Abstract
Purpose
Cryptococcal meningitis is a leading cause of mortality among HIV-infected individuals in sub-Saharan Africa but little is known about its treatment and outcomes in decentralized HIV outpatient settings. We assessed adherence to treatment guidelines and determined predictors of survival.
Design
A computerized laboratory database identified HIV-infected adults with cryptococcal meningitis at Family AIDS Care and Education Services in Nyanza Province, Kenya, between 2005-2009. Medical records were reviewed. Kaplan-Meier survival curves were generated. Bivariate and multivariate Cox proportional hazards models were used to determine associations between key clinical characteristics and survival.
Results
Medical records were located for 79% (71/90). Mortality was 38% (27/71) over a median follow-up period of 201 days [Inter-quartile range (IQR): 10-705 days]. Adherence to local guidelines for treatment of cryptococcal meningitis was 48% (34/71). Higher BMI was associated with improved survival (HR: 0.82, 95%CI [0.68,0.99]) even after controlling for factors such as age, CD4 cell count, receipt of HAART, and treatment with any anti-fungal therapy.
Conclusion
Cryptococcal meningitis diagnosed in routine HIV outpatient settings is largely treated as an outpatient and adherence to treatment guidelines is poor. BMI is a critical independent predictor of outcome. Additional research to determine the most effect strategies to reduce premature mortality is urgently needed.
Keywords: Meningitis, Cryptococcal, AIDS-Related Opportunistic Infection, ambulatory care, Kenya, HIV
Introduction
Meningitis due to Cryptococcus neoformans is a leading cause of mortality among HIV-infected individuals. In sub-Saharan Africa, cryptococcal meningitis may be surpassing tuberculosis as the leading cause of death;[1] cryptococcal meningitis caused nearly 20% of deaths in several sub-Saharan African HIV-infected cohorts.[2, 3] Although the prevalence of cryptococcal infection has not been systematically studied in sub-Saharan Africa, the prevalence of serum cryptococcal antigen (sCrAg) in HIV infected individuals with low CD4 cell counts ranges from 6-14%.[4-7]
Despite highly active anti-retroviral therapy (HAART) and effective anti-fungal treatments, mortality from cryptococcal meningitis in HIV-infected individuals is high even in developed countries, ranging from 9-38%.[8-11] Mortality is dramatically higher in sub-Saharan Africa; cryptococcal meningitis has a mortality of 37-58% even with HAART and amphotericin-based anti-fungal treatments [12-14] and up to 100% without these therapies.[15] However, these studies were conducted in tertiary care referral centers, possibly including more severe cases, and often within the context of a clinical trial. Little is known about treatment for and outcomes of cryptococcal meningitis in decentralized HIV outpatient care settings—now the predominant model of HIV care across much of sub-Saharan Africa[16].
Thus, the main objectives of this evaluation were to: (1) assess adherence to guidelines for treatment of cryptococcal meningitis, and (2) determine the key predictors of survival in cryptococcal meningitis in a decentralized HIV outpatient care setting in western Kenya.
Main Messages
Cryptococcal meningitis was primarily managed as an outpatient with oral therapy in our decentralized HIV program in Kenya
Despite available guidelines for the treatment of cryptococcal meningitis, adherence to these guidelines was poor in this real-world setting
Cryptococcal meningitis had an unacceptably high mortality rate
Mortality was significantly associated with lower body-mass index
Current Research Questions
How can current cryptococcal meningitis treatment guidelines be optimally operationalized in decentralized HIV programs in sub-Saharan Africa?
Can more potent oral regimens for the treatment of cryptococcal meningitis be implemented for more effective outpatient management?
Can cryptococcal meningitis deaths and overall mortality be reduced through early screening for cryptococcal antigenemia?
Study Design
Study Site
Family AIDS Care and Education Services is a decentralized HIV outpatient care and treatment program in Nyanza Province, Kenya. Founded in 2004, FACES is comprised of 62 clinics across 4 predominantly rural districts of Nyanza (as of Oct 2009). Cumulative adult enrollment as of Sept 2009 was 58,674 adults. The clinics provide free basic HIV care and treatment including anti-retroviral therapy.
During the study period, the main diagnostic test available for the diagnosis of cryptococcal meningitis was serum cryptococcal antigen (sCrAg). The test was typically done when a patient presented with signs and symptoms of meningitis. During the study period, screening asymptomatic individuals for sCrAg was not performed at FACES. Because of funding limitations, in our setting a single sCrAg test is performed on an undiluted sample; serial dilutions for sCrAg titers are not performed because each dilution requires an additional test kit. Lumbar punctures are only available in inpatient settings, and the cost of the procedure and the admission must be borne by the patient. Thus, although this would be the preferred method of diagnosis, in reality, lumbar puncture was rarely performed in routine clinical practice in our study setting.
Study Population and Procedures
A computerized laboratory database and a hand search of laboratory registers was used to identify all HIV-infected individuals with a positive sCrAg test (titer≥1:2) (Immuno-Mycologics) between July 2005 and October 2009. Two attempts were made to locate paper charts housed at peripheral sites and charts which could be found were reviewed retrospectively. Clinical and outcome data was abstracted using a standardized form between March and November 2009.
Cerebrospinal fluid cultures and other definitive diagnostic tests were not available in our setting, and documentation of signs and symptoms in the clinical record was poor. Thus, we developed three case definitions relevant to the diagnosis of cryptococcal meningitis in our resource-limited clinical setting: 1) Positive sCrAg; 2) Positive sCrAg plus at least one documented sign/symptom of meningitis; 3) Positive sCrAg plus at least one sign/symptom of meningitis, excluding headache. Signs and symptoms of meningitis included: fever≥38°C, headache, altered mental status, stiff neck, photophobia, seizures, and a focal neurological deficit.
Outcomes
The key outcomes were adherence to clinical guidelines for treatment of cryptococcal meningitis, all-cause mortality within 12 weeks of the sCrAg test, and all-cause mortality at the end of the follow-up period. Survival was defined as time from the date of sCrAg test until date of death. In one case, death was confirmed but date of death was not available, so the date of last clinic visit was used as a substitute. Surviving subjects who were currently in care (i.e. attended clinic within 3 months) were censored at the date of medical record abstraction. For two subjects who were lost-to-follow-up (i.e. not attended clinic within 3 months), date of last clinic visit was used as the censoring point. For 12-week survival, all subjects were censored at 12 weeks.
Data Analysis
First, we assessed adherence to local guidelines for the treatment of cryptococcal meningitis. Due to missing data and attrition due to mortality, the denominators in this analysis are frequently smaller than our sample.
Second, key clinical, laboratory and demographic characteristics were compared among fatal and non-fatal cases using Fisher's exact test for dichotomous variables and Wilcoxon rank-sum test for continuous variables. Binomial exact confidence intervals are presented.
Third, Kaplan-Meier curves were generated to assess survival. Associations between key predictor variables and survival were explored using bivariate Cox proportional hazards regression. Sensitivity analysis was performed using the three case definitions. Multivariate models were limited due to sample size;[17] variables with conceptual importance or significant bivariate associations were included. Sensitivity analysis was performed using 12-week mortality as an outcome (death within 12 weeks of sCrAg test).
Statistical analyses were performed using Stata 11. Significance was defined at p<.05.
Ethics
This program evaluation under FACES was approved by the University of California San Francisco Committee on Human Research, the Kenya Medical Research Institute National Ethical Review Committee, and the Centers for Disease Control and Prevention.
Results
Between July 2005 and October 2009, there were 90 sCrAg positive results at FACES. Charts were located for 79% (71/90) and of these follow-up data was available for 97% (69/71) (Figure 1). Mortality at 12 weeks was 23% (16/71) (95% Confidence Interval (CI): [13%,34%]) and 38% (27/71) (95%CI: [27%,50%]) at the end of follow-up, over a median follow-up period of 201 days [Inter-quartile range (IQR): 10-705 days]. Median survival was 38 days (IQR: 6-184 days) among all fatal cases and total follow-up time was 74.9 person years.
Figure 1.
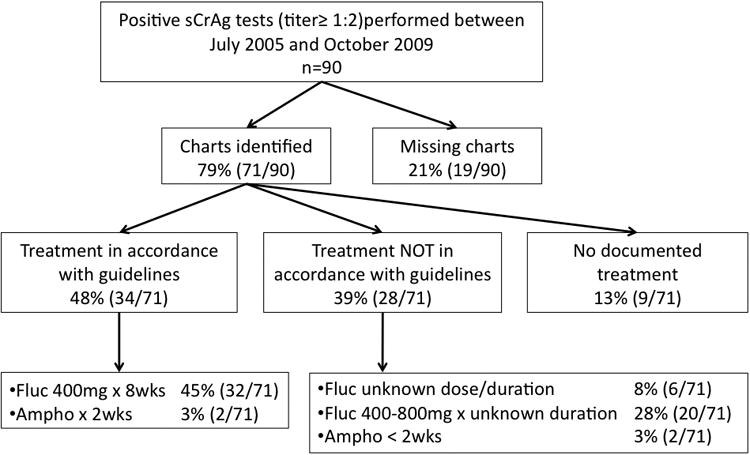
Flow chart describing availability of charts and anti-fungal treatment of individuals with serum cryptococcal antigen titers ≥ 1:2 at Family AIDS Care and Education Services in Nyanza Province Kenya between 2005 and 2009.
Descriptive statistics for individuals meeting case definition 1 are presented in Table 1. Fatal cases were significantly associated with lower BMI (p=.003) and younger age (p=.03). The most common symptom was headache in 88% (60/68). Fever was observed in 18% (11/61), seizures in 10% (7/68) and focal neurological deficits in 9% (6/68). Paradoxically, headache was more common in non-fatal cases (p=.05). Over one-third of individuals were taking anti-retroviral therapy for at least two weeks at the time of sCrAg testing. Overall, the median duration of HAART therapy was 40 days. Fatal cases had shorter duration on HAART than non-fatal cases, but this difference did not reach significance.
Table 1.
Key clinical characteristics compared between fatal and non-fatal outcomes among individuals with serum cryptococcal antigen titers ≥ 1:2 at Family AIDS Care and Education Services in Nyanza Province Kenya between 2005 and 2009.
| Variable | N | Overall n=71* | Fatal Cases n=27 | Non-Fatal Cases n=42 | p† |
|---|---|---|---|---|---|
| Age [median (IQR)] | 69 | 38 (34, 45) | 36 (34, 41) | 41 (35, 47) | .03 |
| Male Gender [% (n)] | 71 | 54% (36) | 52% (14) | 52% (22) | 1.00 |
| CD4 cell count (cells/μl) [median (IQR)]‡ | 68 | 41 (13, 91) | 31 (23, 72) | 48 (11, 95) | .58 |
| Body Mass Index (kg/m2) [median (IQR)] | 66 | 17.7 (16.3, 20.3) | 16.8 (14.0, 18.5) | 18.8 (16.9, 22.3) | .003 |
| On HAART at time of sCrAg [% (n)]** | 56 | 36% (20) | 36% (5) | 37% (15) | 1.00 |
| Duration of HAART (days) [median (IQR)]§ | 22 | 40 (29, 75) | 29.5 (21, 49) | 44.5 (33.5, 133) | .12 |
| Anti-fungal therapy [% (n)] | 71 | ||||
| Fluconazole only | 82% (58) | 78% (21) | 83% (35) | .71 | |
| Amphotericin +/- fluconazole | 6% (4) | 7% (2) | 5% (2) | ||
| Missing or Unknown | 13% (9) | 15% (4) | 12% (5) | ||
| Symptoms [% (n)] | |||||
| Fever ≥38°C | 61 | 18% (11) | 18% (4) | 16% (6) | 1.00 |
| Headache | 68 | 88% (60) | 77% (20) | 95% (38) | .05 |
| Stiff neck | 68 | 26% (18) | 31% (8) | 25% (10) | .78 |
| Photophobia | 68 | 18% (12) | 15% (4) | 18% (7) | 1.00 |
| Altered Mental Status | 68 | 12% (8) | 19% (5) | 8% (3) | .25 |
| Seizures | 68 | 10% (7) | 11% (3) | 10% (4) | 1.00 |
| Focal Neurological Deficit | 68 | 9% (6) | 4% (1) | 13% (5) | .39 |
Abbreviations: HAART: Highly active anti-retroviral therapy
Values for overall sample include two individuals with unknown vital status
Wilcoxon rank-sum test used to compare continuous variables; Fisher's exact for proportions; binomial exact 95% confidence intervals were used for proportions.
CD4 count performed closest to date of serum cryptococcal antigen test
On HAART at least 2 weeks on date of sCrAg
Only calculated for individuals on HAART at the time sCrAg test was performed; duration of HAART measured from HAART initiation to date of sCrAg test: fatal cases (n=6) non fatal cases (n=16)
Adherence to Guidelines
Anti-fungal therapy occurred primarily in the outpatient setting and treatment type varied widely. Hospital admission was documented in only 31% (22/71). None of the patients were treated using current international standards[18] or the preferred anti-fungal treatment in the Kenya National Guidelines (Figure 1)[19]. However, 48% (34/71) received intensive therapy with either amphotericin or fluconazole in accordance with local guidelines (Appendix 1). Over 82% (58/71) received fluconazole monotherapy, 6% (4/71) received amphotericin based and 13% (9/71) had no documented anti-fungal treatment. Among the 48 patients still living at 8 weeks after the sCrAg test, maintenance therapy with fluconazole 200mg was started in 83% (40/48).
All-cause mortality
Increasing age and BMI were positively associated with survival in bivariate Cox proportional hazards regression among individuals meeting case definition 1 (Table 2). In multivariate survival models, higher BMI retained a significant positive association with survival (HR: 0.82, 95%CI [0.68,0.99]) even after controlling for factors such as age, CD4 cell count, receipt of HAART, and treatment with any anti-fungal therapy (Table 2). Sensitivity analysis, performed using case definitions 2 (n=65) and 3 (n=48), did not demonstrate differences in the direction or magnitude of relationships between survival and key variables though only BMI remained significantly associated with survival (data not shown). Sensitivity analysis of bivariate associations using 12-week mortality demonstrated similar relationships.
Table 2.
Bivariate and multivariate Cox proportional hazards regression models for long-term survival and key clinical characteristics among individuals with serum cryptococcal antigen titers ≥ 1:2 at Family AIDS Care and Education Services in Nyanza Province Kenya between 2005 and 2009.
| Bivariate Models | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | P |
| Age (decades) | 0.71 | [0.51, 0.99] | .05 | 0.75 | [0.47, 1.21] | .25 |
| Male gender | 0.95 | [0.45, 2.03] | .91 | |||
| CD4 cell count (100 cells/ul) | 0.59 | [0.30, 1.17] | .13 | 0.65 | [0.20, 2.09] | .47 |
| Body Mass Index (kg/m2) | 0.78 | [0.67, 0.91] | .001 | 0.82 | [0.68, 0.99] | .04 |
| On HAART | 0.80 | [0.26, 2.39] | .69 | 0.80 | [0.23, 2.78] | .72 |
| Any anti-fungal therapy | 0.69 | [0.24, 1.99] | .49 | 0.50 | [0.06, 4.08] | .52 |
Abbreviations: HAART: Highly active anti-retroviral therapy
Discussion
The 12-week mortality in our study was 23%, which is lower than other cohorts in sub-Saharan Africa where mortality ranged from 43-100%[12-15, 20, 21] and within the range of studies from developed countries (8%-27%).[8, 10, 22, 23] The long-term mortality of 39% was also lower than other sub-Saharan African studies where the mortality ranged from 41-100%.[12, 15, 20, 21, 24-26] The relatively low mortality in our study may be a result of using an outpatient sample with less severe disease. Alternatively, this may be due to missing data. If all the charts we could not locate (19/71) as well as the charts with missing outcome data (2/71) were fatal cases, then the mortality rate could potentially be as high as 68% (48/71).
Our sample had similar rates of headache, stiff neck, photophobia, seizures and focal neurological deficits as other studies from inpatient samples from both developed and developing countries.[10, 15, 21, 22, 27, 28] However, in our study only 18% presented with fever, as compared to 42-93% in inpatient samples.[10, 15, 21, 22, 27, 28] Thus, we may have selected for individuals with less clinically severe cases.
Anti-fungal therapy did not exhibit significant associations with survival. This may have resulted from infrequent use of either international or local standards of care.[18, 19] Amphotericin was rarely used; although it is available in some district hospitals in Kenya, the complexity of administration and inability to monitor for side effects may limit the use of this medication. Fluconazole is available for free at most clinic sites through a donation program, but only half of our sample received therapy in accordance with local guidelines. It is clear that in our setting cryptococcal meningitis treatment is predominantly with outpatient oral fluconazole without lumbar puncture for diagnostic or therapeutic purposes. This may be because fluconazole is free while inpatient admission is not, poor acceptability of lumbar puncture [24], or due to other barriers to accessing inpatient care.
In addition, adherence to treatment guidelines was poor. Less than half of the individuals in our sample received cryptococcal meningitis treatment in accordance with local guidelines. However, documentation of dose and duration in the medical record was poor; either dose or duration were not documented for 36% (26/71) of our sample, so this study may underestimate actual adherence to guidelines in clinical practice.
Increasing BMI had a striking positive association with survival even after controlling for potential confounders such as CD4 cell count, duration of HAART and anti-fungal therapy. The association between BMI and mortality among patients with HIV has been described noted previously in a South African study which noted an association between wasting and death from cryptococcal infection [28].
Our study has several limitations. First, there was a lot of missing data—both charts and individual data points were variably recorded. For example, this was a retrospective review of routinely collected data extracted from paper outpatient charts kept on-site at FACES clinics, many of which are quite remote. Therefore, despite at least two attempts to locate them, charts were often not found. Since the charts which could not be found may have been more likely to belong to fatal cases, this could have led to marked underestimation of mortality rates. In addition, data was missing for several important variables such as dose and duration of anti-fungal therapy, and we were unable to review inpatient records. Also, exact dates were often missing from mortality data.
Second, this study reviews all positive sCrAg cases in our clinical setting as the test became available. The test became available at different times—at more central and higher resourced clinics first—so this is not a representative sample of our clinic population. Third, lumbar puncture and cerebrospinal fluid cultures, India Ink stain, and cerebrospinal fluid CrAg test are not available in our clinical settings so we were not able to confirm meningeal infection. However, sCrAg has excellent sensitivity and specificity for meningeal infection in symptomatic HIV infected individuals.[29] Finally, the small sample size limited our ability to draw definitive conclusions about the effects of key variables on survival.
In summary, this descriptive and exploratory study to identify key predictors of outcome from cryptococcal meningitis in a routine rural HIV outpatient care setting in sub-Saharan Africa offers several provocative findings. First, in our experience, cryptococcal meningitis diagnosed in a routine rural HIV outpatient setting is largely treated as an outpatient. Second, adherence to treatment guidelines for cryptococcal meningitis is poor, though this may be an underestimate as documentation of dose and duration of treatment was poor. Third, BMI is a critical independent predictor of outcome.
Ultimately, it is critical to recognize that cryptococcal meningitis is a leading cause of mortality among HIV-infected individuals. This study demonstrates that in HIV care settings like ours treatment for cryptococcal meningitis is currently largely as an outpatient—whether or not this is applicable to other similar programs is an area for future study. Potential strategies to improve outcomes for individuals with cryptococcal meningitis in rural outpatient African settings like ours include improving access to inpatient care; increasing availability of medications which are the standard of care such as amphotericin or flucytosine; building capacity in inpatient settings such that appropriate laboratory monitoring for side effects of amphotericin and flucytosine can be performed; screening individuals with low CD4 T-cell counts for sCrAg and initiating early treatment; increasing adherence to care guidelines; or more effective oral therapies[30]. To reduce the premature mortality due to cryptococcal meningitis, future studies are critical to determine the optimal approaches to operationalize current cryptococcal treatment guidelines in decentralized HIV outpatient care and treatment programs in rural sub-Saharan Africa.
Supplementary Material
Acknowledgments
We would also like to thank the medical officers, clinical officers, nurses, clinic/community health assistants, staff and patients at Family AIDS Care and Education Services for their participation and the Director of the Centre for Microbiological Research and the Director of Kenya Medical Research Institute (KEMRI) for their support. This manuscript is submitted with permission to publish from the Director KEMRI and the Centers for Disease Control and Prevention.
Funding: This study was supported by the President's Emergency Plan for AIDS Relief (U62/CCU924511) (Kendi, Penner, Koech, Nyonda, Cohen, Bukusi), the American Academy of Neurology Foundation Practice Research Training Fellowship (Meyer), and the Fogarty International Clinical Research Fellowship (5 R24 TW00798; 3 R24 TW00798-02S1) from the National Institutes of Health, Fogarty International Center through Vanderbilt University, the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), Office of the Director, National Institutes of Health and the National Institute of Mental Health (NIMH) (Meyer). The Centers for Disease Control & Prevention had a role in editing the final manuscript and approved the manuscript for publication.
Footnotes
Competing Interests: None
References
- 1.Park B, Wannemuehler K, Marston B, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002 May 3;16(7):1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 3.Lawn S, Harries A, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmet P, Kayembe KD, De Vroey C. The value of cryptococcal serum antigen screening among HIV-positive/AIDS patients in Kinshasa, Zaire. AIDS. 1989 Feb 1;3(2):77–8. doi: 10.1097/00002030-198902000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis Joseph N, Lawn Stephen D, Vogt M, et al. Screening for Cryptococcal Antigenemia in Patients Accessing an Antiretroviral Treatment Program in South Africa. CLIN INFECT DIS. 2009 Apr 1;48(7):856–62. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007 Aug 1;12(8):929–35. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 7.Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. CLIN INFECT DIS. 2010 Aug 15;51(4):448–55. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006 Nov 14;20(17):2183–91. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 9.Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992 Jan 9;326(2):83–9. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 10.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997 Jul 3;337(1):15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 11.Mirza SA, Phelan M, Rimland D, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. CLIN INFECT DIS. 2003 Mar 15;36(6):789–94. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 12.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. CLIN INFECT DIS. 2007 Jul 1;45(1):76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 13.Nussbaum Jesse C, Jackson A, Namarika D, et al. Combination Flucytosine and High-Dose Fluconazole Compared with Fluconazole Monotherapy for the Treatment of Cryptococcal Meningitis: A Randomized Trial in Malawi. CLIN INFECT DIS. 2010 Feb 1;50(3):338–44. doi: 10.1086/649861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. CLIN INFECT DIS. 2008 Dec 15;47(12):1556–61. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 15.Mwaba P, Mwansa J, Chintu C, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001 Dec 1;77(914):769–73. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Countries showcase benefits of scaling up HIV/AIDS services using WHO approach. Available from: http://www.who.int/hiv/capacity/IMAI-ICASA/en/
- 17.Vittinghoff E, McCulloch C. Relaxing the rule of ten events per variable in logistic and cox regression. American Journal of Epidemiology. 2007;165(6):710–8. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 18.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010 Feb 1;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Manual for the Management of HIV-related Opportunistic Infections and Conditions. 2nd. Nairobi, Kenya: Kenya Ministry of Health; 2008. [Google Scholar]
- 20.Schaars CF, Meintjes GA, Morroni C, et al. Outcome of AIDS-associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazole. BMC Infect Dis. 2006 Jan 1;6:118. doi: 10.1186/1471-2334-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher D, Mwandumba H. Cryptococcal meningitis in Lilongwe and Blantyre, Malawi. J Infect. 1994 Jan 1;28(1):59–64. doi: 10.1016/s0163-4453(94)94161-0. [DOI] [PubMed] [Google Scholar]
- 22.Antinori S, Galimberti L, Magni C, et al. Cryptococcus neoformans infection in a cohort of Italian AIDS patients: natural history, early prognostic parameters, and autopsy findings. Eur J Clin Microbiol Infect Dis. 2001 Oct 1;20(10):711–7. doi: 10.1007/s100960100616. [DOI] [PubMed] [Google Scholar]
- 23.Jongwutiwes U, Kiertiburanakul S, Sungkanuparph S. Impact of antiretroviral therapy on the relapse of cryptococcosis and survival of HIV-infected patients with cryptococcal infection. Curr HIV Res. 2007 May 1;5(3):355–60. doi: 10.2174/157016207780636551. [DOI] [PubMed] [Google Scholar]
- 24.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. CLIN INFECT DIS. 2008 Jun 1;46(11):1694–701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bicanic T, Muzoora C, Brouwer Annemarie E, et al. Independent Association between Rate of Clearance of Infection and Clinical Outcome of HIV-Associated Cryptococcal Meningitis: Analysis of a Combined Cohort of 262 Patients. CLIN INFECT DIS. 2009 Sep 1;49(5):702–9. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayanja-Kizza H, Oishi K, Mitarai S, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis. 1998 Jun 1;26(6):1362–6. doi: 10.1086/516372. [DOI] [PubMed] [Google Scholar]
- 27.Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989 Sep 21;321(12):794–9. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy KM, Morgan J, Wannemuehler KA, et al. Population-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalence. AIDS. 2006 Nov 14;20(17):2199–206. doi: 10.1097/QAD.0b013e3280106d6a. [DOI] [PubMed] [Google Scholar]
- 29.Antinori S, Radice A, Galimberti L, et al. The role of cryptococcal antigen assay in diagnosis and monitoring of cryptococcal meningitis. J Clin Microbiol. 2005 Nov 1;43(11):5828–9. doi: 10.1128/JCM.43.11.5828-5829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization; Dec, 2011. Rapid Advice: Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children. www.who.int/hiv/pub/cryptococcal_disease2011/en/: [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


