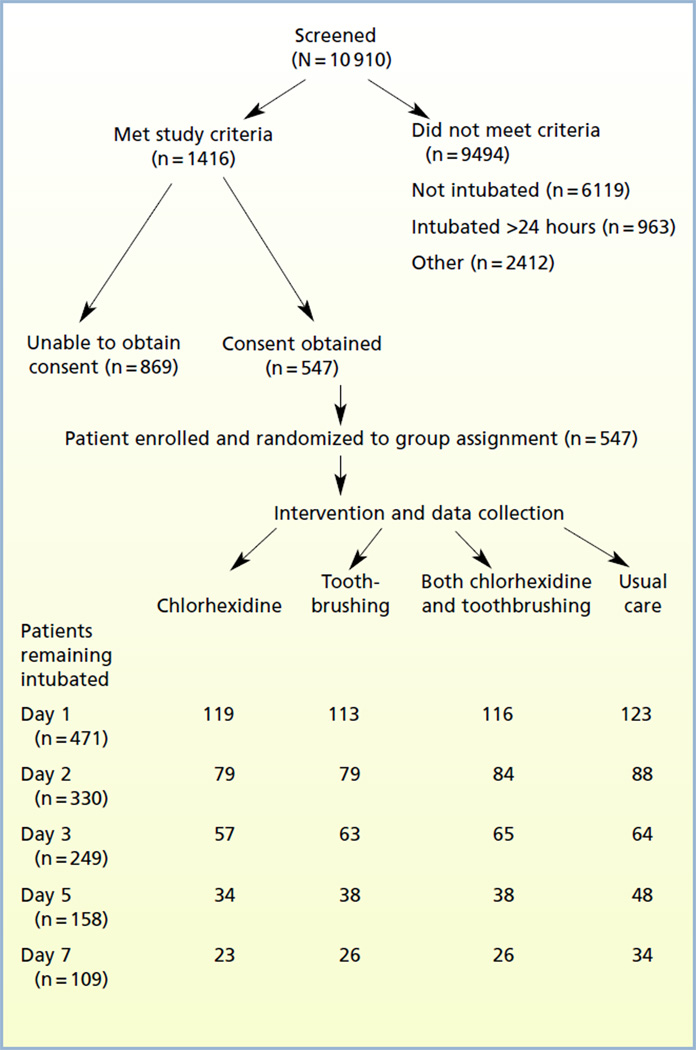
Patients were recruited from 3 ICUs at Virginia Commonwealth University Medical Center, a large urban hospital. All patients older than 18 years (n = 10 913) in medical, surgical/trauma, and neuroscience ICUs were screened for inclusion. The Figure provides information about screening, enrollment, and attrition. Patients were randomized to treatment within each ICU according to a permuted block design developed by the biostatistician (D.K.M.) before the start of the study. Patients receiving mechanical ventilation were enrolled within 24 hours of intubation. Because reintubation increases the risk for VAP,23,24 patients who had had a previous endotracheal intubation during the current hospital admission were excluded. Edentulous patients were excluded because dental plaque could not be assessed. Patients with a clinical diagnosis of pneumonia at the time of intubation were excluded because determination of nosocomial pneumonia is confounded in patients with preexisting pneumonia. Consent was obtained for 547 patients.
Figure.
Study flow.
Patients remained in the study for a maximum of 7 days unless extubated; for patients extubated before day 7, participation in the study ended on the day of extubation. This step was necessary because VAP was scored by using the CPIS, and variables necessary for computation of the CPIS (eg, number of times endotracheal suctioning was performed, ventilator settings, endotracheal tube cultures) were not available after extubation. The primary outcome of the study was the CPIS on day 3. On day 3, the analysis sample consisted of 192 patients, 116 patients remained in the analysis sample on day 5, and 76 patients were analyzed on day 7.
Oral Care
Patients were randomly assigned to 1 of 4 treatments: a 0.12% solution of chlorhexidine gluconate (chlorhexidine) 5 mL by oral swab twice daily (at 10 am and 10 pm), toothbrushing 3 times a day (at 9 am, 2 pm, and 8 pm), combination care (toothbrushing 3 times a day and chlorhexidine every 12 hours), or control (usual care). A 0.12% solution of chlorhexidine gluconate was used because this is the formulation approved by the Food and Drug Administration in the United States. Chlorhexidine and toothbrushing were provided by study personnel. Study personnel who provided oral care had no knowledge of patients′ CPIS results.
The toothbrushing protocol was developed in consultation with dental hygienist faculty and was based on recommendations of the American Dental Association for healthy populations. Each patient′s mouth was divided into 4 dental quadrants (right upper, right lower, left upper, left lower), and each quadrant was brushed in a defined pattern. In each quadrant, every tooth was brushed for 5 strokes on lingual, buccal, and biting surfaces with a soft pediatric toothbrush and toothpaste (Biotene toothpaste, Laclede, Inc, Rancho Dominguez, California). The palate and tongue were also brushed. Each quadrant, the palate, and the tongue were rinsed with mouth-wash (Biotene), 2.5 mL per area, by using a transfer pipette. A Yankauer suction catheter was used as needed to suction excess saliva and water from the mouth as the intervention was performed. Finally, a measured amount of moisturizing gel (OralBalance, Laclede, Inc) was applied to all soft surfaces of the oral cavity and lips by using a green Toothette swab (Sage Products, Inc, Cary, Illinois).
Chlorhexidine was applied in a defined pattern by using a green Toothette swab to evenly coat each tooth, the tongue, and the palate. A commercially available chlorhexidine solution was dispensed by the investigational drug pharmacy for use in the study.
Measurement and Quantification of Key Variables
Ventilator-Associated Pneumonia
Development of VAP was quantified by using the CPIS.25 Microbial cultures of tracheal aspirates were performed by the hospital′s clinical support laboratory (which has Clinical Laboratory Improvement Amendments certification) by laboratory personnel who had no knowledge of any patient′s treatment assignment. Chest radiographs were interpreted by an intensivist board certified in critical care medicine (C.S.) who had no knowledge of any patient′s treatment assignment. Data for CPIS calculation were collected on study days 1, 3, 5, and 7.
For the CPIS, points are assigned to 6 easily obtained variables: temperature, white blood cell count, tracheal secretions, oxygenation (calculated as PaO2 divided by the fraction of inspired oxygen), findings on chest radiographs (no infiltrate, diffuse infiltrate, localized infiltrate), and results of culturing of tracheal aspirates (microscopic examination and semiquantitative culture of tracheal secretions, scored by using the same scale as that used for the oral cultures). Points for each variable of the CPIS are summed, yielding a total CPIS, which provides a range of scores from 0 to 12 for data analysis. Although the CPIS has been used by some investigators26,27 as a dichotomous measure of VAP (defining CPIS ≥6 as a diagnosis of pneumonia and CPIS <6 as absence of pneumonia), other investigators16,25,28 have used the entire CPIS range of scores to describe the clinical development and progression of pulmonary infection over time. In this study, analyses were planned to examine the effect of interventions on both the range of CPIS scores and on dichotomous categories of the presence (CPIS ≥6) or absence (CPIS <6) of VAP.
Influencing Factors
Several other risk factors may contribute to the development of VAP. Descriptive data related to these risk factors were also collected, including demographics and severity of illness as determined by scores on the Acute Physiology, Age, and Chronic Health Evaluation (APACHE) III.29 Demographic data included sex, race, age, prior antibiotic use, reason for intubation, intubation process (elective, urgent, emergent), and reason for admission to the ICU. A count of decayed, missing and filled teeth (a standardized dental assessment tool) was done at the time of admission to the study as a measure of general oral health status. Additional information on established risk factors for VAP, including elevation of the head of the bed, ventilator support data, enteral nutrition data, and use of selected medications was collected to establish equivalence among groups.
Patients receiving mechanical ventilation were enrolled within 24 hours of intubation.
Procedures
Study personnel conducted daily screening of all patients for eligibility. If a patient met the inclusion criteria, the study was explained to the patient′s legally authorized representative and consent was obtained. Toothbrushing (3 times daily) and administration of chlorhexidine (twice daily) were performed by study personnel according to each patient′s group assignment. Data were collected from time of admission to the study through day 7 of intubation or until extubation. The CPIS was assessed 4 times during the study: at the time of admission into the study (study day 1), at study day 3 (corresponding to the definition of early-onset VAP30), at day 5, and at day 7 (corresponding to late-onset VAP30). Data related to other VAP risk factors were also collected daily and included information on ventilator support, enteral nutrition, and selected medications.
Ventilator-associated pneumonia was measured by using the Clinical Pulmonary Infection Score.
Data Analysis
Descriptive statistics were used to summarize the characteristics of the study population; percentages for discrete variables and means and standard deviations for continuous variables were calculated. Analysis of covariance was used to compare CPIS values by treatment group. The interaction of toothbrushing and chlorhexidine was initially included in each model; because the interaction of toothbrushing and chlorhexidine was not significant, the interaction was not included in final analysis models. The final models included terms for the main effects of toothbrushing and chlorhexidine along with the covariates ICU (a stratification variable) and baseline CPIS score. Logistic regression was used to compare proportions of patients in each group with pneumonia (CPIS score ≥6) in a similar manner, again with controls for ICU and baseline CPIS score. Analysis was performed on data of all patients in the analysis sample, as well as data of the subset of patients who had a CPIS less than 6 at baseline.
The study was designed to have an interim analysis, with Bonferroni correction used to adjust the P value. A single interim analysis was performed and did not provide sufficient evidence to stop the study, so the investigation continued to completion. Thus, a comparison was statistically significant when P<.025.
Results
Description of the Sample
Characteristics of the sample are summarized in Table 1. A total of 60% were male, 59% were nonwhite (56% black, 2% other/unknown), and 98% were non-Hispanic. Mean age was 47.9 years (SD, 17.5); mean APACHE III score was 77 (SD, 25.6). Clinical characteristics did not differ significantly among groups. Of the 547 enrolled patients, 249 were still endotracheally intubated on study day 3; of these, 209 patients had complete day 3 CPIS data. Because of missing values on some of the components of the CPIS, only 192 patients had CPIS values on both days 1 and 3, and their data could be analyzed completely. Comparison of the 192 patients in this analysis sample with the rest of the patients who were enrolled indicated no significant differences in baseline variables (sex, intubation process or reason, ICU, use of antibiotics at the time of admission, age, and count of decayed, missing, and filled teeth) except for the APACHE III score; those in the analysis sample had higher APACHE III scores than the other patients did. Patients in the analysis sample also had longer lengths of stay than did the other patients but did not have greater mortality. Comparison of data for patients in the day 3 analysis sample (n= 192) with data for patients missing CPIS components on either day 1 or 3 showed no significant differences in baseline variables.
Table 1.
Characteristics of enrolled sample and of day 3 analysis sample
| Day 3 analysis sample (n = 192) by randomized intervention group |
|||||
|---|---|---|---|---|---|
| Variablea | Enrolled sample (n = 547) |
Toothbrush only (n = 49) |
Chlorhexidine only (n = 44) |
Both (n = 48) |
Control (n = 51) |
| Sex | |||||
| Male | 328 (60) | 28 (57) | 26 (59) | 28 (58) | 37 (73) |
| Female | 219 (40) | 21 (43) | 18 (41) | 20 (42) | 14 (27) |
| Race | |||||
| White | 227 (41) | 22 (45) | 22 (50) | 19 (40) | 22 (43) |
| Nonwhite | 320 (59) | 27 (55) | 22 (50) | 29 (60) | 29 (57) |
| Intensive care unit | |||||
| Medical respiratory | 147 (27) | 9 (18) | 13 (30) | 13 (27) | 12 (24) |
| Neurosurgical | 168 (31) | 13 (27) | 12 (27) | 18 (38) | 12 (24) |
| Surgical trauma | 232 (42) | 27 (55) | 19 (43) | 17 (35) | 27 (53) |
| Intubation process | |||||
| Elective | 206 (38) | 19 (39) | 15 (34) | 18 (38) | 16 (31) |
| Urgent | 171 (31) | 14 (29) | 17 (39) | 13 (27) | 20 (39) |
| Emergent | 170 (31) | 16 (33) | 12 (27) | 17 (35) | 15 (29) |
| Antibiotics at admissionb | |||||
| Yes | 85 (18) | 11 (22) | 7 (16) | 6 (12) | 8 (16) |
| No | 381 (82) | 38 (78) | 37 (84) | 42 (83) | 43 (84) |
| Age, mean (SD), y | 47.9 (17.5) | 47.1 (15.7) | 46.1 (18.2) | 47.3 (18.8) | 46.8 (16.4) |
| No. of decayed, missing, and filled teeth, mean (SD) | 9.3 (8.5) | 10.0 (8.3) | 8.4 (7.9) | 8.3 (7.7) | 10.3 (8.5) |
| Severity of illness (Acute Physiology and Chronic Health Evaluation III) score, mean (SD) | 73.1 (27.3) | 76.4 (23.3) | 80.4 (28.7) | 76.2 (25.5) | 76.2 (3.3) |
| Length of stay in intensive care unit, median, days | 6.0 | 10.8 | 10.7 | 11.7 | 14.2 |
| Length of stay in hospital, median, days | 13.0 | 23.4 | 22.1 | 19.6 | 24.3 |
| Outcome | |||||
| Discharged alive | 430 (79) | 39 (80) | 31 (70) | 36 (75) | 42 (82) |
| Died during hospitalization | 117 (21) | 10 (20) | 13 (30) | 12 (25) | 9 (18) |
All values are No. (%) unless indicated otherwise. Because of rounding, not all percentages total 100. The intervention groups did not differ significantly in any variable.
Only 466 of the 547 patients enrolled in the study had data for this variable.
On day 5, a total of 158 patients remained intubated (116 patients in analysis sample), and 109 patients remained intubated through day 7 (76 patients in analysis sample). Because patients remained in the study only while intubated and receiving mechanical ventilation, an important reason for attrition was extubation; patients′ death related to critical illness was another source of attrition.
Baseline counts of decayed, missing, and filled teeth (Table 1) indicated that many patients had oral health problems before admission.
Although patients with a clinical diagnosis of pneumonia were excluded from the study, we unexpectedly found that many patients met our research definition of pneumonia (CPIS ≥6) at the time data were collected for admission to the study. We were not able to use CPIS ≥6 as a criterion for exclusion from the study because doing so would have resulted in a substantial delay in initiation of study interventions; the CPIS cannot be calculated until results of chest radiographs and microbial cultures are available, and unblinding study personnel to patients′ baseline CPIS scores would have been necessary. Thus, at the time of data analysis, we identified 2 subsets of patients on the basis of initial CPIS scores at the time of admission to the study: patients without pneumonia at baseline (ie, did not meet the research definition of pneumonia of CPIS <6 on day 1) and patients with pneumonia at baseline (ie, did meet the research definition of pneumonia of CPIS ≥6 on day 1 even though they did not have a clinical diagnosis of pneumonia).
Effects of Intervention on VAP
Table 2 presents results for the main effects of toothbrushing and of chlorhexidine on the basis of CPIS values at day 3. The interaction of toothbrushing and chlorhexidine was not significant, so the interaction was not included in final analysis models. Thus, the tables present results for main effects of toothbrushing and chlorhexidine by treatment, rather than by treatment group assignment. When the entire sample was considered, neither chlorhexidine nor toothbrushing had significant effects on CPIS values or on pneumonia (CPIS ≥6). The interaction of toothbrushing and chlorhexidine was not significant. However, in the subset of patients who did not already have a CPIS ≥6 on day 1, patients who received chlorhexidine had significantly lower CPIS values on day 3, and pneumonia (CPIS ≥6) developed in significantly fewer patients by day 3; toothbrushing was not associated with lower day 3 CPIS values or less pneumonia in this subset.
Table 2.
Comparison of baseline and day 3 outcomes by treatment
| All patients (n = 192) |
Patients without pneumonia at baseline (n = 87) |
|||||
|---|---|---|---|---|---|---|
| Outcomes | Day 1 | Day 3 | Pa | Day 1 | Day 3 | Pb |
| Clinical Pulmonary Infection Score, mean (SD) | ||||||
| Chlorhexidine | .29 | .02c | ||||
| Yes | 5.36 (2.17) | 5.26 (2.44) | 3.56 (1.29) | 4.36 (2.11) | ||
| No | 5.70 (2.35) | 5.78 (2.20) | 3.36 (1.16) | 5.36 (2.08) | ||
| Toothbrushing | .95 | .30 | ||||
| Yes | 5.66 (2.38) | 5.58 (2.34) | 3.49 (1.30) | 5.02 (2.28) | ||
| No | 5.41 (2.16) | 5.48 (2.33) | 3.43 (1.17) | 4.66 (2.01) | ||
| Pneumonia, % | ||||||
| Chlorhexidine | .13 | .006c | ||||
| Yes | 51.1 | 41.3 | —d | 24 | ||
| No | 58.0 | 55.0 | — | 52 | ||
| Toothbrushing | .86 | .54 | ||||
| Yes | 55.7 | 49.5 | — | 40 | ||
| No | 53.7 | 47.4 | — | 36 | ||
P comparing those with and without the specific intervention, for all patients; analyses controlling for intensive care unit (strata), baseline Clinical Pulmonary Infection Score, and presence of other intervention.
P comparing those with and without the specific intervention, for patients without pneumonia at baseline; analysis controlling for intensive care unit (strata), baseline Clinical Pulmonary Infection Score, and presence of other intervention.
Statistically significant (P≤.025).
Dash indicates not applicable.
Many subjects had oral health problems before admission.
Data for days 5 and 7 are presented in Tables 3 and 4. Significant relationships between groups were not apparent, perhaps because of smaller sample sizes related to attrition. The number of patients with data for analysis at day 5 decreased from 192 to 11 6 (from 87 to 51 without pneumonia) and further decreased at day 7 (to 76 and 37 without pneumonia).
Table 3.
Comparison of baseline and day 5 outcomes by treatment
| All patients (n = 116) |
Patients without pneumonia at baseline (n = 51) |
|||||
|---|---|---|---|---|---|---|
| Outcomes | Day 1 | Day 5 | Pa | Day 1 | Day 5 | Pb |
| Clinical Pulmonary Infection Score, mean (SD) | ||||||
| Chlorhexidine | .48 | .94 | ||||
| Yes | 5.32 (2.32) | 5.71 (2.39) | 3.33 (1.36) | 5.26 (2.21) | ||
| No | 5.65 (2.26) | 5.72 (2.49) | 3.33 (1.27) | 5.25 (2.21) | ||
| Toothbrushing | .37 | .84 | ||||
| Yes | 5.63 (2.37) | 5.52 (2.22) | 3.43 (1.44) | 5.35 (2.21) | ||
| No | 5.37 (2.22) | 5.89 (2.61) | 3.25 (1.20) | 5.18 (2.21) | ||
| Pneumonia, % | ||||||
| Chlorhexidine | .24 | .84 | ||||
| Yes | 51.8 | 53.6 | —c | 44 | ||
| No | 60.0 | 48.3 | — | 42 | ||
| Toothbrushing | .27 | .23 | ||||
| Yes | 57.4 | 55.6 | — | 52 | ||
| No | 54.8 | 46.8 | — | 36 | ||
P comparing those with and without the specific intervention, for all patients; analyses controlling for intensive care unit (strata), baseline Clinical Pulmonary Infection Score, and presence of other intervention.
P comparing those with and without the specific intervention, for patients without pneumonia at baseline; analysis controlling for intensive care unit (strata), baseline Clinical Pulmonary Infection Score, and presence of other intervention.
Dash indicates not applicable.
Table 4.
Comparison of baseline and day 7 outcomes by treatment
| All patients (n = 76) |
Patients without pneumonia at baseline (n = 37) |
|||||
|---|---|---|---|---|---|---|
| Outcomes | Day 1 | Day 7 | Pa | Day 1 | Day 7 | Pb |
| Clinical Pulmonary Infection Score, mean (SD) | ||||||
| Chlorhexidine | .35 | .59 | ||||
| Yes | 5.11 (2.49) | 5.36 (2.29) | 3.21 (1.58) | 4.89 (2.69) | ||
| No | 5.70 (2.14) | 6.15 (2.33) | 3.78 (1.00) | 5.33 (1.78) | ||
| Toothbrushing | .77 | .87 | ||||
| Yes | 5.59 (2.46) | 5.85 (2.18) | 3.56 (1.41) | 5.12 (2.09) | ||
| No | 5.29 (2.21) | 5.71 (2.46) | 3.43 (1.33) | 5.10 (2.45) | ||
| Pneumonia, % | ||||||
| Chlorhexidine | .46 | .52 | ||||
| Yes | 47 | 53 | — | 53 | ||
| No | 55 | 50 | — | 33 | ||
| Toothbrushing | .21 | .25 | ||||
| Yes | 53 | 59 | — | 56 | ||
| No | 50 | 45 | — | 33 | ||
P comparing those with and without the specific intervention, for all patients; analyses controlling for intensive care unit (strata), baseline Clinical Pulmonary Infection Score, and presence of other intervention.
P comparing those with and without the specific intervention, for patients without pneumonia at baseline; analysis controlling for intensive care unit (strata), baseline Clinical Pulmonary Infection Score, and presence of other intervention.
Discussion
VAP is associated with increased health care costs, morbidity, and mortality. Chlorhexidine oral swabbing was effective in reducing early VAP in patients in medical, surgical/trauma, and neuroscience ICUs who did not have pneumonia at baseline. Toothbrushing did not reduce the incidence of VAP, and combining toothbrushing and chlorhexidine did not provide additional benefit over use of chlorhexidine alone.
This project was a randomized, controlled clinical trial to determine if 2 oral care interventions, chlorhexidine and toothbrushing, would reduce the risk for VAP during the first week of intubation in critically ill adults receiving mechanical ventilation. The sample was diverse in race and included both men and women. Severity of illness was appropriate for a large urban medical center, and the VAP rate observed was similar to published rates.31
In our previous studies on the effect of oral health status on development of VAP, we found that increased dental plaque was predictive of pneumonia in patients with lower baseline CPIS values.32 At that time, we speculated that patients who did not yet have indications of pulmonary infection might derive the most benefit from interventions to reduce dental plaque. The finding in the current study that chlorhexidine was beneficial on day 3 in the subset of patients who had baseline CPIS values <6 but not in the total sample supports the idea of a differential benefit of a reduction in the number of oral organisms based on level of pulmonary infection, with more benefit in those without preexisting infection.
The toothbrushing protocol did not have a significant effect on VAP. Although both chlorhexidine and toothbrushing control organisms in dental plaque, chlorhexidine has bactericidal activity, whereas toothbrushing mechanically reduces the number of organisms without residual activity on the organisms remaining in the mouth. The intermittent reduction in the number of organisms by toothbrushing was insufficient to reduce the risk for pneumonia.
The most recent (2004) Centers for Disease Control and Prevention recommendations9 for prevention of nosocomial bacterial pneumonia in patients receiving mechanical ventilation specifically address the importance of oral microbial flora in the development of VAP. Recommendations for patients having elective cardiac surgery include the use of chlorhexidine during the perioperative period and are based on the results of studies19–21 in which patients began using chlorhexidine before hospital admission for elective cardiac surgery and chlorhexidine use was continued throughout the hospital stay. However, for other critically ill patients, the oral care recommendations are much more general, and evidence available when the guidelines were updated was insufficient for making a recommendation for use of chlorhexidine in the general ICU population.
Toothbrushes are generally regarded as the best tool for mechanical oral care in healthy populations. In this study we hypothesized that a defined toothbrushing intervention would reduce risk of VAP; it did not. Despite great enthusiasm among bedside nurses regarding the theorized effect of oral care on VAP reduction, few data support the effectiveness of mechanical oral care procedures. Most studies are anecdotal or a nonexperimental design was used, and many studies included oral care as one component of a VAP reduction program that included interventions with proven efficacy (including elevation of the head of the bed), with all interventions tested together as a bundle.
Chlorhexidine reduced rates of ventilator-associated pneumonia in patients without pneumonia at baseline.
For example, in a study often used as supporting evidence for the efficacy of oral care in reducing the rate of VAP, Zack et al33 conducted an observational study of VAP rates in a single hospital 12 months before and 12 months after providing an educational self-study program about VAP reduction to respiratory care practitioners and ICU nurses. A decrease in VAP (P < .001) was noted in the year after the educational program. However, oral care (″provide oral hygiene at least once daily″) was only 1 of 14 recommendations, which also included extubating patients as soon as possible, elevating the head of the bed, reducing unnecessary use of antibiotics, and ventilatory circuit management). The direct contribution of toothbrushing to VAP reduction was not ascertainable. In a follow-up study34 conducted by the same group using the same design in 4 hospitals (a pediatric teaching hospital, an adult teaching hospital, and 2 community hospitals in an integrated health system), combined VAP rates decreased significantly (P < .001) even though the recommendation for routine oral hygiene was omitted.
Toothbrushing alone did not reduce ventilator-associated pneumonia, and combining toothbrushing with chlorhexidine did not provide additional benefit over chlorhexidine alone.
Chlorhexidine is a broad-spectrum antibacterial agent that has been used extensively in healthy populations as an oral rinse to control dental plaque and to prevent and treat gingivitis.35,36 Microbial resistance to chlorhexidine has not been demonstrated, and the drug has minimal side effects. Three investigative teams19–21 have shown the effectiveness of oral chlorhexidine in reducing nosocomial respiratory tract infections in patients having elective cardiac surgery. Importantly, in each of these studies, the intervention was begun preoperatively (before intubation) and was continued throughout the ICU stay. However, cardiac surgery patients who have elective surgery most likely have different comorbid conditions and better physiological status at the time of intubation than do patients in the general adult ICU population. Studies in patients having elective cardiac surgery focused broadly on nosocomial infection (including surgical infection and tracheobronchitis) rather than on VAP.
Risk of ventilator-associated pneumonia begins with intubation; so too should prevention efforts.
Recently, chlorhexidine has been investigated in other ICU populations as well. Koeman et al37 randomized patients to a control group or to oral topical application of either 2% chlorhexidine or 2% chlorhexidine with colistin. Both chlorhexidine groups had reduced daily risk of VAP compared with control patients (chlorhexidine vs control, P = .01; chlorhexidine plus colistin, P = .03). Of note, the concentration of chlorhexidine used by Koeman et al was higher than the dental solution of 0.12% approved by the Food and Drug Administration that was used in our study and in other reported studies. In a randomized controlled trial of 0.2% chlorhexidine vs placebo in 228 ICU patients, Fourrier et al38 found no effect of chlorhexidine on VAP rate, with reported VAP rates of 11 % in each group. In our current study, topical application of chlorhexidine 0.12% solution to the oral cavity significantly reduced the incidence of pneumonia on day 3 among patients who did not have pneumonia at baseline (P = .006).
The smaller sample sizes on days 5 and 7 did not allow conclusions about the effect of the interventions on late-onset VAP. The target population of critically ill adults is difficult to study because of their heterogeneity of underlying medical conditions, rapid changes in health status, numerous intervening variables, and uncontrollable attrition due to death or extubation. Additionally, our study design specified recruiting patients within the first 24 hours of intubation and obtaining prospective informed consent from potential patients’ legally authorized representatives during a stressful period. These requirements further limited enrollment of patients.
Conclusion
VAP remains an important clinical problem for critically ill patients. Further research to prevent VAP is needed. A different toothbrushing protocol might yield different results. Although the finding is not statistically significant, patients who received the toothbrushing intervention tended to have higher CPIS values on days 3, 5, and 7 than did patients in the other groups. Because dislodgement of dental plaque organisms during toothbrushing could provide a larger pool of organisms for translocation from the mouth to subglottic secretions or the lung, further investigation of potential risks of toothbrushing is warranted. Additionally, the role of endotracheal tube stabilization and manipulation in provision of oral care is an area for future research. We excluded edentulous patients because dental plaque was an outcome measure in our study, but VAP may also develop in edentulous patients, and optimal oral care practices for such patients have not yet been tested.
Risk of VAP begins with intubation; so too should VAP prevention efforts. Cardenosa Cendrero et al39 found that 80 of 110 patients had tracheal colonization during the first day of mechanical ventilation. Thus, interventions will most likely have greatest effect on the incidence of early colonization and early VAP if they are begun very early in the ICU stay. Additional strategies to reduce VAP, such as beginning interventions earlier in the course of intubation, should be developed and tested.
Acknowledgments
FINANCIAL DISCLOSURES
This research was supported by grant NIH R01 NR07652 from the National Institutes of Health to Cindy L. Munro, principal investigator.
REFERENCES
- 1.Cook D. Ventilator associated pneumonia: perspectives on the burden of illness. Intensive Care Med. 2000;26(suppl 1):S31–S37. doi: 10.1007/s001340051116. [DOI] [PubMed] [Google Scholar]
- 2.Byers JF, Sole ML. Analysis of factors related to the development of ventilator-associated pneumonia: use of existing databases. Am J Crit Care. 2000;9:344–349. [PubMed] [Google Scholar]
- 3.Rodriguez JL, Gibbons KJ, Bitzer LG, Dechert RE, Steinberg SM, Flint LM. Pneumonia: incidence, risk factors, and outcome in injured patients. J Trauma. 1991;31:907–912. [PubMed] [Google Scholar]
- 4.Leu HS, Kaiser DL, Mori M, Woolson RF, Wenzel RP. Hospital-acquired pneumonia: attributable mortality and morbidity. Am J Epidemiol. 1989;129:1258–1267. doi: 10.1093/oxfordjournals.aje.a115245. [DOI] [PubMed] [Google Scholar]
- 5.Haley RW, Schaberg DR, Crossley K, Von Allmen SD, McGowan JE., Jr Extra charges and prolongation of stay attributable to nosocomial infections: a prospective inter-hospital comparison. Am J Med. 1981;70:51–58. doi: 10.1016/0002-9343(81)90411-3. [DOI] [PubMed] [Google Scholar]
- 6.Munro CL, Grap MJ. Oral health and care in the intensive care unit: state of the science. Am J Crit Care. 2004;13:25–33. [PubMed] [Google Scholar]
- 7.Munro CL, Grap MJ, Hummel R, Elswick RK, Sessler C. Oral health status: effect on VAP [abstract] Am J Crit Care. 2002;11(3):280. [Google Scholar]
- 8.Bearman GM, Munro C, Sessler CN, Wenzel RP. Infection control and the prevention of nosocomial infections in the intensive care unit. Semin Respir Crit Care Med. 2006;27:310–324. doi: 10.1055/s-2006-945534. [DOI] [PubMed] [Google Scholar]
- 9.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 10.Alp E, Voss A. Ventilator associated pneumonia and infection control. Ann Clin Microbiol Antimicrob. 2006;5:7. doi: 10.1186/1476-0711-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute for Healthcare Improvement. Implement the ventilator bundle. [Accessed June 19, 2009];Institute for Healthcare Improvement Web site. http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/ImplementtheVentilatorBundle.htm.
- 12.Craven DE, Driks MR. Nosocomial pneumonia in the intubated patient. Semin Respir Infect. 1987;2:20–33. [PubMed] [Google Scholar]
- 13.Torres A, el-Ebiary M, Gonzalez J, et al. Gastric and pharyngeal flora in nosocomial pneumonia acquired during mechanical ventilation. Am Rev Respir Dis. 1993;148:352–357. doi: 10.1164/ajrccm/148.2.352. [DOI] [PubMed] [Google Scholar]
- 14.Greene R, Thompson S, Jantsch HS, et al. Detection of pooled secretions above endotracheal-tube cuffs: value of plain radiographs in sheep cadavers and patients. AJR Am J Roentgenol. 1994;163:1333–1337. doi: 10.2214/ajr.163.6.7992723. [DOI] [PubMed] [Google Scholar]
- 15.Valles J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995;122:179–186. doi: 10.7326/0003-4819-122-3-199502010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Abele HM, Dauber A, Bauernfeind A, et al. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD) Intensive Care Med. 1997;23:187–195. doi: 10.1007/s001340050314. [DOI] [PubMed] [Google Scholar]
- 17.Johanson WG, Jr, Seidenfeld JJ, de los Santos R, Coalson JJ, Gomez P. Prevention of nosocomial pneumonia using topical and parenteral antimicrobial agents. Am Rev Respir Dis. 1988;137:265–272. doi: 10.1164/ajrccm/137.2.265. [DOI] [PubMed] [Google Scholar]
- 18.Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20:740–745. doi: 10.1097/00003246-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 19.DeRiso AJ, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–1561. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 20.Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care. 2002;11:567–570. [PubMed] [Google Scholar]
- 21.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296:2460–2466. doi: 10.1001/jama.296.20.2460. [DOI] [PubMed] [Google Scholar]
- 22.Pineda LA, Saliba RG, El Solh AA. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta-analysis. Crit Care. 2006;10(1):R35. doi: 10.1186/cc4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–528. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 24.Torres A, Gatell JM, Aznar E, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152(1):137–341. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- 25.Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 26.Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Yende SP, Wunderink RG. Clinical Pulmonary Infection Score (abstract) Chest. 1998;114:324S. [Google Scholar]
- 28.Papazian L, Thomas P, Garbe L, et al. Bronchoscopic or blind sampling techniques for the diagnosis of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1995;152(6 pt 1):1982–1991. doi: 10.1164/ajrccm.152.6.8520766. [DOI] [PubMed] [Google Scholar]
- 29.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Am J Respir Crit Care Med. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 30.Langer M, Cigada M, Mandelli M, Mosconi P, Tognoni G. Early onset pneumonia: a multicenter study in intensive care units. Intensive Care Med. 1987;13(5):342–346. doi: 10.1007/BF00255791. [DOI] [PubMed] [Google Scholar]
- 31.Bauer TT, Ferrer R, Angrill J, Schultze-Werninghaus G, Torres A. Ventilator-associated pneumonia: incidence, risk factors, and microbiology. Semin Respir Infect. 2000;15:272–279. doi: 10.1053/srin.2000.20938. [DOI] [PubMed] [Google Scholar]
- 32.Munro CL, Grap MJ, Elswick RK, Jr, McKinney J, Sessler CN, Hummel RS., III Oral health status and development of ventilator-associated pneumonia: a descriptive study. Am J Crit Care. 2006;15:453–460. [PubMed] [Google Scholar]
- 33.Zack JE, Garrison T, Trovillion E, et al. Effect of an education program aimed at reducing the occurrence of ventilator-associated pneumonia. Crit Care Med. 2002;30:2407–2412. doi: 10.1097/00003246-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Babcock HM, Zack JE, Garrison T, et al. An educational intervention to reduce ventilator-associated pneumonia in an integrated health system: a comparison of effects. Am J Respir Crit Care Med. 2004;125:2224–2231. doi: 10.1378/chest.125.6.2224. [DOI] [PubMed] [Google Scholar]
- 35.Iacono VJ, Aldredge WA, Lucks H, Schwartzstein S. Modern supragingival plaque control. Int Dent J. 1998;48:290–297. doi: 10.1111/j.1875-595x.1998.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 36.Newman HN. The rationale for chemical adjuncts in plaque control. Int Dent J. 1998;48:298–304. doi: 10.1111/j.1875-595x.1998.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 37.Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173(12):1348–1355. doi: 10.1164/rccm.200505-820OC. [DOI] [PubMed] [Google Scholar]
- 38.Fourrier F, Dubois D, Pronnier P, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo-controlled multicenter study. Crit Care Med. 2005;33:1728–1735. doi: 10.1097/01.ccm.0000171537.03493.b0. [DOI] [PubMed] [Google Scholar]
- 39.Cardenosa Cendrero JA, Sole-Violan J, Bordes BA, et al. Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 1999;116:462–470. doi: 10.1378/chest.116.2.462. [DOI] [PubMed] [Google Scholar]