Abstract
Two primary animal models persist for assessing hallucinogenic potential of novel compounds and for examining the pharmacological and neurobiological substrates underlying the actions of classical hallucinogens, the two-lever drug discrimination procedure and the drug-induced head-twitch response (HTR) in rodents. The substituted amphetamine hallucinogen, serotonin 2 (5-HT2) receptor agonist, 2,5-dimethoxy-4-iodoamphetamine (DOI) has emerged as the most popular pharmacological tool used in HTR studies of hallucinogens. Synthesizing classic, recent, and relatively overlooked findings, addressing ostensibly conflicting observations, and considering contemporary theories in receptor and behavioural pharmacology, this review provides an up-to-date and comprehensive synopsis of DOI and the HTR model, from neural mechanisms to utility for understanding psychiatric diseases. Also presented is support for the argument that, although both the two-lever drug discrimination and the HTR models in rodents are useful for uncovering receptors, interacting proteins, intracellular signalling pathways, and neurochemical processes affected by DOI and related classical hallucinogens, results from both models suggest they are not reporting hallucinogenic experiences in animals.
Keywords: 5-HT2; serotonin; DOI; HTR; model; 2,5-dimethoxy-4-iodoamphetamine; phenylisopropylamine; psychedelic; hallucinogen
A brief chemistry and timeline of DOI
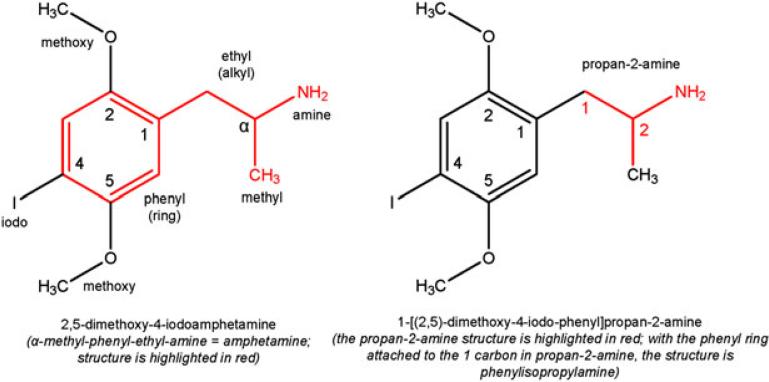
The hallucinogen, 2,5-dimethoxy-4-iodoamphetamine (DOI), is in the broad class of compounds called phenylalkylamines. As the name suggests, phenylalkylamines consist of a phenyl ring attached to an alkyl chain that is attached to an amine. Having a two-carbon chain, the alkyl in DOI is ethyl, rendering phenethylamine. DOI has a methyl group attached to the α carbon of phenethylamine making its core structure, amphetamine (α-methyl-phenethylamine). Attached to the 2 and 5 carbons of the phenyl ring are methoxy groups, and attached to the 4 carbon is iodine. Amphetamine is also named phenylisopropylamine, and thus DOI is interchangeably identified as a substituted amphetamine or phenylisopropylamine. A detailed description of DOI's chemical make-up and its common nomenclature are shown in Figure 1.
Figure 1.
Commercial (a) and IUPAC (b) nomenclature for DOI.
DOI possesses one chiral centre and can exist as two enantiomers, (R)-(–)-DOI or (S)-(+)-DOI. Although enantiomers of many compounds exhibit vastly different affinities for their target receptors, this does not appear to be the case for DOI; the (–) enantiomer was reported as having only moderately (~2-fold) higher affinity than the racemate (±) or (+) enantiomer for rat cortical serotonin (5-hydroxytryptamine, 5-HT), 5-HT2A receptors[1,2] and human recombinant 5-HT2A and 5-HT2C receptors,[3] and had slightly lower (< 2 fold) affinity than the (+) enantiomer for human recombinant 5-HT2B receptors.[3] There are reports that the behavioural potencies of the enantiomers of DOI, however, are substantially different in mice (induction of an ear-scratch response),[4] rats (drug discrimination),[5] and humans (subjective psychedelic effects),[6,7] with (–)-DOI reported as more potent than (+)-DOI or the racemate. Also, the chemically similar DOM (2,5-dimethoxy-4-methylamphetamine) was shown to have different potencies to induce hyperthermia in rabbits whether the (–) (more potent) or (+) (less potent) enantiomer was used; the racemate showed an intermediate potency, though somewhat overlapped with the (–) enantiomer.[8] The greater potency of (–)-DOM was corroborated by behavioural studies in rats.[9] Regarding the closely related analogue, DOB (2,5-dimethoxy-4-bromoamphetamine), (–)-DOB showed much greater potency and efficacy to activate 5-HT2C receptors in rat choroid plexus compared to (+)-DOB,[10] an effect recapitulated in rat behavioural studies, wherein (–)-DOB was much more effective than (+)-DOB for substituting for the DOI discriminative cue in rats.[11]
Of the 2,5-dimethoxy-4 series of substituted amphetamine hallucinogens, synthesis of DOM was reported first in the scientific literature in 1970,[12] followed later by DOB,[13] and DOI.[14] In their recent book, however, Shulgin et al. note that Shulgin synthesized DOM in 1963, and its psychedelic effects in humans were discovered in 1964.[15] The radiolabelled DOI, [[125]I]-DOI, which has high specific activity, was used to identify DOI binding sites in the rat brain in 1987.[16] Since then, [[125]I]-DOI has become a frequently used tool to label high affinity (or non-hydrolyzable GTP-sensitive) 5-HT2 (5-HT2A, 5-HT2B, or 5-HT2C) receptors for determination of ligand affinities of novel compounds in both recombinant DNA in vitro assays and ex vivo. Also, competition binding using [[125]I]-DOI to label high affinity receptor binding sites may be part of a first-pass screen to test for 5-HT2 agonist activity of novel compounds.[17]
DOI has become one of the most common tools to study mechanisms of classical hallucinogens in animals, with over 100 scientific publications reporting its use since 2000. There are a few reasons for this: (1) of the substituted amphetamine hallucinogens, it is one of the few that is not specifically scheduled in the United States; (2) it is chemically stable even in solution; (3) it is relatively selective for activating 5-HT2 receptors, activation of which likely underlies the psychoactive effects of classical hallucinogens.[18]
Pharmacology of DOI
Most discussion regarding the pharmacology of DOI has centered on the 5-HT2 G protein-coupled receptors (GPCRs) that include 5-HT2A, 5-HT2B, and 5-HT2C subtypes. DOI has high affinity and is a potent agonist for each of the 5-HT2 receptor subtypes, with respect to the traditional signalling pathways that they share.[10,19–21] For example, using agonist radiolabelling, the Ki values of (±)-DOI for human 5-HT2A, 5-HT2B, and 5-HT2C receptors were 0.7, 20, and 2.4 nM, respectively,[20] and (±)-DOI's agonist potencies for stimulating recombinant human 5-HT2A, 5-HT2B, and 5-HT2C receptor-mediated calcium signalling were 0.9, 1.4, and 7.9 nM, respectively.[19] DOI has relatively low affinity (Ki values greater than 1 mM) for all other 5-HT receptors investigated.[22,23] Though DOI's affinity for 5-HT3 receptors has not been reported to our knowledge, it is likely that DOI also has low affinity for this receptor.[2] Unique among the 5-HT2 receptor subtypes, the 5-HT2C receptor can be edited post-transcriptionally to produce more than 20 different isoforms.[24,25] DOI's potency (both affinity and efficacy) is affected by RNA editing of the 5-HT2C receptor, having lower potency for fully edited compared to non-edited 5-HT2C receptors.[26,27] RNA editing of 5-HT2C receptors also affects their desensitization and trafficking in the cell.[28] Thus, when examining the effects of DOI (and other compounds) at 5-HT2C receptors, the isoform(s) studied should be taken into consideration. This is feasible when assessing recombinant 5-HT2C receptor isoforms in vitro, as expression of a specific isoform of the receptor is controlled by the experimenter. In vivo, however, it is not possible to determine the specific 5-HT2C receptor protein isoform(s) that are examined in the brain from a wild-type animal using common techniques (e.g. radioligand binding). For example, the relative proportion of different htr2C (genetic nomenclature for 5-HT2C) RNA isoforms that can be assessed by many existing methods does not directly correlate with the relative proportion of different 5-HT2C receptor protein isoforms expressed in the cell membrane.[29] This challenging issue warrants further attention.
Depending on the signalling pathway examined, DOI has been shown to be, in vitro, either a partial or a full agonist for 5-HT2A, 5-HT2B, and 5-HT2C receptors. At recombinant human 5-HT2 receptors, DOI generally behaves as a potent partial agonist for stimulating 5-HT2-mediated Ca++ currents[19] (used the 5-HT2C-VSV isoform) and the phospholipase-C pathway, generating inositol phosphates (PLC-IP)[30–32] (5-HT2C isoform was not identified). DOI is a full agonist for activating 5-HT2C receptors coupled to the phospholipase-A (PLA) signalling pathway,[31,32] and is a partial agonist for activating 5-HT2A receptors coupled to PLA.[32] DOI's efficacy for activating 5-HT2 receptors is also related to receptor expression level, displaying greater efficacy in cells expressing relatively higher receptor densities. For example, DOI behaves as a partial agonist (i.e. 53% efficacy relative to 5-HT) for 5-HT2C-VSV receptor-mediated PLC-IP activity when receptors are expressed at ~300 fmol/mg,[33] and as a full agonist (i.e. 101% efficacy relative to 5-HT) for 5-HT2C-VSV receptor mediated PLC-IP activity when receptors are expressed at ~20 pmol/mg, suggesting a significant receptor reserve.[34] Ex vivo, DOI exhibits partial agonism at 5-HT2 receptors, for example at 5-HT2A receptors expressed on GABAergic interneurons in piriform cortex,[35] and 5-HT2C receptors expressed in choroid plexus.[10] To our knowledge, DOI's efficacy for activating 5-HT2B receptors in the brain has not been tested, likely owing to their scant expression in the rodent brain.[36]
DOI's pharmacology at receptors other than 5-HT receptors has received considerably less attention, but warrants discussion. A recent report[23] presents binding affinity (Ki) data for DOI (among many other psychedelic compounds) at more than 40 unique receptors known to be expressed in the brain. DOI exhibited affinities, considered by the author to be noteworthy, for 19 receptors. The highest Ki value presented as noteworthy, however, was 2.72 μM, i.e. DOI's affinity for the M1 muscarinic receptor (shown in the supplementary material), an affinity that is tacitly considered low or arguably not physiologically relevant (but see section on dosing issues). The Ki of DOI was less than 400 nM for only 6 receptors: 5-HT2A (165.4 nM), 5-HT2B (336 nM), 5-HT2C (45.8 nM)a, α2A-adrenergic (73.7 nM), α2B-adrenergic (340 nM), and β2-adrenergic (138.6 nM) receptors.
The reported affinities of DOI for α2- and β2-adrenergic receptors should be taken into account when considering DOI's behavioural effects. For example , Benloucif et al.[37] showed that DOI (above 12 nmol) increases dopamine release in the anterior striatum of anesthetized rats, an effect that was not blocked by the 5-HT2 antagonist, ritanserin (4 nmol), suggesting it may be mediated by DOI acting on α2-adrenergic receptors, which are known to regulate dopamine release in brain tissue. Also, cortical application of the α1/2 adrenergic antagonist, prazosin, blocked the 5-HT releasing effect of DOI in the brain.[38]
The head-twitch response (HTR)
The first drug-elicited HTR
To our knowledge, the first description of a drug-elicited HTR was reported in 1956 by Keller and Umbreit[41] following intravenous administration of lysergic acid diethylamide (LSD) to mice. They described an easily observed and quantifiable response that could be elicited by a light touch near the back of the head, similar to the reflexive response observed when the ear is touched or pinched (the pinna reflex response), or following LSD administration (which became ‘permanent’ in a subset of mice following an injection of indole followed by LSD). In their words:
The response consists of a rapid and violent head shaking. . . The head-twitch response does not occur in normal mice, and with a little experience the response is easy to detect. It is only rarely that one is uncertain whether a particular animal possesses the head twitch or not. . ..Independent observations by different workers are remarkably consistent, so that this provided a suitable tool for the behavioral studies. (p. 723)
In our experience, the HTR is indeed easily quantifiable, with a high concordance of counts between independent observers, and shows a reliable and remarkably low level of within-subject (when animals are tested repeatedly) and between-subject (across animals within a particular treatment condition) variability. Following this initial observation with LSD, Corne et al.[42] demonstrated in a convincing and comprehensive manner that the serotonin precursor 5-hydroxytryptophan (which is decarboxylated into serotonin) elicits a HTR, and characterized the pharmacology of the response. Several classes of drugs (including serotonin antagonists, antihistamines, and opioids) attenuated the response, MAO inhibitors (e.g. harmine, iproniazid, pheniprazine, and tranylcypromine) potentiated the HTR, whereas other drugs had no effect (e.g. barbituates). Several years later, Corne and Pickering[43] demonstrated that a variety of hallucinogens produce the HTR. These drugs included the classical hallucinogens LSD, psilocybin, psilocin, N,N-dimethyltryptamine (DMT), and mescaline, as well as drugs such as phencyclidine (PCP), atropine, and yohimbine. Interestingly, there was a correlation between potency of these compounds to elicit the HTR and the dose that was hallucinogenic in humans. Subsequently, a number of serotonergic compounds (predominantly serotonin-releasing agents and direct 5-HT2 agonists) have been shown to produce the HTR in rodents, including quipazine, 5-MeO-DMT, 5-MeO-DIPT, DPT, 2C-T-7, 1-methylpsilocin, TCB-2, fenfluramine, DOM, MK-212, DOB, ergometrine, and to a lesser extent 2,5-DMA, 2C-D, 2C-B, and 2C-I.[32,44–51] It should be noted that there are compounds from other pharmacological classes that also produce the HTR (e.g. CB1 cannabinoid antagonists, see the section on Hallucinogenesis).
The first DOI-elicited HTR
To the best of our knowledge, Glennon et al.[5] first studied DOI's behavioural properties in animals (rats), showing that DOI fully substituted for DOM in a drug discrimination task. Two years later, based on a robust correlation between rat 5-HT2 affinity and behavioural potency, Glennon et al. reported that the 5-HT2 receptor was likely responsible for DOI's discriminative stimulus effects.[52] With the discovery of 5-HT2B and 5-HT2C receptors, the 5-HT2 receptor was reclassified as 5-HT2A.[53,54] Henceforth, when discussing the entire class of serotonin 2 receptors, i.e. 5-HT2A, 5-HT2B, and 5-HT2C, 5-HT2 will be used. The first study examining the HTR elicited by DOI was reported by Arnt and Hyttel[55] who used rats, and demonstrated dose-dependent increases in the number of head-twitches; this behavioural effect was blocked by administration of the fairly selective 5-HT2A antagonist ketanserin, as well as the 5-HT1A agonist 8-OH-DPAT. Later studies confirmed 5-HT2A receptors are the primary, direct mediators of the response, while 5-HT1A receptors perform a modulatory function. Many investigators describe the HTR effect in the rat as a ‘head shake’, however, it appears to be essentially the same response as the HTR in mice, at least in regards to similarity in appearance and 5-HT2A receptor dependence. A report by Darmani et al.[56] was the first to demonstrate a DOI-elicited HTR in the mouse. In this paper, DOI produced dose-dependent increases in HTRs (tested up to 5.0 mg/kg), the (–) isomer was demonstrated to be more effective in eliciting an HTR, and the 5-HT2A antagonists, ketanserin and spiperone, as well as the 5-HT1A agonist, 8-OH-DPAT, attenuated the response. Similar but less commonly studied 5-HT2A receptor-dependent behavioural responses have been elicited in other species including the rabbit (head bobs[57,58]) and the least shrew.[59] In contrast, there are several other behavioural responses elicited by serotonergic compounds that are not 5-HT2A receptor dependent. For example, the ‘serotonin syndrome’ consists of a number of responses (e.g. flat body posture, hind limb abduction, forepaw treading, lower lip retraction, backwards walking) that are primarily a consequence of agonism at 5-HT1 receptor subtypes.[60] The ‘ear-scratch response’ observed in mice (and also elicited by DOI) appears to be primarily mediated by activation of 5-HT2C receptors.[61]
How to quantify the HTR and dose-related effects
There are several methods that have been used to quantify drug-elicited HTRs. Quantal methods determine the percentage of animals that show an HTR in a given period of time following particular treatments. This allows a relatively high-throughput method of quantifying the HTR, as large groups of animals can be observed at the same time (e.g. for an extended discussion of these methods see Corne et al.[42]). More commonly, a graded response is obtained simply by counting the number of HTRs observed in a single animal during a testing period; these data are obtained by trained observers either in real time, or video-recorded and later quantified. Depending on the question of interest, either a single animal's behaviour will be observed over time (e.g. to determine the time-course of the drug effect; Figure 2)[62,63] or more commonly a particular set of pretreatment and observation times are established. As an example, we and other investigators have adopted a protocol in which DOI is administered 10 min before the testing session, and then the mouse is observed for 10 min. These parameters allow for quantification of a robust HTR with consistent frequency throughout the observation period. Numerous studies have demonstrated dose-dependent effects of DOI in rats and mice, such that increasing the doses results in increases in HTRs over a dose range from 0.1 mg/kg (essentially no HTRs) up to 2–10 mg/kg (maximal levels of HTR).[48,55,56,64–71] Some investigators have reported biphasic dose-effect curves, i.e. increases followed by decreases in HTR frequency with increasing doses. For example, Pranzatelli[72] describes a ‘descending limb’ of the dose-effect curve from 12–20 mg/kg in rats, whereas Fantegrossi et al.[73] describe peak HTRs at 1.0 mg/kg, and decreases in HTR frequency at 3.0 mg/kg in Swiss NIH mice. The reasons for the differences in the shape of the dose-effect curve are not known, although species and strain differences are likely to contribute (see extensive discussion of some of these issues below).
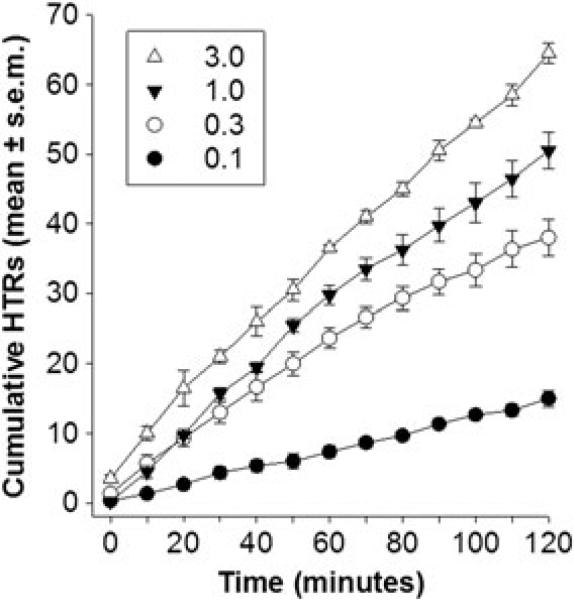
Figure 2.
Cumulative number of head-twitch responses (HTRs) following administration of various doses of (–)-DOI (mg/kg; IP) to male, C57Bl/6J mice. Each mouse (n = 4) was tested with all four doses (in random order) at approximately 1-week intervals. HTRs were counted in 2-min periods, every 10 min, beginning immediately following administration of drug. Note that (–)-DOI elicited HTRs began within the first 2-min period (Time 0), the frequency of HTRs were dose-dependent, and HTRs continued throughout the 2-h testing period.
Effects of repeated administration
Most studies use a particular experimental subject one time, presumably to eliminate the potential influence of repeated drug administration (i.e. the development of tolerance or sensitization) and repeated exposure to the testing apparatus. However, there have been a number of studies examining the effects of repeated DOI administration on HTR and subsequent sensitivity to DOI. As observed following the administration of essentially all drugs, the pattern of behavioural effects and change in subsequent sensitivity depend on the dose administered, frequency of administration, and time since the last injection. In early studies, it was demonstrated that a single injection of DOI (2.5 mg/kg) in mice results in tolerance to a second dose administered 24 h later, but ‘supersensitivity’ (an increase in responsiveness) to a second dose administered 48 h later.[61,62,70] To further investigate the effects of repeated drug exposure, 2.5 mg/kg DOI was administered daily for 13 days, and separate groups of mice were challenged following discontinuation of the chronic drug treatment. Tolerance immediately developed to the effects of DOI as indicated by ~40–50% decrease in the number of DOI-elicited HTRs following a 24-h period. This tolerance, however, disappeared over a 13-day period such that the number of HTRs increased and returned to control levels by day 6. Following chronic administration, a challenge dose of DOI (2.5 mg/kg), after a 48-h abstinence period, revealed a ‘supersensitivity’ (i.e. increase in HTR) that lasted for approximately 1 week.[70] In a more recent paper, Dougherty and Aloyo[74] administered 1 mg/kg DOI daily for 8 days (and 0.75 mg/kg on the 9th day). Tolerance (responding decreased by ~40–50%) developed following the first injection and was maintained throughout the 9-day testing period. The reasons for the discrepancy between the maintenance of tolerance in this study and the loss of tolerance upon repeated administration in the earlier studies by Darmani et al. are unclear; however, the dose used in the earlier studies was significantly higher (2.5 versus 1.0 mg/kg) and different strains of mice (ICR versus CB57Bl/6 N) were used (see the section on species and strain differences for further discussion). To our knowledge, no papers have examined the effects of DOI administered repeatedly every 48 h to determine whether the increased sensitivity is maintained over time. However, it has been demonstrated that when DOI is administered at weekly intervals, there appears to be no change in sensitivity over the course of 4 weeks.[75] This finding is important as it suggests that within-subject experiments could be conducted to allow animals to act as their own controls, potentially increasing statistical power and sensitivity to identifying treatment effects.
Influence of various physiological and environmental factors
Age
Relatively few studies have examined the effects of non-pharmacological factors on the DOI-elicited HTR. With regards to age, the dose-effect curve for DOI was shifted slightly downward (i.e. DOI was less effective) in older (3–3½-month-old) animals relative to younger (5–6-week-old) animals;[76] however, a relatively limited age range was examined. Darmani et al.[77] examined the ontogeny of various DOI-elicited behaviours and found that the HTR elicited by 1.0 and 2.5 mg/kg became apparent at 18 days of age and remained at high levels through 180 days of age; there were gradual non-significant decreases over time. In a genetic model of aging, the senescence accelerated mouse-prone (SAMP) was significantly more sensitive to DOI (more than twice as many HTRs following 0.3 and 1.0 mg/kg) relative to SAM-resistant mice.[78] At this point, there are not enough data to draw strong conclusions regarding the role of age in the DOI-elicited HTR, although it has been demonstrated that 5-HT2A receptor levels decrease with age in both humans and rodents.[79–82]
Sex
Few studies have examined the influence of sex and/or oestrus cycle on the HTR. In the Darmani paper described earlier,[77] male and female mice were not differentially sensitive to DOI. Using 5-HTP to elicit the HTR, Boulton and Handley[83] demonstrated that males were more sensitive to the effects of 5-HTP, but that oestrus cycle had no effect on the female mice. There are simply not enough data making direct comparisons to clearly identify the potential influence of sex and hormonal status on the DOI-elicited HTR.
Environmental factors
The effects of housing conditions (e.g. the number of animals per cage) on the DOI-elicited HTR were reported in one study.[84] Grouped-housed animals (5–6 ddY mice per cage) were significantly less sensitive to (±)-DOI (2.5 mg/kg) relative to singly-housed mice (13 vs. 24 HTRs in a 10-min observation period). These data contrast with a study showing that 5-HTP-elicited HTRs are extraordinarily low in singly-housed mice, and the frequency of the 5-HTP-induced HTRs increases along with the number of mice living in a particular group.[83] However, in the first paper to describe the HTR,[41] the authors noted that the HTR elicited by LSD was topographically identical to the HTR elicited by ‘solitary confinement’ (i.e. moving an animal from group-housing to singly-housed conditions). Given that housing conditions and social ranking within a colony can have dramatic effects on the 5-HT2A receptor system,[85] a systematic study of the effects of these factors on the sensitivity to DOI would be useful.
Yamata et al.[86] examined the effects of a number of ‘stressors’ on DOI-elicited responses in rats. Tail pinch for 30 min, immobilization for 60 min, and repeated foot shock for 30 min rendered animals less sensitive to DOI. Administration of diazepam (benzodiazepine receptor agonist) 5 min before the immobilization stress prevented this loss of sensitivity to DOI. This effect was blocked by the co-administration of flumazenil (benzodiazepine inverse agonist). Swim stress for 10 min similarly produced a decreased sensitivity to DOI, i.e. 64–73% decrease in HTRs depending on the time of observation.[87] In apparent contrast to these findings, stress induced by 3 h of restraint enhanced sensitivity to the effects of 1.0 mg/kg (but not 2.0 mg/kg) DOI in rats.[88] Interestingly, obese Zucker rats that have an overactive hypothalamic-pituitary-adrenal system, resulting in a model of ‘hypercorticism’ with chronic, high levels of circulating corticosterone (which generally increases in rodents when exposed to stressful conditions) show a dramatically attenuated response to DOI compared to lean littermates.[89] Finally, a couple of other studies have failed to show any effect of stress on sensitivity to DOI.[86,90]
Receptor systems involved in the HTR induced by DOI
5-HT2A
Activation of the 5-HT2A receptor is necessary for DOI's (and all other classical hallucinogens) head-twitch-inducing effects in rodents.[18] Corpuses of studies support this claim, which is undisputed, and which has been thoroughly reviewed elsewhere.[18,91–93] Briefly, the HTR in mice and rats (and the mole-like shrew) induced by DOI (and other serotonin-releasing compounds) is blocked by a plethora of relatively selective 5-HT2A antagonists; mice lacking the 5-HT2A receptor do not show an HTR to DOI; and restoration of 5-HT2A receptors to cortical (presumably glutamate) neurons in 5-HT2A receptor knockout mice restores the ability of DOI to induce an HTR in mice.[94] Furthermore, blockade of 5-HT2A receptors by one of the most selective 5-HT2A receptor antagonists, M100907,[95] occludes the discriminative stimulus effects of psychedelic amphetamines in rodents[18] and monkeys,[96] suggesting the 5-HT2A receptor underlies the discriminative stimulus effects of psychedelic amphetamines across species. Finally, modulation of the DOI HTR by any class of compounds, noted in the next several sections, can be blocked by selective 5-HT2A antagonists. Thus, the 5-HT2A receptor is necessary for the DOI HTR, and most of the following receptors, and neurotransmitter systems are best considered modulators of the DOI HTR.
5-HT2B
Owing to its relatively low expression level in the brain,[36,97,98] the 5-HT2B receptor has not received much of the limelight as a possible regulator of the DOI-induced HTR. We are unaware of papers reporting effects of selective 5-HT2B receptor antagonists on the DOI HTR. The DOI HTR-inducing effects have also not been described in 5-HT2B receptor knockout mice. Interestingly, although the ergoline analogue, lisuride, has 5-HT2A receptor agonist activity, it does not produce the HTR, and some (but not all) studies show that it is a potent antagonist of 5-HT2B receptors.[99] Other studies suggest it is worth considering the 5-HT2B receptor as a potential modulator of the behavioral effects of DOI. For example, like 5-HT2A and 5-HT2C receptors, 5-HT2B receptor affinity of hallucinogenic phenylisopropalamines is highly correlated with their human hallucinogenic potency.[20] Also, recent studies with rats show that the 5-HT2B receptor is required for the anorexient effects of the serotonin releaser dexfenfluramine[100] and for the serotonin-releasing and hyperlocomotion effects of 3,4-methylenedioxymethamphetamine (MDMA),[101] findings that argue brain 5-HT2B receptors may be involved in cognitive and/or behavioural effects of psychoactive drugs.
5-HT2C
Concerning their putative roles in the behavioural effects of psychedelic phenylisopropalamines, of the three 5-HT2 receptors, most contention emanates from the 5-HT2C receptor. A number of studies have substantiated a role, albeit a modulatory one, for the 5-HT2C receptor.[65,102,103] Like the other 5-HT2 receptors, the potency of phenylisopropalamines is highly correlated with their affinities for 5-HT2C receptors.[20] Also, the hallucinogenic potency of DOI, DOB, DOM and MDA decreases in parallel with decreasing efficacy to activate 5-HT2C receptors.[10] The (–)-DOM discriminative stimulus-potentiating effects of citalopram, furthermore, are attenuated by the highly selective 5-HT2C antagonist, SB242084,[104,105] and mCPP, considered a selective, albeit poorly selective, 5-HT2C agonist (relative to 5-HT2A receptors), also potentiated the discriminative stimulus effects of (–)-DOM.[106]
Regarding the HTR in rats, direct frontal cortex injections of mCPP induced an HTR, an effect that was attenuated by systemic pretreatment with the selective 5-HT2C receptor antagonist SDZ SER-082.[107] Interestingly, mCPP was more potent and produced more HTRs than DOI when injected centrally,[107] though its affinity and efficacy for stimulating 5-HT2 receptors is substantially lower than DOI's.[22] Also intriguing, when administered systemically, mCPP actually attenuates HTRs induced by a number of 5-HT2A agonists, including DOI.[108,109] Nevertheless, both the 5-HT2B/2C selective antagonist/inverse agonist, SB206553,[110] and the 5-HT2C antagonist, SB242084, attenuated the HTR elicited by DOI by 50% in C56BL/6J and DBA/2J mice, and the DOI HTR was attenuated by ~50% in 5-HT2C knockout mice, relative to their wild-type littermates.[65] Moreover, 5-HT2C antagonists also attenuated the discriminative stimulus effects of (+/–)-DOI in C57Bl/6J mice.[111] Collectively, these data suggest that activation of 5-HT2C receptors regulates the HTR and a portion of the behavioural effects of phenylisopropalamines in mice and rats. Nevertheless, caveats and conflicting data abound.
A role for the 5-HT2C receptor in mediating some of the cognitive and behavioural effects of psychedelic amphetamines is usually downplayed due to observations of opposing actions of 5-HT2A and 5-HT2C receptors.[73,112] Moreover, SB242084 did not affect a rat's ability to discriminate DOI,[11] and did not block the HTR in rats elicited by DOI,[64,113] nor did SDZ SER-082,[107] or the selective 5-HT2B/2C receptor antagonist SB 200646A.[114] Furthermore, behavioural tolerance to DOI after chronic administration (i.e. decreased discriminative stimulus effect), paralleled a decrease in 5-HT2A receptor-binding sites in the cortex and claustrum, yet was not associated with a decrease in 5-HT2C receptor-binding sites in the striatum or choroid plexus, determined by autoradiography.[11] It should be noted, however, that quantification of 5-HT2C-receptor binding sites (which are much fewer in number than 5-HT2A receptor binding sites) in the cortex, which is arguably the site of action for the discriminative stimulus effects of DOI,[115] could not be ascertained with this method.[11]
Adding to these findings were reports that the selective 5-HT2C agonists (relative to 5-HT2A), Ro 60–0175 and CP 809 101,[116,117] did not induce an HTR in rats on their own,[113,117] and actually dose-dependently decreased the (–)-DOI-elicited HTR in Swiss NIH mice (Ro 60–0175)[73] and rats (CP 809 101).[117] When co-administered with SB242084 to putatively unmask its agonist activity for 5-HT2A receptors, Ro 60–0175 produced an HTR, though at a lower frequency than the HTR induced by DOI.[113] Based on these data, and that SB242084 pretreatment in Swiss NIH mice can potentiate the maximum HTR induced by DOI by ~20%,[73] it is inferred that 5-HT2C receptor activation suppresses the psychoactive effects of DOI. Further elaborating on this point, counts of HTRs with increasing doses of DOI in Swiss NIH mice reveals an inverted-U shaped dose–response curve, and experiments combining M100907 or SB242084 with (–)-DOI suggest that the ascending part of the curve, at low DOI doses (from 0.3 to 1 mg/kg), is due to activation of 5-HT2A receptors, while the descending part of the curve, at high doses (from 1 mg/kg to 10 mg/kg), is due to increasing activation of 5-HT2C receptors.[73] How these data are reconciled with the similar agonist potencies and efficacies of DOI for both 5-HT2A and 5-HT2C receptors, and the reports of linear increases in hallucinogenic effects in humans with increasing doses of DOI, was not addressed. These data may suggest, however, that the HTR response observed may not map to hallucinogenic activity in humans. Nevertheless, in C57Bl/6J and DBA/2J mice, which show a more robust HTR to DOI than Swiss NIH mice, the DOI HTR dose–response curve increased linearly then plateaued at doses up to 12.8 mg/kg.[65] In this set of experiments, mice appeared sick at higher doses of DOI; thus, large doses were administered to only a few mice (unpublished observations). Similar linear DOI HTR dose–response curves up to 5 mg/kg have also been reported in albino ICR mice.[56] Again, strain and species differences appear to be major factors to consider when interpreting DOI HTR data.
It is also worth noting that 1-methylpsilocin is a 5-HT2C agonist, with more than 50 times greater affinity and potency for activating human 5-HT2C-INI compared to 5-HT2A receptors,[118] yet causes a dose-dependent increase in the HTR in C57Bl/6J mice,[47] suggesting that 5-HT2C receptor activation (if the pharmacology of 1-methylpsilocin at human receptors is recapitulated at mice receptors) does not suppress 5-HT2A receptor-mediated HTRs. Finally, the novel indazole 5-HT2 agonist, AL-38022A, showed several-fold higher potency for human 5-HT2C receptors (isoform unspecified), compared to 5-HT2A and 5-HT2B receptors, and yet fully generalized to the DOM stimulus in both rat and monkey drug discrimination studies.[96]
5-HT1
In the first papers describing the DOI-elicited HTR in rats and mice, the 5-HT1A agonist 8-OH-DPAT was shown to attenuate the response.[55,56] This finding was replicated a number of times,[64,69,109] including demonstration that direct infusion of 8-OH-DPAT into the medial prefrontal cortex inhibits the effects of systemically administered DOI.[107] These findings have been extended to a number of relatively selective 5-HT1A agonists, with varying degrees of efficacy, including buspirone, flesinoxan, MKC242, S14671, and S14506.[64,69,84] The effects of the non-selective 5-HT1A/2 agonist 5-MeO-DMT are interesting in that this compound can produce HTRs when administered alone.[47] When combined with DOI, however, 5-MeO-DMT dose-dependently decreases the number of DOI-elicited HTRs,[56] an effect attributed to 5-MeO-DMT's agonist activity for 5-HT1A receptors. Antagonism of the 5-HT1A receptor (e.g. with WAY-100635) fails to alter the DOI-elicited HTR.[69,84,119] Endogenous 5-HT1A receptor activity, thus, may be too low to affect DOI-induced 5-HT2 receptor activity.
Other 5-HT receptors and the serotonin transporter
In general, it appears that selective serotonin transporter inhibitors (i.e. SSRIs), such as fluoxetine, imipramine, fluvoxamine, and citalopram, fail to alter the DOI-elicited HTR.[63,120] However, if the target of these compounds (i.e. the serotonin transporter) is genetically deleted, the ability of DOI to elicit HTRs is greatly reduced and/or eliminated.[119,121] This effect is attributed to adaptations, e.g. desensitization and decreased expression of 5-HT2A receptors, due to life-long elevated levels of serotonin.
Chronic administration of serotonin, norepinephrine transporter inhibitors (i.e. SNRIs) decrease the DOI-elicited HTR.[122,123] Similarly, repeated exposure to MDMA, which acts partially through interactions with the serotonin, dopamine, and norepinephrine transporters, may alter sensitivity to DOI. Although one early paper suggested no lasting effect of MDMA administration,[124] a more recent report found that repeated MDMA treatment resulted in an enhanced sensitivity to DOI at a higher (3.0 mg/kg) but not a lower dose (0.5 mg/kg).[66]
Administration of quipazine, a mixed 5-HT2/5-HT3 agonist results in a robust HTR in both mice and rats.[64,125,126] Co-administration of the 5-HT3 receptor antagonist, ondansetron, does not substantially alter the DOI-elicited or quipazine-elicited HTR;[120] we recently replicated this finding in our lab using C57Bl/6J mice (unpublished observations). These data suggest that the 5-HT3 receptor may not alter 5-HT2 receptor function. It is worth noting that some early studies suggested that quipazine was hallucinogenic in man,[127] and a recent case report noted that quipazine produced a full hallucinogenic response when it was administered with a 5-HT3 receptor antagonist, presumably to prevent nausea/vomiting associated with 5-HT3 receptor activation.[15]
The 5-HT7 receptor has been generating increasing interest as a potential target for drug development for the treatment of psychiatric disorders, including schizophrenia.[128,129] To our knowledge, no selective 5-HT7 compound has been tested in the DOI-induced HTR procedure, although all antipsychotic compounds are active in this model.
Glutamate
There has been considerable interest in the role of glutamatergic systems in the DOI-elicited HTR. Much of this interest stems from the putative involvement of 5-HT2 receptors and glutamatergic systems in schizophrenia,[130] and that the DOI-elicited HTR may be relevant as a model of schizophrenia. To this end, numerous studies have examined pharmacological manipulations of both the ionotropic NMDA and AMPA receptors, and the metabotropic mGluR receptor systems. For example, antagonism of the NMDA receptor by MK801 or ketamine,[131–133] or antagonism of mGluR2/3 autoreceptors by LY341495[75,134] consistently increase prefrontal cortical levels of glutamate and enhance the effects of DOI, indicated by increased HTRs elicited by a particular dose or shifts in the DOI dose–response curve to the left and upwards.
An equally compelling story has emerged regarding glutamatergic interventions that block the effects of DOI. Activation of mGluR2/3 autoreceptors with LY379268 and LY354740, which presumably leads to decreased extracellular levels of glutamate, decreased sensitivity to DOI.[75,134] Similarly, administration of the glutamate release inhibitor riluzole or the glutamate analog theanine (which functions as a competitive antagonist) reduces DOI-induced HTRs.[135] Recent studies have demonstrated that selective AMPA glutamate antagonists, such as GYKI52466, LY203558, and NBWX, attenuate DOI-induced HTRs.[131,135] To complicate matters, it was recently demonstrated that the mGluR2-receptor knockout mice are insensitive to HTR-inducing effects of DOI (and LSD).[136] Although the neuropharmacological mechanisms underlying all of these interactions are not delineated, these data suggest a prominent role for glutamate receptor systems in the behavioural effects of DOI, and a strong interaction between glutamatergic and 5-HT2 receptor systems. Notably, it was recently discovered that 5-HT2A and mGluR2 receptors form heteromeric complexes that have unique signalling properties.[137]
GABA
Several allosteric modulators of the GABAA receptor have been examined for their influence on the DOI HTR. Both the high efficacy benzodiazepine agonist diazepam and the inverse agonist flumazenil appear to have no influence on DOI-elicited HTRs,[67,86,120,138] while the partial inverse agonist Ro154513 attenuates the DOI-induced HTR.[67,138] Furthermore, the direct GABAA agonist bicuculline attenuates DOI-induced head twitches.[138] More comprehensive studies directly comparing a range of compounds with various pharmacological actions at this receptor will help elucidate the potential modulatory role of GABAA receptors. A single paper has examined the role of metabotropic GABAB receptors in DOI's behavioural effects.[139] These authors demonstrated that both an agonist, CGP44532, and a positive allosteric modulator, GS39783, attenuated DOI-induced HTRs, which was reversed by two antagonists, CGP51176 and CGP36742, that had no effect on their own.
Dopamine
Given the potential relevance of the behavioural effects of DOI as a model of schizophrenia, a number of studies have examined the influence of typical, dopamine D2 antagonist, anti-psychotic compounds on the HTR. For example, haloperidol, chlorpromazine, and pimozide inhibit the HTR elicited by DOI.[64,68,72,120,140,141] Similarly, chronic administration of haloperidol results in a decreased sensitivity to DOI.[140] Although they possess excellent affinity for D2 receptors, these compounds are also 5-HT2A receptor antagonists.[22,142] The more selective D2 receptor antagonists, raclopride and sulpiride, show inconsistent effects or do not affect the DOI-elicited HTR.[64,72,141] The dopamine D1 receptor antagonists, SCH23390 and SCH39166, which also have affinity for some serotonin receptors, inhibit DOI's behavioural effects;[64,141] these findings are consistent with observations that the D1 receptor modulates glutamate and serotonin interactions in the prefrontal cortex.[143] Cocaine, a drug which blocks the reup-take of dopamine, 5-HT, and norepinephrine, attenuates DOI-elicited HTRs.[71,144,145] Although many of cocaine's behavioural effects have been attributed to activity at the dopamine transporter, Darmani et al. have demonstrated repeatedly that attenuation of the HTR by cocaine can be reversed by administration of the 5-HT1A antagonist, alprenolol, or the non-selective α2-adrenergic receptor antagonist, yohimbine, suggesting that cocaine's actions are mediated indirectly by increases in 5-HT1A and α2- adrenergic activity.[71,144,145]
Cannabinoids
The role of the cannabinoid system is interesting as the CB1 receptor antagonist SR141716A (Rimonabant) produces an HTR when administered alone.[146–148] Although SR141716A has no affinity for 5-HT receptors,[149] it is of interest is that the 5-HT2A/2C antagonist SR46349B potently reversed the effects of SR141716A, suggesting that activation of 5-HT2 receptors may be related to the behavioural effects of SR141716A.[147] Pretreatment with all CB1 agonists tested thus far, i.e. THC (Δ9 and Δ8), anandamide, HU210, CP55940, and WIN55212 inhibit DOI's behavioural effects.[146,150] Similarly, indirect cannabinoid agonists such as the anandamide transporter inhibitor AM404 and the FAAH inhibitor URB597, inhibit the HTR elicited by DOI.[135] These data suggest close interactions between 5-HT2 and cannabinoid receptors in vivo, which recent studies support.[151,152]
Acetylcholine
Acute and chronic administration of nicotine (direct agonist) and donepezil (acetylcholinesterase inhibitor; indirect agonist), two common drugs used in the treatment of Tourette's Syndrome, attenuate DOI-elicited HTRs. Based on these findings, it has been suggested that the DOI-elicited HTR may be useful as a model of Tourette's Syndrome.[140,153,154] The neurobiological mechanisms underlying these interactions are unknown, however, chronic administration of nicotine and donepezil results in decreased cortical 5-HT2A receptor density.[140]
Norepinephrine
The role of the norepinephrine system in regulating effects of hallucinogens has been studied mostly utilizing hallucinogens other than DOI, for example, 5-MeO-DMT, quipazine, and 5-HTP.[126,155–158] Fewer studies have examined the role of norepinephrine in regulating the DOI-induced HTR; however, it was demonstrated that prazosin, an α1/2 adrenergic receptor antagonist, completely blocks the DOI-elicited HTR.[141] In this study, behavioural potency of 9 compounds (including prazosine) was significantly correlated with binding at 5-HT2A and α1 adrenergic receptors. The finding that DOI itself has appreciable affinity for α2 receptors[23] suggests that DOI may directly affect the norepinephrine system, which may contribute to the behavioural effects of DOI. We are currently investigating this possibility.
Adenosine
One comprehensive paper has demonstrated a role for adenosine systems.[159] Adenosine receptors are expressed in practically all neural systems in the brain, and their activation generally suppresses neural activity. For example, activation of adenosine A1 receptors in the cortex reduces glutamate release.[160] Thus, it follows that the adenosine A1 agonist, N6-cyclohexyladenosine, attenuates the DOI-elicited HTR, an effect that is reversed by the adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine.[159] Of interest, the antagonist administered in combination with DOI enhances the HTR suggesting either that there is some basal level of adenosine receptor stimulation that modulates 5-HT2A receptor activity or that the drug is non-selective for adenosine A1 receptors.
Other systems
The mu opioid agonists, morphine and buprenorphine, and various atypical opiates, for example, tramadol and levorphanol, can attenuate the DOI-induced HTR in a naloxone-reversible manner;[63,161] for an exception see Wettstein et al.[120] The neuropeptide, galanin, was demonstrated to attenuate DOI-elicited HTRs, and completely blocked HTRs elicited by DOB and mescaline.[162,163] Lastly, a series of papers examined the effects of the compounds rubidium, caesium, and quinine (all compounds that interact with K+ channels and likely alter 5-HT release), and all tended to enhance the number of DOI-elicited HTRs.[164–166]
Summary
Taken together, the data described above suggest a number of neurotransmitter systems can alter behavioural effects elicited by the relatively selective 5-HT2 agonist, DOI, and highlight the wide-ranging and complex interactions associated with 5-HT2 receptors.
Neural and cellular systems underlying the psychoactive effects of DOI
Neural systems
McKenna et al.[16] first showed the distribution of DOI binding sites in rat brain using [[125]I]-(–)-DOI; the highest density of binding sites were observed in the cortex, claustrum, and olfactory bulb. Although the entire spectrum of psychedelic effects are likely the result of emergent outputs from communication between several neural systems,[167,168] several studies employing pharmacological, physiological, molecular, imaging, and/or genetic tools, have provided converging evidence that the cortex is the most primary site of action of the psychedelic effects of classical hallucinogens in man (e.g. psilocybin[167,169]), their drug discrimination stimulus effects in rats (e.g. LSD[115]), and their head-twitch-inducing effects in rats and mice (e.g. DOI[94,107]). Regarding DOI specifically, there are no studies (of which we are aware) aimed at deciphering its neuroanatomical site of psychedelic action in man, likely relating to its long duration of action, i.e. 16–30 h,[6] which would render clinical studies arduous to perform. However, based on results from similar classical hallucinogens, the cortex is probably the primary site mediating its hallucinogenic effects in man.
Whether or not there is a specific cortical region(s) that is necessary for producing the psychedelic effects of classical hallucinogens is not fully elucidated, but data point to the cingu-late gyri, a neural system located dorsomedially in the cortex, and implicated in decision making, emotion, and pain processing.[170] Psilocybin increases cerebral metabolic activity in the cingulate cortex in humans.[169] Also, direct injections of d-LSD into the cingulate cortex of rats dose-dependently substituted for systemic injections of d-LSD in a drug discrimination task; injections outside this region, including rostrally, into the orbital cortex, did not substitute.[115] Also, direct injections of DOI into the cingulate cortex dose-dependently induced the HTR in rats.[107] Moreover, restoration of expression of 5-HT2A receptors solely in the cortex, including the cingulate cortex, of 5-HT2A receptor knockout mice restored the ability of DOI to induce an HTR.[94]
Cellular systems: neurophysiology and neurochemistry
Many more studies have examined the cellular, both physiological and genetic, effects of DOI. In rodents, primates, and humans, 5-HT2A receptors, primary targets of DOI, are highly expressed in cortical structures where they are mostly (but not solely) postsynaptic; fewer 5-HT2A receptors are found in subcortical structures.[171–174] 5-HT2A receptors are most abundant on glutamatergic pyramidal neurons and GABAergic interneurons of the pre-frontal cortex, where they robustly stud somatodendritic regions of layer V.[171,172,175–178] Thus, much focus, regarding the cellular effects of DOI, has been on layer V of the cortex.
5-HT2 receptor activation by DOI significantly alters glutamate release and glutamatergic pyramidal neuron activity in the medial prefrontal cortex (layer V, predominantly), an effect that recent studies show is likely post-synaptically mediated and intrinsic to cortical-cortical circuits.[179,180] Results from both in vitro and in vivo studies in rats show that DOI increases spontaneous and evoked excitatory currents in layer V pyramidal cells (EPSCs),[181,182] increases the firing rate of a majority of neurons, but also decreases the firing rate of a significant number of layer V cells[183] and also unidentified medial prefrontal cortex cells,[184] reduces low-frequency oscillations, and desynchronizes pyramidal cell discharge from active phases of slow oscillations of medial prefrontal cortex cells.[179] The majority of these effects are abolished by M100907 or other 5-HT2A antagonists, suggesting they are 5-HT2A receptor-mediated. However, for full disclosure, it should be noted that Puig et al.[183] reported that the DOI-induced increase in firing rate was not reversed by systemic injections of M100907 (up to 0.5 mg/kg) in 33% (3/9) of the cells examined. Furthermore, the excitatory effects of the 5-HT2 agonist, am-5-HT, were mostly, yet not completely absent in cortical pyramidal cells from 5-HT2A receptor knockout mice; indeed 23% (9/39) of cells examined from these mice showed measurable am-5-HT-induced increases in EPSCs that were subsequently blocked by the selective 5-HT2C antagonist, SB242084.[180] Also, although the increase in EPSCs by am-5-HT was completely abolished by M100907 in pyramidal cells from wild-type mice, the concentration used, 300 nM,[180] was approximately 6-fold higher than its Ki for 5-HT2C (human, INI isoform) receptors.[3] Thus, the direct neurophysiological effects of DOI in pyramidal cells of layer V of the cortex likely involve activation of both 5-HT2A and 5-HT2C receptors, and subsequent increases in local glutamate release.
DOI also increases 5-HT, dopamine, and acetylcholine release in the frontal cortex, as well as other neural systems in rodents.[38,185–189] These effects of DOI, however, are likely secondary to activation of 5-HT2 receptors on cortical pyramidal neurons, which send efferent projections to regulate neurotransmitter release from subcortical 5-HT, dopamine, and acetylcholine-producing neurons. For example, systemic and direct injections of DOI in the frontal cortex increase 5-HT, dopamine, and acetylcholine release there, and the increases are prevented by direct injections of 5-HT2 antagonists into the frontal cortex.[38,187–189] Moreover, the DOI-induced increase in cortical dopamine release is absent in 5-HT2A receptor, but not 5-HT2C receptor knockout mice, suggesting this effect is 5-HT2A mediated.[190] Notably, 5-HT2C receptor knockout mice show, relative to wild-type mice, increased firing of dopaminergic substantia nigra neurons.[191] Whether this effect is related to 5-HT2C receptors on cells in the substantia nigra or is an indirect effect is unknown to us. Similarly, the increases in 5-HT in the medial prefrontal cortex in rats produced by direct DOI injections were blocked by co-application of the glutamate AMPA receptor antagonist, NBQX, but not SB242084, suggesting the DOI-induced 5-HT release in the cortex is mediated by 5-HT2A receptors on pyramidal neurons; though local 5-HT1A and alpha-adrenergic receptors were also involved.[38]
Cellular systems: intracellular proteins
At the molecular level, DOI causes strong expression, in cortical layer V neurons, of the immediate early gene c-fos, an effect blocked by pretreatment with M100907, but not by SB206553 (up to 6 mg/kg,[192]), and not observed in 5-HT2A knockout mice,[193] suggesting it is specifically 5-HT2A receptor mediated.[192] The induction of c-Fos protein in layer V by DOI likely does not, however, mediate its HTR-inducing effects, as the non-HTR-producing 5-HT2A agonist, lisuride, also induces c-Fos expression in the cortex.[94] DOI, but not lisuride, induced cortical expression of egr-1 and egr-2.[94] Though these genetic markers, also, do not underlie DOI's HTR-inducing effects, as their maximal induction was not observed until an hour after injection of DOI, whereas the HTR to DOI was observed within minutes of injection; furthermore, mice lacking egr-1 show a normal response to DOI.[194]
5-HT2 receptors share a common signalling pathway involving coupling with Gq/11 proteins that leads to activation of phospholipase Cβ, production of diacylglycerol, release of Ca++ from intracellular stores, and modulation of activity of several other intracellular proteins, including PKC and extracellular signal-regulated kinase (ERK).[195] Mice lacking the Gq protein show a reduction in number, but not an elimination of HTRs induced by DOI.[196] Although alterations in density of 5-HT2 receptors are not associated with alterations in the level of Gq proteins,[197,198] to our knowledge the converse is not known. Nevertheless, these data suggest that G proteins, other than Gq, that interact with 5-HT2 receptors, including Gi and G12/13 (5-HT2C receptors,[199,200]) may also contribute to its HTR-producing effect. Also notable, DOI-induced c-Fos expression in the medial prefrontal cortex was completely abolished in Gq knockout mice,[196] providing further evidence that c-Fos is not a critical molecular component of the DOI HTR.
Other intracellular contributors to the HTR induced by DOI include PKCγ. Mice lacking the neuronal specific, PKCγ, show enhanced DOI-induced phospholipase C activation and increased number of DOI-induced HTRs, suggesting a suppressive effect of PKCγ on DOI-mediated intracellular functions.[201] Importantly PKCγ knockout mice do not show alterations in brain 5-HT2A receptor density.[201]
Several new studies have begun to elucidate the G-protein independent β-arrestin pathway, which is modulated by 5-HT2A receptors. Recently it was shown that the DOI-induced HTR is unaffected by knockout of β-arrestin2 in mice, yet the HTR from the hallucinogens N-methyl-5-HT and 5-MeO-DMT is significantly enhanced in these mice; on the other hand, the HTR induced by the non-hallucinogenic compound, 5-HTP, is severely diminished, relative to wild-type littermates.[202,203] Moreover, the HTR induced by all of these compounds is blocked by M100907, suggesting unique 5-HT2-mediated signalling pathways induced by DOI and other HTR producing compounds[202,203] that may be activated by stabilization of agonist-specific receptor conformations.
Schmid et al.[202] and Abbas et al.[204] showed that DOI induces phosphorylation of ERK in cortical cells. This event, however, does not seem to be necessary for the discriminative stimulus effects of DOI, as systemic injections of SL327 that attenuated cerebral ERK phosphorylation, did not lessen the ability of mice to discriminate DOI from vehicle (Sanders-Bush et al., unpublished observations). Whether blockade of cortical ERK phosphorylation affects the DOI-induced HTR has not been reported to our knowledge.
Recent studies have described a substantial function of scaffolding and other 5-HT2 interacting proteins in the cellular and behavioural effects of DOI. For example, mice lacking the receptor-scaffolding protein, postsynaptic density protein of 95 kDa (PSD-95), traditionally considered a glutamatergic scaffolding protein, show a reduction, yet not abolishment of the HTR induced by DOI.[204] PSD-95 knockout mice showed decreases in brain 5-HT2A and 5-HT2C receptor expression (yet not 5-HT1A receptors), and 5-HT2C receptor function, but did not show reductions in 5-HT2A or 5-HT2C mRNA levels. Furthermore, 5-HT2A receptor trafficking to the cortical dendritic compartment in PSD-95 knockout mice was reduced. These data suggested the effect of PSD-95 elimination on the DOI HTR was mediated by reduced 5-HT2 receptor expression in the cortex.[204]
Interestingly, other studies have shown that DOI induces region-specific induction of BDNF mRNA, including induction in layer V of the rat cortex,[205,206] which can be suppressed by the mGlu2/3 agonist, LY354740, and enhanced by the mGlu2/3 antagonist LY341495.[206] Also, BDNF induces transport of PSD95 to cortical dendritic spines.[208] In BDNF knockout mice, 5-HT2A receptor binding sites were significantly decreased in the cortex compared to wild-type mice; the HTR to DOI, nevertheless, was not altered in BDNF knockout mice (though the ear-scratch response to DOI was significantly different[208]). Finally, there are several other 5-HT2A and 5-HT2C-interacting proteins that could regulate the effects of DOI, including PDZ domain-containing proteins, such as Mupp-1, and non-PDZ-domain-containing proteins, such as Arf-1, caveolin, RSK2, and MAPs.[209]
Caveats: resolution of conflicting data
Species and strain differences
Behavioural differences
There are many discrepancies in the DOI HTR literature that clearly appear related to the animal subject tested. For example, as shown in Table 1, mice and rats exhibit widely varying numbers of DOI-elicited HTRs within a defined time period, with mice typically showing a greater number of HTRs than rats. There are also large differences, up to 10-fold, between strains within a given species, and even across sub-strains, i.e. C57Bl/6J mice (Jackson Laboratories)[65,76] compared to C57Bl/6 N mice (Harlan Laboratories),[74] as shown in Table 1. The time between injection of DOI and the start of HTR counting, however, was notably different, i.e. 10 min[65,76] compared to 0 min;[74] we have observed that the frequency of HTRs after peripheral injections of DOI increases within the first 2 min (Figure 2), similar to Darmani et al.[56] Thus, these differences may appear more striking than they are. Experimenter variability does not likely account for most of the variance observed, as reports from several experiments directly comparing mouse strains show significant differences in HTR following DOI injections.
Table 1.
Head-twitch response (HTR) induced by DOI (mg/kg, i.p. or s.c.) across rodent species and strains.
| Species and strains | Dose, enantiomer | HTRs | References |
|---|---|---|---|
| Rat | |||
| Wistar | 1.0-2.5, NR | 5-12 | [55,90,64] |
| Wistar | 0.22-5.0, (±) | 5-6 | [109,86] |
| Sprague-Dawley | 1.0-3.0, (±) | 4-10 | [72,159]; |
| Sprague-Dawley | 1.25-3.0, NR | 5-9 | [114,32,131,66] |
| Listar | 1.0, (±) | 8 | [113] |
| OFA S-D | 1.0-3.0, (±) | 5-7 | [120] |
| Mouse | |||
| SAMP6 | 1.0, NR | 83 | [78] |
| DBA-2J | 1.0, (±) | 70 | [65] |
| C57Bl/6J (background) | 1.0,1.5, (±) | 48,68 | [119,121] |
| C57Bl/6J | 1.0, (+) | 46 | [76] |
| C57Bl/6J | 1.0, (±) | 30,40 | [48,65] |
| CAST/Ei | 1.0, (+) | 44 | [76] |
| SAMR1 | 1.0, NR | 33 | [78] |
| 129S6/SvEv | 2.0, NR | 32 | [94] |
| Aston MF1 | 0.5, (±) | 32 | [153] |
| ICR | 1.0, (±) | 28,29 | [67,138] |
| ICR | 1.0, NR | 25,26 | [68,154] |
| C3HeB/FeJ | 1.0, (+) | 28 | [76] |
| Aston MF1 | 1.0, NR | 27 | [141] |
| 129Sv | 2.0, NR | 21 | [194] |
| C57Bl/6N | 1.0, NR | 19 | [74] |
| Balb/cJ | 1.0, (+) | 18 | [76] |
| NIH Swiss | 1.0, (−) | 18 | [73] |
| CBA | 2.5, NR | 16 | [87] |
| Swiss Webster | 1.0, (−) | 15 | [73] |
| Albino ICR | 1.0, (±) | 20,25 | [61,146] |
| Albino ICR | 1.25-2.5, (±) | 8,15 | [4,56] |
| Albino ICR | 2.5, (−) | 15 | [56] |
| Albino ICR | 1.0, NR | 8,9 | [140,252] |
| Albino ICR | 2.5, (+) | 8 | [56] |
| A/J | 1.0, (+) | 9 | [76] |
| NMRI | 1.0, (±) | 8 | [236] |
| Albino Swiss | 2.5, (±) | 5-9 | [122,123,134,139] |
Results are averages from published reports. This list is not exhaustive, but is for illustrative purposes. Experiments using doses of, or close to 1.0 mg/kg were chosen for consistency. HTR counts began between 0 and 30 min after DOI injection. Results from observation periods greater than 10 min were divided to obtain a 10-min average. NR (not reported)
Different receptor densities
A recent report noted that mice and rats have different ratios of 5-HT2A/2C receptor binding site densities in the brain, with the former showing a greater ratio than the latter, i.e. relatively more 5-HT2A receptors than 5-HT2C receptors in the mouse brain.[74] It could be argued that these observations explain some of the variability between DOI HTRs in rats and mice. Some studies have suggested that activation of 5-HT2C receptors inhibits 5-HT2A receptor function induced by DOI (discussed above in the section on 5-HT2C and below in the section, “Dosing issues: problem of non-selectivity”). Following this logic, because there are fewer 5-HT2C receptors potentially suppressing 5-HT2A receptor activity in mice relative to rats, mice exhibit a greater number of HTRs elicited by DOI. Neither 5-HT2A nor 5-HT2C receptor binding site densities, however, explained the differences in DOI-elicited HTRs between C57Bl/6J and DBA/2J mice. DBA/2J mice exhibited an almost 2-fold greater frequency in DOI-induced HTRs relative to C57Bl/6J mice, yet had approximately equivalent 5-HT2A and 5-HT2C receptor binding site densities in brain areas examined;[65] though not reaching statistical significance, a closer look at the data, however, suggest that 5-HT2A receptors in the posterior striatum may be more dense in DBA/2J than C57Bl/6J mice. As suggested previously,[11] autoradiography techniques are relatively poor for deciphering subtle, but potentially important, differences in receptor binding site density.
The fact that there are substantially greater numbers of 5-HT2A receptors, compared to 5-HT2B and 5-HT2C receptors, expressed in the brains of most species, is pertinent when considering the relative contribution of these receptors to the effects of DOI. Namely, 5-HT2A receptors may have a greater, and more easily detectable, role in DOI's effects simply because they exist in greater numbers and therefore have greater control over neuro-physiological processes in regions where the three receptors are co-expressed. Thus, the potential 5-HT2B and 5-HT2C receptor-mediated functions in hallucinogen HTR studies and drug discrimination tasks may be masked by 5-HT2A receptors when all three receptors are activated simultaneously, as occurs with DOI. Considering a hypothetical example, if a mouse model were created that over-expressed 5-HT2A receptors, and another 5-HT2B receptors, and another 5-HT2C receptors, and each was trained to discriminate DOI from saline, it seems logical that 5-HT2A selective antagonists would more readily block the discriminative cue in 5-HT2A over-expressing mice (similar to wild-type mice), but would be less effective in blocking the cue in 5-HT2B over-expressing mice, and likewise for all other iterations.
Finally, although the translated DNA sequences for 5-HT2 receptors are ~98% identical between rats and mice, and ~90% identical between mice and humans,[210] it is not currently known whether selective 5-HT2 ligands have the same affinities and functions at both mouse and rat 5-HT2 receptors (discussed below). It is well known that changing even a single residue in a critical region of the transmembrane domain (where most ligands bind) of a G protein-coupled receptor can substantially alter ligand affinity and function (e.g. for 5-HT2A and 5-HT2C receptors[211,212]). Though these regions of the 5-HT2 receptors tend to be conserved across species, potentially other, unidentified regions of the receptors that differ between species may be important in determining a given ligand's affinity for and function at these receptors. Notably, the differences between rodent and human 5-HT2A receptors result in considerable differences in the pharmacology of N-1 substituted tryptamines and ergolines.[209,213,214]
Dosing issues: problem of non-selectivity
The issue of drug dose is a critical one in behavioural and neuropharmacological studies, and is regularly overlooked, as it is sometimes presumed that when a compound is described as ‘selective’, it means ‘specific’. 5-HT2 receptors share approximately 80% sequence homology in their transmembrane domains, which makes development of very specific 5-HT2 subtype-selective compounds a tedious endeavour, and thus determination of the 5-HT2 receptor subtypes involved in the behavioural effects of DOI also an ongoing challenge. Although the antagonists/inverse agonists, M100907, SB206553, and SB242084, which for human receptors, show at least 100-fold selectivity for 5-HT2A, 5-HT2B/2C, and 5-HT2C receptors, respectively, were a practical boon for the field, their relative affinities for 5-HT2 receptors from a range of laboratory species have not yet been reported. In addition, it is important to keep in mind that although M100907, for example, has extremely high affinity (0.1 nM Ki) for the human 5-HT2A receptor, its affinity for human 5-HT2C receptors (~50 nM Ki) is not dismissible.
Recent studies suggest that the activities of these newer and commonly used compounds may differ at mouse and rat 5-HT2 receptors. SB206553, for example, dose-dependently, but partially, attenuated the stimulus effects of 2.5 mg/kg (±)-DOI in a drug discrimination paradigm in C57Bl/6J mice,[111] findings that did not extend to Sprague–Dawley rats, despite using a lower dose of DOI (0.5 mg/kg) and much higher doses of SB206553.[11] M100907 completely blocked the DOI stimulus effect in both studies, though a 0.25 mg/kg dose was required for maximal effect in mice, whereas a 0.02 mg/kg dose produced maximal blockade in rats.[11,111]
A recent neuroendocrine study in Sprague–Dawley rats that included a full dose–response curve showed that SB242084 up to 10 mg/kg did not block corticosterone increases caused by 0.3 mg/kg DOI, yet M100907 completely blocked corticosterone increases caused by DOI with an ED50 value of 0.0035 mg/kg,[215] suggesting this effect is 5-HT2A-mediated. However, these data should not suggest that all of the behavioral effects of M100907 are solely due to antagonism of 5-HT2A receptors. For example, in a drug discrimination study using Wistar rats, although it was reported that neither SB206553, nor SB242084 at doses up to 10 mg/kg significantly generalized to 0.16 mg/kg M100907, there was approximately 40% generalization with 0.16 mg/kg SB206553 and 20% generalization with 2.5 mg/kg SB242084, yet 0% generalization with the 5-HT2B selective antagonist SB204741.[216] These data illustrate that the stimulus cue elicited by 0.16 mg/kg M100907 may involve some blockade of 5-HT2C receptors, or may involve other unidentified receptors targeted by these compounds.
As another example, in Wistar rats trained to discriminate the selective 5-HT2C receptor agonist, Ro 60–0175 (2.5 mg/kg), from saline, SB 206553 (0.63 mg/kg) was 100%, SB 242084 (0.63 mg/kg) was 80%, and M100907 (0.16 mg/kg) was 40% effective at blocking the discriminative cue.[217] It must be emphasized that although touted as a highly selective and potent 5-HT2C agonist, Ro 60–0175 also likely causes behavioural effects mediated by receptors other than 5-HT2C, such as 5-HT2A and 5-HT2B receptors. Comparing affinities for each of the human 5-HT2 receptors, Ro 60–0175 has highest affinity for 5-HT2B receptors (the 5-HT2C-VSV isoform was tested),[34] and has greatest potency for activating 5-HT2B receptors.[19] Moreover, the neuroendocrine stimulating effects (including release of corticosterone) of Ro 60–0175 in Sprague–Dawley rats, which occurred above 2.5 mg/kg, were not blocked by pretreatment with SB242084 (at doses up to 5 mg/kg) or M100907 (0.01 mg/kg).[218] Taken together, these data illustrate an often ignored point that ‘selective’ does not translate to ‘specific’. Thus, a titration of doses for any given 5-HT2A, 2B, or 2C selective compound is necessary to begin to determine specific 5-HT2 receptor subtypes that may be involved in a particular behavioural outcome. Also, many compounds, such as SB206553,[219] have affinity for off-target receptors and other proteins that may play a role in their behavioural effects.
As discussed in a previous section (5-HT2C), similar dosing issues and discrepant findings are apparent using the DOI HTR model. For example, although Ro 60–0175 at 2.5 mg/kg was an effective training dose in drug discrimination studies,[217] caused significant neuroendocrine activation,[218] and significant changes in release of cerebral dopamine[220,221] in rats, 3.0 mg/kg was ineffective at attenuating the HTR induced by 1.0 mg/kg ( )-DOI; 10 mg/kg was required to attenuate the HTR in mice, and– even the highest dose tested, 30 mg/kg, was ineffective at abolishing the HTR in these mice.[73]
SB242084 up to 3 mg/kg does not block the HTR in Listar rats induced by 0.2 mg/kg (±)-DOI,[113] yet blocks 50% of the HTRs induced by 1 mg/kg (±)-DOI in C57Bl/6J and DBA/2J mice; SB206553 at 0.3 and 3 mg/kg led to the same result,[65] a finding (with C57Bl/6J mice) we recently confirmed at a different institution (unpublished observations). Nevertheless, this general finding was not observed in Swiss NIH mice.[73] One possibility for these discrepant findings is that 5-HT2C antagonists, such as the 5-HT2C/2B antagonist SB206553, block 5-HT2A receptors in C57Bl/6J but not Swiss NIH mice. EEDQ irreversible binding of 5-HT2A receptors, however, was not attenuated by pretreating C57Bl/6J mice with 3 mg/kg SB206553, yet was by pretreatment with 0.25 mg/kg M100907,[65] suggesting this is not the answer.
Finally, in Wistar rats, SB206553 at 1.8 mg/kg inhibited the HTR induced by the 5-HT2/3 receptor agonist quipazine.[222] Proteins other than 5-HT2 receptors, including 5-HT2 interacting proteins[204,209] and perhaps enzymes, such as monoamine oxidase, are likely involved in regulating the HTR and may differ between species (as noted in studies aimed at deciphering the species differences in toxicity caused by MPTP injections[223]). Obviously, dose is not the only issue here.
Several pharmacological studies have suggested there are different and often opposing neurochemical functions of 5-HT2A and 5-HT2C receptors; the most widely reproduced observation is that 5-HT2A receptor activation increases, whereas 5-HT2C receptor activation decreases dopamine release in cortex, dorsal striatum, and nucleus accumbens,[190,220,221] which could suggest that DOI has auto-opposing (or auto-regulating) neurochemical effects. Regarding 5-HT2C receptor function, the 5-HT2C agonist, Ro 60–0175 most effectively decreased dopamine release at concentrations at or above 2.5 mg/kg.[220,221] Although both 5-HT2C receptor antagonists increased dopamine release, the effects of SB206553 (combined 5-HT2B/2C antagonist) were more robust than SB242084, and the increase in dopamine release following SB206553, but not SB242084, injections in Sprague–Dawley rats were dose-dependent up to 10 mg/kg.[221] This dose is notably very high, considering that 0.63 mg/kg SB206553 (and SB242084) is sufficient to block 100% (and 80%, respectively) of the Ro 60–0175 (2.5 mg/kg) discriminative cue in rats,[217] and suggests that receptors in addition to 5-HT2C receptors, including nicotinic acetylcholine receptors[219] and 5-HT2B receptors, may also be regulating the increases in dopamine release by SB206553. Alternatively, the differences between SB206553 and SB242084 may relate to the observations that the former behaves as a 5-HT2C receptor inverse agonist, while the latter behaves as a partial 5-HT2C receptor agonist, able to attenuate inverse agonist functions of SB206553 (in vitro, recombinant receptors, isoform unspecified, in CHO cells, PLC function).[221] These data suggest that constitutive activity of 5-HT2C receptors may be as important as endogenous 5-HT induced activation of 5-HT2C receptor for regulation of basal dopamine release.
DOI injections (2.5 mg/kg) increased dopamine release in the rat cortex, an effect not observed in 5-HT2A receptor knockout mice, but preserved in 5-HT2C receptor knockout mice.[190] Curiously, DOI's effects on dopamine release were not potentiated in 5-HT2C receptor knockout mice,[190] and yet this group reported that increases in the concentration of DOI injected systemically (0, 1.25, 2.5, and 5 mg/kg) produced an inverted U-shaped dose–response curve for dopamine release, and suggested, though did not show, that increased activation of 5-HT2C receptors by DOI mediated the descending part of the curve.[224]
DOI at 1 mg/kg, as noted previously, reliably induces a HTR that is blocked by 5-HT2 antagonists, yet this dose was not sufficient to induce increases in acetylcholine in the rat hippocampus; 2 mg/kg was.[188] Finally, also intriguing are results from neurophysiology studies of DOI. In cortical cells, ex vivo, DOI at a concentration of 300 nM, well above its reported Ki and EC50 values (in vitro) for 5-HT2 receptors,[19] had no effect on electrochemical activity; 1 mM was needed to alter activity, and 30 nM M100907 blocked the effects.[35]
Drug-specific stabilization of receptor conformations
It is becoming increasingly clear that different ligands that bind to the same GPCR induce or stabilize unique receptor conformations, and furthermore, that the conformational changes may be different when receptors are expressed in different cell types.[225] The stabilization of unique receptor conformations by different ligands positions the receptor to have selective interactions with G-protein subtypes or even with signalling proteins other than G-proteins (e.g. β-arrestin); these unique interactions confer activation (or inactivation) of potentially several specific cellular signalling pathways, a phenomenon known as functional selectivity or biased agonism.[32,226] Thus, as described by Kenakin,[227] G-protein coupled receptors are no longer viewed as ‘on-off switches’, but are more akin to ‘microprocessors’ regulated by interacting proteins and ligands that bind them.
Therefore, designation of a ligand simply by its efficacy as an agonist (partial or full), antagonist (competitive or non-competitive), or inverse agonist of a particular receptor, is not adequately informative without specifying the relative endpoint that is measured. For example, a ligand at 5-HT2A receptors could be a full agonist for the recombinant rat 5-HT2A receptor-Gq-PLC-IP pathway plus a partial agonist for the 5-HT2A-G12-PLD pathway when the receptor is expressed in Chinese hamster ovary cells. When examined in the rat claustrum in vivo, however, the same ligand may show weak partial agonist activity for the 5-HT2A receptor-Gq-PLC pathway, and no activity at the 5-HT2A-G12-PLD pathway; thus the ligand may actually behave as an antagonist of endogenous serotonin. Many of the conflicting data regarding DOI's behavioural pharmacology may be explained by unique functionally selective activity at several receptors, expressed in vivo, on different neural cells, on physically distinct parts of the cells, across species and strains.
Most 5-HT2 receptor agonists that are hallucinogenic are partial agonists, with similar potencies and efficacies (for stimulation of PLC-IP and others) for 5-HT2A and 5-HT2C receptors. So, why are other 5-HT2A/2C agonists, such as the phenylpiperazine, mCPP, and the benzazapine, lorcaserin[19,228–230] generally considered non-hallucinogenic? That is, why don't these compounds produce psychedelic effects comparable to LSD? Besides having notably poorer potency for activating either or both 5-HT2A and 5-HT2C receptors, another argument (discussed in detail previously) is that 5-HT2C receptor activation suppresses (or alters) 5-HT2A receptor function, and 5-HT2 agonists lacking LSD-like effects are generally considered relatively more potent and efficacious 5-HT2C receptor agonists compared to 5-HT2A receptors. This argument seems untenable, though, simply because hallucinogens that produce LSD-like effects are 5-HT2C agonists; if 5-HT2C receptors suppressed hallucinogenic effects, LSD wouldn't be hallucinogenic, a point lost in many discussions. It should be noted, also, that several phenylpiperazines fully substituted for the mCPP stimulus in rats, yet LSD and (–)-DOM only partially substituted. The efficacy of phenylpiperazines for stimulating 5-HT2C receptor-linked PLC signalling, moreover, was not directly related to their stimulus generalization effects.[231] Thus, these data suggest that the stimulus effects of phenylpiperazines are not mediated by the primary 5-HT2C receptor signalling pathway.
Regardless, a more parsimonious explanation is that certain 5-HT2 receptor-activating compounds, such as DOI and psilocin, cause specific conformations of 5-HT2 receptors that lead to LSD-like psychedelic effects.[94,232] Moreover, although the 5-HT2 agonists mCPP, lorcaserin, and quipazine, are often considered ‘non-hallucinogenic’, they actually do produce perceptual, cognitive, and emotional changes that may be considered ‘hallucinogenic’.[15,233,234] These effects, however, are generally not considered LSD-like. Finally, it is noteworthy that novel, partial 5-HT2A/D2 receptor agonists also attenuate the DOI-elicited HTR.[235,236] These observations provide further support for the idea that DOI potentially stabilizes a 5-HT2A receptor conformation unique from other 5-HT2A receptor agonists. It would be interesting to know whether pre-treatment with LSD or DOM attenuates the DOI-induced HTR, as the outcome could potentially strengthen the argument for ligand-specific receptor conformations. Molecular modelling and computational studies of different 5-HT2A agonists docked in the 5-HT2A receptor binding pocket would also help clarify this point.
Animal models
Animal models of human behaviour or psychiatric disorders are typically used to mimic some aspect of the human condition. As is true for all models it is imperative to clearly and explicitly identify the purpose of the model – what are you trying to ‘model’? The degree to which any particular animal model is useful can then be assessed through various forms of validity. The purpose of a particular model can vary, and may include:
mimicking an entire behavioural or psychiatric syndrome;
modelling a particular behavioural phenomenon or psychiatric symptom, i.e. an endophenotype of a disease; and
predicting the outcome a particular experimental intervention (e.g. an environmental, behavioural, pharmacological, or neurobiological manipulation).
Model validity
Although a wide variety of validity types have been described, one common categorization of validity for assessing a model of ‘normal’ or ‘pathological’ behaviour includes the following:
Face validity refers to the phenomenological similarities between the behaviour of interest in the animal and the behaviour (pathological or not) of the ‘real-world’ human; that is, do the behaviours resemble each other on the ‘face’ of it. This is not required for an animal model, and is oftentimes detrimental as it can imply inaccurately similar underlying mechanisms in the model and the human condition.
Predictive validity refers to the ability of the model to predict something about the human condition; that is, does the outcome of testing in the animal model allow you to predict the response in humans? This is the most important and desirable aspect of a model, and is oftentimes assessed by similar outcomes of neurobiological or pharmacological interventions in the animal model and humans.
Etiological validity refers to situations in which the animal and the human condition are “caused” by the same mechanism; that is, the etiology of the behavioural or psychiatric phenomenon is identical. Advances in the study of genetic vulnerability to various psychiatric disorders and our ability to manipulate the genetic makeup of animals are leading to increases in model development along these lines, although this presumes that we know the cause of a particular disorder (which is often not the case).
Construct validity refers to the notion that the animal model is measuring what it was intended to measure; that is, there is close functional homology between the behavioural phenomena in the animal model and the human condition. This requires a high degree of theoretical and/or conceptual understanding of the phenomena, and although much can be learned about the behavioural or pathological condition of interest, construct validity is not necessary for an effective animal model.
What do DOI-elicited HTRs model?
The DOI-elicited HTR has been considered as an animal model of a variety of behavioral and psychiatric conditions including hallucinogenesis, schizophrenia, obsessive-compulsive disorders, Tourette's syndrome, and more generally as a model of 5-HT2 function/activity. Although the DOI-elicited HTR has some validity as a model for each of these conditions, it is likely most suitable as a model of 5-HT2 receptor activity, as described below.
Hallucinogenesis
Many hallucinogenic 5-HT2 receptor agonists produce the HTR in rodents, and early animal studies of hallucinogens suggested that this response may be a model of hallucinating.[43] The consistently reproduced observations that 5-HT2 receptor antagonists, such as those that block the hallucinogenic effects of classical hallucinogens, such as psilocybin,[169] completely block the hallucinogen-induced HTR bolster support for the HTR as a model of hallucinating, and for 5-HT2 receptor activation as the underlying mediator of hallucinogenesis. Moreover, nonhallucinogenic compounds with 5-HT2A agonist activity, for example, lisuride, fail to produce the HTR.[94] The HTR model itself (excluding the DOI-elicited HTR model), however, falls short of directly modeling hallucinations in humans, because a much larger list of compounds, including but not limited to the serotonin precursor, 5-HTP, the 5-HT releasing agent, fenfluramine, and the cannabinoid receptor antagonist, SR141716A, that are known to lack hallucinogenic activity in man, nevertheless produce the HTR in rodents.[43,51,147,237] Also, compounds, including DMT, that are hallucinogenic in man do not reliably produce the HTR.[46] However, the HTR is a good model of 5-HT2 receptor activation in the rodent, as to our knowledge 5-HT2 receptor antagonists have blocked the HTR induced by every compound tested, and all compounds of which we are aware that have been shown consistently to be potent b and selective agonists for 5-HT2A, 2B, and 2C receptors induce the HTR. Nevertheless, the DOI-elicited HTR itself may still be useful as a tool to investigate (as best as the rodent brain will permit) neural mechanisms that may produce or underlie hallucinogenic effects caused by psychedelic drugs.
For reasons similar to the HTR model, the rodent drug-discrimination model also fails to model hallucinogenesis. For example, both LSD and DOM stimuli generalize with the non-hallucinogenic compounds, fenfluramine and lisuride,[238,239] and much of the stimulus effect of lisuride is likely mediated by 5-HT2A receptor activation.[239] In other words, 5-HT2A receptor activation in the rodent by lisuride and LSD may produce similar cognitive effects in rodents, i.e. rodents may not always be able to distinguish hallucinogenic (LSD) from non-hallucinogenic (lisuride). Moreover, there is some evidence that activity at 5-HT1A receptors can modulate the discriminative stimulus effects of hallucinogens (e.g. 5-HT1A agonists produce partial substitution and enhance, while antagonists can partially block the effects of LSD),[240,241] yet results from a plethora of studies suggest 5-HT1A receptor activation does not cause hallucinogenic effects in humans.[18,92] Indeed, antagonism of 5-HT1A receptors by pindolol potentiates the psychological responses to DMT in humans.[242] Again a disclaimer arises, as pindolol may have weak partial agonist activity at 5-HT1A receptors in vivo. In conclusion, subjective (i.e. drug discrimination) as well as observable (i.e. HTR) behavioural effects observed in rodents after administration of hallucinogens are better conceived as pharmacological outcomes, and not models of hallucinating.
Schizophrenia
Shortly after the discovery of psychedelic hallucinogens, similarities between the subjective effects of these compounds and the perceptual effects associated with schizophrenia and related psychosis-related disorders were noted.[243,244] Early reports suggested that administration of hallucinogenic drugs to animals could function as a model of schizophrenia.[245] It may be that drug-elicited head-twitches are a simple model of the ‘positive symptoms’ (in particular, hallucinations) associated with schizophrenia rather than a model of the entire syndrome of schizophrenia. This would be a classic example of an animal model that initially is proposed as a model of the entire syndrome of interest, which is later tapered down to potentially being a model of an endophenotype of a particular syndrome.[246]
Current conceptions of the pathophysiology of schizophrenia have been derived largely from pharmacological studies and can be summarized as follows. Dopamine agonists such as amphetamine, 5-HT2A receptor agonists that are psychedelics (e.g. LSD), and glutamate NMDA receptor antagonists including PCP, MK-801, and ketamine can all produce psychosis-like behavioural and subjective effects in humans. Conversely, dopamine D2 antagonists, 5-HT2A receptor antagonists, and glutamate mGluR2/3 receptor agonists are to some extent effective antipsychotic medications.
Data obtained using DOI-elicited HTRs and pharmacological challenges are generally consistent with this notion. In particular, much data suggests that glutamate NMDA receptor antagonists exacerbate the HTR, mGluR2/3 receptor agonists and antagonists attenuate and enhance the HTR respectively, 5-HT2A antagonists block the HTR, and dopamine D2 receptor and D1 receptor antagonists block the HTR. Post-mortem study of brains from schizophrenic patients,[137,247] and PET studies of untreated, first-episode psychotic patients demonstrated altered levels of 5-HT2A receptors which appear to corroborate these findings.
Of particular interest regarding the use of this model as a model of schizophrenia is that essentially every effective antipsychotic medication used in humans is effective in attenuating the HTR elicited by DOI administration. That is, there seem to be relatively few ‘false negatives’ – drugs that are useful as antipsychotic medications that fail to block the HTR. While this is an interesting set of findings, one issue that has recently become apparent is that blockade of the DOI-elicited HTR in mice does not necessarily translate to antipsychotic activity in humans. This is most clearly demonstrated by clinical studies examining the highly selective 5-HT2A receptor antagonist M100907, which has extraordinary potency at blocking the DOI-elicited HTR. Although originally developed as a novel antipsychotic,[248] M100907 capparently failed in clinical trials for the treatment of schizophrenia.[249] Although we believe that positive results in DOI-elicited HTR behavioural procedures may be indicative of antipsychotic activity, these results highlight the fact that findings obtained from one animal model are not enough to draw strong conclusions about pharmacotherapeutic implications. Rather, convergent evidence from the use of many animal models is required. So although M100907 was not effective in human trials, the fact that it may show up as a ‘false positive’ in DOI-elicited HTR studies does not necessarily indicate the model is useless. Rather, this animal model represents one tool at researchers’ disposal that may help to identify a desired preclinical profile indicative of antipsychotic activity in humans.
One particularly interesting study exploring the relationship between DOI-elicited HTR and schizophrenia was one in which the effects of various other symptoms commonly associated with the disorder, i.e. poorly-structured sleep and suppressed endogenous opioid function, were assessed in combination with DOI-elicited HTRs.[250] In this study, the effects of sleep disturbances were modelled by a period of sleep deprivation and suppressed opioid function was induced by administration of the opioid antagonist naloxone. Neither treatment alone had effects on the sensitivity of HTRs to DOI. The combination, however, of sleep deprivation and naloxone treatment significantly enhanced sensitivity to DOI.
Epidemiologically, gestational exposure to infection is associated with high risk of developing schizophrenia in adult offspring.[251] Another recent and interesting paper using mice demonstrated that maternal influenza viral infection increased sensitivity to DOI (approximately twice as many HTRs) in the adult (but not prepubertal) offspring.[136] Continued exploration of the model in this matter may provide substantial etiological validity that could contribute to the development of novel treatments for schizophrenia.
Obsessive-compulsive disorder (OCD) and Tourette's syndrome
OCD incorporates a variety of related behavioural pathologies, and Tourette's syndrome is typically considered as part of the OCD spectrum. One symptom associated with these disorders is the presence of a tic, and the DOI-elicited HTR has been described as an analogous behavioural outcome. As an animal model of tic disorders, the DOI-elicited HTR seems to have some face validity (i.e. the HTR may look like a tic). It is possible, although unknown at this point in time, that there is some etiological validity if tics are indeed produced by an over-stimulation of 5-HT2A receptors. Importantly, the model appears to have some predictive validity in one direction: the only medications that appear to be useful in treating tics, such as haloperidol (primarily a dopamine D2 antagonist), atypical opioids (levorphanol, tramadol), nicotine (acetylcholine agonist), and donepezil (acetylcholine esterase inhibitor; indirect acetylcholine agonist), are effective in suppressing DOI-elicited head-twitches.[63,140,153,154,252] Unfortunately the preclinical literature and clinical outcome research exploring the potential relationship between DOI-elicited HTR and OCD/Tourette's syndrome is relatively limited, so firm conclusions cannot be made at this time.
Involvement of 5-HT2A receptor activity and status of the 5-HT2A system
It may be relatively safer to suggest that the HTR elicited by DOI and related compounds can be effectively used as in vivo behavioural models to study 5-HT2A receptor pharmacology, however as noted above, drugs from many pharmacological classes can alter DOI-elicited HTRs. Thus, if a novel drug attenuates or enhances the DOI-elicited HTR, one cannot with certainty conclude anything about the pharmacological mechanism of action.
A number of investigators have used sensitivity to DOI (measuring the HTR) as a measure of the functional status of an animal's 5-HT2A receptor system. That is, the animal is exposed to some environmental, pharmacological, or genetic manipulation, and the state of the 5-HT2A system is inferred from the number of HTRs elicited by DOI administration. In many instances, sensitivity to DOI is highly correlated with the number and/or function of 5-HT2A receptors; for example, Rinaldi-Carmona et al.[253] demonstrated that chronic administration of a novel 5-HT2A antagonist upregulated 5-HT2A receptor density and increased sensitivity to DOI (see Dougherty and Aloyo[74] for a more comprehensive discussion of repeated drug administration, changes in receptor density, and sensitivity to DOI). It should be noted, however, that there are situations (e.g. across mouse strains) where sensitivity to DOI does not correlate with 5-HT2A density.[65] Thus, interpretations about the 5-HT2A receptor system following a single experiment with DOI should be corroborated using additional models to provide convergent evidence regarding a particular scientific question.
Summary
The DOI-elicited HTR has been used as an animal model of a variety of behavioural and psychiatric conditions. As described above, there is reasonable evidence that these models may have some predictive validity, especially regarding pharmacological interventions in each case. However, simply because a model has some form of validity, one should not infer that the model incorporates all aspects of the behaviour or condition under investigation. That is, DOI-elicited HTRs should not be considered hallucinogenic episodes, or a way to examine the entire syndromes called schizophrenia, Tourette's syndrome, or obsessive-compulsive disorder. Similarly, the neuropharmacology of DOI, along with the pharmacology of essentially every other psycho-active drug ever examined is relatively complex. Furthermore, activity in the head-twitch model itself is not de facto proof of action at the 5-HT2A receptor as described above. Rather these procedures can be useful as one of many tools for examining a particular aspect of these disorders, and that progress will only be made when there is convergent evidence using a number of models.
Conclusions
The DOI-induced HTR is a relatively straightforward behavioural and pharmacological procedure to set up and quantify, and can be used to ask interesting questions about neuropharmacological mechanisms and complex behavioural and psychiatric conditions. Various unresolved issues remain regarding behavioural neuropharmacological effects of DOI; therefore, there is much reason to continue using this procedure. There is increasing interest in the therapeutic potential of both 5-HT2A, as well as 5-HT2C, receptor agonists and antagonists for a variety of conditions. In addition to various psychiatric disorders mentioned above (e.g. psychosis-related disorders and OCD), the 5-HT2 receptor system has been identified as a desirable drug target for the treatment of depression, anxiety, post-traumatic stress disorder, obesity, numerous pain conditions (inflammation, neuropathic pain, cluster headaches), cardiovascular disease, and various sleep disorders (recent reviews[254,259]). Hallucinogenic 5-HT2 receptor agonists, in addition, are receiving renewed attention as potential psychotherapeutic agents.[260,261] Others have noted, however, that 5-HT2A receptor activation may potentially limit the therapeutic properties of other neuropsychiatric medications (e.g. SSRIs), suggesting combination therapies that include 5-HT2A antagonists.[262] Notably, there is not a lot of support to develop selective 5-HT2B agonists, as activation of 5-HT2B receptors in heart tissue is associated with valvular heart disease.[263,264] Given the great potential for therapeutic agents targeting 5-HT2 systems, relevant animal models need to be used and improved, in order to advance drug discovery programs. The DOI-induced HTR is one model that can help contribute to this effort.
In conclusion, DOI's hallucinogenic effects may be likened to a symphony orchestra conducted in the brain, with the receptors it activates, the sections or instruments. With this analogy, DOI-induced activation of 5-HT2A receptors sets the melody of the experience, and the other instruments, such as 5-HT2B, 5-HT2C, and potentially adrenergic receptors, play accompanying roles. With each receptor an instrument, DOI has a number of them to play. To argue that performance of only one instrument, i.e. the 5-HT2A receptor, produces the total psychedelic experience while the other instruments are plugged in and turned on but not played, seems indefensible. In other words, as pointed out by other researchers,[18,93,238] activation of 5-HT2A receptors, may be necessary, but not sufficient to produce the full range of effects of DOI.
Acknowledgements
The authors thank Krishnakanth Kondabolu for assistance in collecting the data presented in Figure 2. The authors were partially supported by a grant from the National Institutes of Health (R01 DA030989) awarded to Raymond G. Booth.
Footnotes
Notably, the Ki values of DOI for 5-HT2 receptors from[23] are at least 2-fold higher than previous reports[17,34,39,40] that used [3H]-ketanserin (5-HT2A) or [3H]-mesulergine (5-HT2B, 5-HT2C) as radiolabels, and greater than 10-fold higher than Ki values reported using [[125]I]-DOI.[20]
notably DMT has relatively poor affinity for 5-HT2A receptors,[40] yet we could not find in the literature DMT's affinity for 5-HT2C receptors.
This compound is currently being evaluated for the treatment of insomnia and treatment resistant depression: www.clinicaltrials.gov.
References
- 1.Shannon M, Battaglia G, Glennon RA, Titeler M. 5-HT1 and 5-HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA). Eur. J. Pharmacol. 1984;102:23. doi: 10.1016/0014-2999(84)90333-9. [DOI] [PubMed] [Google Scholar]
- 2.McKenna DJ, Peroutka SJ. Differentiation of 5-hydroxytrypta-mine2 receptor subtypes using 125I-R-(−)2,5-dimethoxy-4-iodo phenylisopropylamine and 3H-ketanserin. J. Neurosci. 1989;9:3482. doi: 10.1523/JNEUROSCI.09-10-03482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radio-ligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. N-S. Arch. Pharmacol. 2004;370:114. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- 4.Darmani NA, Martin BR, Pandey U, Glennon RA. Pharmacological characterization of ear-scratch response in mice as a behavioral model for selective 5-HT2-receptor agonists and evidence for 5-HT1B- and 5-HT2-receptor interactions. Pharmacol. Biochem. Be. 1990;37:95. doi: 10.1016/0091-3057(90)90047-l. [DOI] [PubMed] [Google Scholar]
- 5.Glennon RA, Young R, Benington F, Morin RD. Behavioral and serotonin receptor properties of 4-substituted derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane. J. Med. Chem. 1982;25:1163. doi: 10.1021/jm00352a013. [DOI] [PubMed] [Google Scholar]
- 6.Shulgin AT, Shulgin A. Pihkal: A Chemical Love Story. Transform Press; Berkeley, CA, USA: 1990. [Google Scholar]
- 7.Shulgin AT. Stereospecific requirements for hallucinogenesis. J. Pharm. Pharmacol. 1973;25:271. doi: 10.1111/j.2042-7158.1973.tb10642.x. [DOI] [PubMed] [Google Scholar]
- 8.Aldous FA, Barrass BC, Brewster K, Buxton DA, Green DM, Pinder RM, Rich P, Skeels M, Tutt KJ. Structure-activity relationships in psychotomimetic phenylalkylamines. J. Med. Chem. 1974;17:1100. doi: 10.1021/jm00256a016. [DOI] [PubMed] [Google Scholar]
- 9.Benington F, Morin RD, Beaton J, Smythies JR, Bradley RJ. Comparative effects of stereoisomers of hallucinogenic amphetamines. Nat. New Biol. 1973;242:185. doi: 10.1038/newbio242185a0. [DOI] [PubMed] [Google Scholar]
- 10.Sanders-Bush E, Breeding M. Choroid plexus epithelial cells in primary culture: A model of 5HT1C receptor activation by hallucinogenic drugs. Psychopharmacology (Berl) 1991;105:340. doi: 10.1007/BF02244428. [DOI] [PubMed] [Google Scholar]
- 11.Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: Down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology (Berl) 1999;144:248. doi: 10.1007/s002130051000. [DOI] [PubMed] [Google Scholar]
- 12.Shulgin AT. 4-aklyl-dialkoxy-alpha-methyl-phenethylamines and their pharmacologically-acceptable salts. 1970 U.S. Patent 3,547,999. Issued Dec. 15.
- 13.Shulgin AT, Sargent T, Naranjo C. 4-Bromo-2,5-dimethoxyphenylisopropylamine, a new centrally active amphetamine analog. Pharmacology. 1971;5:103. doi: 10.1159/000136181. [DOI] [PubMed] [Google Scholar]
- 14.Coutts RT, Malicky JL. Synthesis of some analogs of the hallucinogen 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM). Can. J. Chem. 1973;51:1402. [Google Scholar]
- 15.Shulgin AT, Manning T, Daley PF. The Shulgin Index: Volume One. Transform Press; Berkeley, CA, USA: 2011. [Google Scholar]
- 16.McKenna DJ, Mathis CA, Shulgin AT, Sargent T, 3rd, Saavedra JM. Autoradiographic localization of binding sites for 125I-DOI, a new psychotomimetic radioligand, in the rat brain. Eur. J. Pharmacol. 1987;137:289. doi: 10.1016/0014-2999(87)90239-1. [DOI] [PubMed] [Google Scholar]
- 17.Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT (2A) and 5f-HT(2C) receptors. Synapse. 2000;35:144. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Nichols DE. Hallucinogens. Pharmacol. Therapeut. 2004;101:131. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Brit. J. Pharmacol. 1999;128:13. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson DL, Lucaites VL, Wainscott DB, Glennon RA. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. N-S. Arch. Pharmacol. 1999;359:1. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- 21.Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL. Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: Evidence for species differences. J. Pharmacol. Exp. Ther. 1996;276:720. [PubMed] [Google Scholar]
- 22.Roth BL, Kroeze WK, Patel S, Lopez E. The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrasment of riches? The Neuroscientist. 2000;6:252. [Google Scholar]
- 23.Ray TS. Psychedelics and the human receptorome. PLoS One. 2010;5:e9019. doi: 10.1371/journal.pone.0009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 25.Sanders-Bush E, Fentress H, Hazelwood L. Serotonin 5-ht2 receptors: Molecular and genomic diversity. Mol. Interv. 2003;3:319. doi: 10.1124/mi.3.6.319. [DOI] [PubMed] [Google Scholar]
- 26.Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J. Biol. Chem. 1999;274:9472. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald LW, Iyer G, Conklin DS, Krause CM, Marshall A, Patterson JP, Tran DP, Jonak GJ, Hartig PR. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology. 1999;21:82S. doi: 10.1016/S0893-133X(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 28.Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J. Biol. Chem. 2004;279:2945. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- 29.Olaghere da Silva UB, Morabito MV, Canal CE, Airey DC, Emeson RB, Sanders-Bush E. Impact of RNA editing on functions of the serotonin 2 C receptor in vivo. Front. Neurosci. 2010;4:26. doi: 10.3389/neuro.23.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Gimenez JF, Villazon M, Brea J, Loza MI, Palacios JM, Mengod G, Vilaro MT. Multiple conformations of native and recombinant human 5-hydroxytryptamine(2a) receptors are labeled by agonists and discriminated by antagonists. Mol. Pharmacol. 2001;60:690. [PubMed] [Google Scholar]
- 31.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: Evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94. [PubMed] [Google Scholar]
- 32.Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J. Pharmacol. Exp. Ther. 2007;321:1054. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- 33.Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RB, Clarke WP. RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. Brit. J. Pharmacol. 2001;134:386. doi: 10.1038/sj.bjp.0704255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cussac D, Newman-Tancredi A, Quentric Y, Carpentier N, Poissonnet G, Parmentier JG, Goldstein S, Millan MJ. Characterization of phospholipase C activity at h5-HT2C compared with h5-HT2B receptors: influence of novel ligands upon membrane-bound levels of [3H]phosphatidylinositols. N-S. Arch. Pharmacol. 2002;365:242. doi: 10.1007/s00210-001-0505-y. [DOI] [PubMed] [Google Scholar]
- 35.Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J. Pharmacol. Exp. Ther. 1996;278:1373. [PubMed] [Google Scholar]
- 36.Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KC. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997;76:323. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- 37.Benloucif S, Keegan MJ, Galloway MP. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J. Pharmacol. Exp. Ther. 1993;265:373. [PubMed] [Google Scholar]
- 38.Bortolozzi A, Amargos-Bosch M, Adell A, Diaz-Mataix L, Serrats J, Pons S, Artigas F. In vivo modulation of 5-hydroxytryptamine release in mouse prefrontal cortex by local 5-HT(2A) receptors: Effect of antipsychotic drugs. Eur. J. Neurosci. 2003;18:1235. doi: 10.1046/j.1460-9568.2003.02829.x. [DOI] [PubMed] [Google Scholar]
- 39.Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 40.Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M. Hallucinogenic drug interactions at human brain 5-HT2 receptors: Implications for treating LSD-induced hallucino-genesis. Psychopharmacology (Berl) 1989;98:495. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- 41.Keller DL, Umbreit WW. Permanent alteration of behavior in mice by chemical and psychological means. Science. 1956;124:723. doi: 10.1126/science.124.3225.723. [DOI] [PubMed] [Google Scholar]
- 42.Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Brit. J. Pharmacol. Chemother. 1963;20:106. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- 44.Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol. Biochem. Be. 2008;88:358. doi: 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol. Biochem. Be. 2006;83:122. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 2005;181:496. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- 47.Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 2010;25:1548. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox M, French H, LaPorte J, Blackler A, Murphy D. The serotonin 5-HT2A receptor agonist TCB-2: A behavioral and neurophysiological analysis. Psychopharmacology. 2010;212:13. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- 49.Clineschmidt BV, McGuffin JC, Pflueger AB. Central serotonin-like activity of 6-chloro-2-[1-piperazinyl]-pyrazine (CPP; MK-212). Eur. J. Pharmacol. 1977;44:65. doi: 10.1016/0014-2999(77)90117-0. [DOI] [PubMed] [Google Scholar]
- 50.Balsara JJ, Bapat TR, Nandal NV, Gada VP, Chandorkar AG. Head-twitch response induced by ergometrine in mice: Behavioural evidence for direct stimulation of central 5-hydroxytrypta-mine receptors by ergometrine. Psychopharmacology (Berl) 1986;88:275. doi: 10.1007/BF00180824. [DOI] [PubMed] [Google Scholar]
- 51.Darmani NA. Cocaine and selective monoamine uptake blockers (sertraline, nisoxetine, and GBR 12935) prevent the d-fenflura-mine-induced head-twitch response in mice. Pharmacol. Biochem. Be. 1998;60:83. doi: 10.1016/s0091-3057(97)00548-0. [DOI] [PubMed] [Google Scholar]
- 52.Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- 53.Humphrey PP, Hartig P, Hoyer D. A proposed new nomenclature for 5-HT receptors. Trends Pharmacol. Sci. 1993;14:233. doi: 10.1016/0165-6147(93)90016-d. [DOI] [PubMed] [Google Scholar]
- 54.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994;46:157. [PubMed] [Google Scholar]
- 55.Arnt J, Hyttel J. Facilitation of 8-OHDPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur. J. Pharmacol. 1989;161:45. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- 56.Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol. Biochem. Be. 1990;36:901. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- 57.Scarlota LC, Harvey JA, Aloyo VJ. The role of serotonin-2 (5-HT2) and dopamine receptors in the behavioral actions of the 5-HT2A/2C agonist, DOI, and putative 5-HT2C inverse agonist, SR46349B. Psychopharmacology (Berl) 2011;213:393. doi: 10.1007/s00213-010-1928-2. [DOI] [PubMed] [Google Scholar]
- 58.Dave KD, Harvey JA, Aloyo VJ. A novel behavioral model that discriminates between 5-HT2A and 5-HT2C receptor activation. Pharmacol. Biochem. Be. 2002;72:371. doi: 10.1016/s0091-3057(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 59.Darmani NA, Mock OB, Towns LC, Gerdes CF. The head-twitch response in the least shrew (Cryptotis parva) is a 5-HT2- and not a 5-HT1C-mediated phenomenon. Pharmacol. Biochem. Be. 1994;48:383. doi: 10.1016/0091-3057(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 60.Tricklebank MD, Forler C, Fozard JR. The involvement of sub-types of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur. J. Pharmacol. 1984;106:271. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- 61.Darmani NA, Gerdes CF. Temporal differential adaptation of head-twitch and ear-scratch responses following administration of challenge doses of DOI. Pharmacol. Biochem. Be. 1995;50:545. doi: 10.1016/0091-3057(94)00340-8. [DOI] [PubMed] [Google Scholar]
- 62.Darmani NA, Martin BR, Glennon RA. Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J. Pharmacol. Exp. Ther. 1992;262:692. [PubMed] [Google Scholar]
- 63.Rojas-Corrales MO, Gibert-Rahola J, Mico JA. Role of atypical opiates in OCD. Experimental approach through the study of 5-HT(2A/C) receptor-mediated behavior. Psychopharmacology (Berl) 2007;190:221. doi: 10.1007/s00213-006-0619-5. [DOI] [PubMed] [Google Scholar]
- 64.Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J. Pharmacol. Exp. Ther. 1995;273:101. [PubMed] [Google Scholar]
- 65.Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2 C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 2010;209:163. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biezonski DK, Courtemanche AB, Hong SB, Piper BJ, Meyer JS. Repeated adolescent MDMA (“Ecstasy”) exposure in rats increases behavioral and neuroendocrine responses to a 5-HT2A/2C agonist. Brain Res. 2009;1252:87. doi: 10.1016/j.brainres.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 67.Miyata S, Hirano S, Kamei J. Diabetes inhibits the DOI-induced head-twitch response in mice. Psychopharmacology (Berl) 2004;177:224. doi: 10.1007/s00213-004-1942-3. [DOI] [PubMed] [Google Scholar]
- 68.Yamada J, Sugimoto Y, Horisaka K. Serotonin2 (5-HT2) receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) inhibits chlorpromazine- and haloperidol-induced hypothermia in mice. Biol. Pharm. Bull. 1995;18:1580. doi: 10.1248/bpb.18.1580. [DOI] [PubMed] [Google Scholar]
- 69.Kleven MS, Assie MB, Koek W. Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J. Pharmacol. Exp. Ther. 1997;282:747. [PubMed] [Google Scholar]
- 70.Darmani NA, Martin BR, Glennon RA. Withdrawal from chronic treatment with (+/−)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur. J. Pharmacol. 1990;186:115. doi: 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- 71.Darmani NA, Martin BR, Pandey U, Glennon RA. Inhibition of 5-HT2 receptor-mediated head-twitch response by cocaine via indirect stimulation of adrenergic alpha 2 and serotonergic 5-HT1A receptors. Pharmacol. Biochem. Be. 1991;38:353. doi: 10.1016/0091-3057(91)90290-i. [DOI] [PubMed] [Google Scholar]
- 72.Pranzatelli MR. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropane)-2 (DOI). Neurosci. Lett. 1990;115:74. doi: 10.1016/0304-3940(90)90520-j. [DOI] [PubMed] [Google Scholar]
- 73.Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J. Pharmacol. Exp. Ther. 2010;335:728. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dougherty JP, Aloyo VJ. Pharmacological and behavioral characterization of the 5-HT2A receptor in C57BL/6N mice. Psychopharmacology (Berl) 2011;215:581. doi: 10.1007/s00213-011-2207-6. [DOI] [PubMed] [Google Scholar]
- 75.Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 76.Weiss KC, Kim DY, Pawson CT, Cordes SP. A genetic screen for mouse mutations with defects in serotonin responsiveness. Brain Res. Mol. Brain Res. 2003;115:162. doi: 10.1016/s0169-328x(03)00205-5. [DOI] [PubMed] [Google Scholar]
- 77.Darmani NA, Shaddy J, Gerdes CF. Differential ontogenesis of three DOI-induced behaviors in mice. Physiol. Behav. 1996;60:1495. doi: 10.1016/s0031-9384(96)00323-x. [DOI] [PubMed] [Google Scholar]
- 78.Niimi K, Takahashi E, Itakura C. Hallucinogenic 5-hydroxytrypta-mine 2A receptor agonist effects in senescence-accelerated mice. Exp. Anim. 2010;59:441. doi: 10.1538/expanim.59.441. [DOI] [PubMed] [Google Scholar]
- 79.Parsons MJ, Benca RM, Brownfield MS, Behan M. Age-associated changes in the serotonergic system in rat superior colliculus and pretectum. Brain Res. Bull. 2001;55:435. doi: 10.1016/s0361-9230(01)00537-8. [DOI] [PubMed] [Google Scholar]
- 80.Yew DT, Yeung LY, Wai MS, Mak YT. 5-HT 1A and 2A receptor positive cells in the cerebella of mice and human and their decline during aging. Microsc. Res. Tech. 2009;72:684. doi: 10.1002/jemt.20717. [DOI] [PubMed] [Google Scholar]
- 81.Uchida H, Chow TW, Mamo DC, Kapur S, Mulsant BH, Houle S, Pollock BG, Graff-Guerrero A. Effects of aging on 5-HT(2A) R binding: a HRRT PET study with and without partial volume corrections. Int. J. Geriatr. Psychiatry. 2011;26:1300. doi: 10.1002/gps.2682. [DOI] [PubMed] [Google Scholar]
- 82.Moses-Kolko EL, Price JC, Shah N, Berga S, Sereika SM, Fisher PM, Coleman R, Becker C, Mason NS, Loucks T, Meltzer CC. Age, sex, and reproductive hormone effects on brain serotonin-1A and serotonin-2A receptor binding in a healthy population. Neuropsychopharmacology. 2011;36:2729. doi: 10.1038/npp.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boulton CS, Handley SL. Factors modifying the head-twitch response to 5-hydroxytryptophan. Psychopharmacologia. 1973;31:205. doi: 10.1007/BF00422511. [DOI] [PubMed] [Google Scholar]
- 84.Sakaue M, Ago Y, Sowa C, Sakamoto Y, Nishihara B, Koyama Y, Baba A, Matsuda T. Modulation by 5-HT2A receptors of aggressive behavior in isolated mice. Jpn. J. Pharmacol. 2002;89:89. doi: 10.1254/jjp.89.89. [DOI] [PubMed] [Google Scholar]
- 85.Preece MA, Dalley JW, Theobald DE, Robbins TW, Reynolds GP. Region specific changes in forebrain 5-hydroxytryptamine1A and 5-hydroxytryptamine2A receptors in isolation-reared rats: an in vitro autoradiography study. Neuroscience. 2004;123:725. doi: 10.1016/j.neuroscience.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Yamada S, Watanabe A, Nankai M, Toru M. Acute immobilization stress reduces (+/−)DOI-induced 5-HT2A receptor-mediated head shakes in rats. Psychopharmacology (Berl) 1995;119:9. doi: 10.1007/BF02246047. [DOI] [PubMed] [Google Scholar]
- 87.Pericic D. Swim stress inhibits 5-HT2A receptor-mediated head twitch behaviour in mice. Psychopharmacology (Berl) 2003;167:373. doi: 10.1007/s00213-002-1357-y. [DOI] [PubMed] [Google Scholar]
- 88.Chaouloff F, Baudrie V, Coupry I. Effects of chlorisondamine and restraint on cortical [3H]ketanserin binding, 5-HT2A receptor-mediated head shakes, and behaviours in models of anxiety. Neuropharmacology. 1994;33:449. doi: 10.1016/0028-3908(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 89.Chaouloff F, Coupry I, Baudrie V. Cortical [3H]ketanserin binding and 5-HT2A receptor-mediated behavioral responses in obese Zucker rats. Pharmacol. Biochem. Be. 1995;50:309. doi: 10.1016/0091-3057(94)00297-v. [DOI] [PubMed] [Google Scholar]
- 90.Zamfir O, Broqua P, Baudrie V, Chaouloff F. Effects of cold stress on some 5-HT1A, 5-HT1C and 5-HT2 receptor-mediated responses. Eur. J. Pharmacol. 1992;219:261. doi: 10.1016/0014-2999(92)90304-m. [DOI] [PubMed] [Google Scholar]
- 91.Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 2008;75:17. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glennon RA. Animal Models for Assessing Classical Hallucinogens. In: Boulton AA, Baker GB, Wu PH, editors. Animal Models of Drug Addiction. Humana Press; Clifton, NJ, USA: 1992. pp. 345–381. [Google Scholar]
- 94.Gonzalez-Maeso J, Weisstaub NV, Zhou M, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Kehne JH, Baron BM, Carr AA, et al. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J. Pharmacol. Exp. Ther. 1996;277:968. [PubMed] [Google Scholar]
- 96.May JA, Sharif NA, Chen HH, Liao JC, Kelly CR, Glennon RA, Young R, Li JX, Rice KC, France CP. Pharmacological properties and discriminative stimulus effects of a novel and selective 5-HT2 receptor agonist AL-38022A [(S)-2-(8,9-dihydro-7H-pyrano[2,3-g]indazol-1-yl)-1-methylethylamine]. Pharmacol. Biochem. Be. 2009;91:307. doi: 10.1016/j.pbb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kursar JD, Nelson DL, Wainscott DB, Baez M. Molecular cloning, functional expression, and mRNA tissue distribution of the human 5-hydroxytryptamine2B receptor. Mol. Pharmacol. 1994;46:227. [PubMed] [Google Scholar]
- 98.Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Brit. J. Pharmacol. 1995;115:622. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hofmann C, Penner U, Dorow R, Pertz HH, Jahnichen S, Horowski R, Latte KP, Palla D, Schurad B. Lisuride, a dopamine receptor agonist with 5-HT2B receptor antagonist properties: Absence of cardiac valvulopathy adverse drug reaction reports supports the concept of a crucial role for 5-HT2B receptor agonism in cardiac valvular fibrosis. Clin. Neuropharmacol. 2006;29:80. doi: 10.1097/00002826-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 100.Banas SM, Doly S, Boutourlinsky K, Diaz SL, Belmer A, Callebert J, Collet C, Launay J-M, Maroteaux L. Deconstructing antiobesity compound action: Requirement of serotonin 5-HT2B receptors for dexfenfluramine anorectic effects. Neuropsychopharmacology. 2011;36:423. doi: 10.1038/npp.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doly S, Valjent E, Setola V, Callebert J, Herve D, Launay JM, Maroteaux L. Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J. Neurosci. 2008;28:2933. doi: 10.1523/JNEUROSCI.5723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology (Berl) 2009;203:251. doi: 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]
- 103.Winter JC, Fiorella DJ, Timineri DM, Filipink RA, Helsley SE, Rabin RA. Serotonergic receptor subtypes and hallucinogen-induced stimulus control. Pharmacol. Biochem. Be. 1999;64:283. doi: 10.1016/s0091-3057(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 104.Bromidge SM, Duckworth M, Forbes IT, et al. 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): The first selective and brain penetrant 5-HT2C receptor antagonist. J. Med. Chem. 1997;40:3494. doi: 10.1021/jm970424c. [DOI] [PubMed] [Google Scholar]
- 105.Kennett GA, Wood MD, Bright F, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 106.Eckler JR, Reissig CJ, Rabin RA, Winter JC. A 5-HT(2C) receptor-mediated interaction between 2,5-dimethoxy-4-methylampheta-mine and citalopram in the rat. Pharmacol. Biochem. Be. 2004;79:25. doi: 10.1016/j.pbb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 107.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J. Pharmacol. Exp. Ther. 1997;282:699. [PubMed] [Google Scholar]
- 108.Simansky KJ, Schechter LE. Properties of some 1-arylpiperazines as antagonists of stereotyped behaviors mediated by central serotonergic receptors in rodents. J. Pharmacol. Exp. Ther. 1988;247:1073. [PubMed] [Google Scholar]
- 109.Berendsen HH, Broekkamp CL. Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Brit. J. Pharmacol. 1990;101:667. doi: 10.1111/j.1476-5381.1990.tb14138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Forbes IT, Ham P, Booth DH, Martin RT, Thompson M, Baxter GS, Blackburn TP, Glen A, Kennett GA, Wood MD. 5-Methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole: a novel 5-HT2C/5-HT2B receptor antagonist with improved affinity, selectivity, and oral activity. J. Med. Chem. 1995;38:2524. doi: 10.1021/jm00014a004. [DOI] [PubMed] [Google Scholar]
- 111.Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/−)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 2003;166:61. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- 112.Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol. Biochem. Be. 2001;69:643. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 114.Kennett GA, Wood MD, Glen A, Grewal S, Forbes I, Gadre A, Blackburn TP. In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist. Brit. J. Pharmacol. 1994;111:797. doi: 10.1111/j.1476-5381.1994.tb14808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J. Pharmacol. Exp. Ther. 2007;320:662. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- 116.Bos M, Jenck F, Martin JR, Moreau JL, Sleight AJ, Wichmann J, Widmer U. Novel agonists of 5HT2C receptors. Synthesis and biological evaluation of substituted 2-(indol-1-yl)-1-methylethylamines and 2-(indeno[1,2-b]pyrrol-1-yl)-1-methylethylamines. Improved therapeutics for obsessive compulsive disorder. J. Med. Chem. 1997;40:2762. doi: 10.1021/jm970030l. [DOI] [PubMed] [Google Scholar]
- 117.Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. CP-809,101, a selective 5-HT2C agonist shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 118.Sard H, Kumaran G, Morency C, Roth BL, Toth BA, He P, Shuster L. SAR of psilocybin analogs: discovery of a selective 5-HT 2C agonist. Bioorg. Med. Chem. Lett. 2005;15:4555. doi: 10.1016/j.bmcl.2005.06.104. [DOI] [PubMed] [Google Scholar]
- 119.Fox MA, Stein AR, French HT, Murphy DL. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT). Brit. J. Pharmacol. 2010;159:879. doi: 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI). Prog. Neuro-Psychoph. 1999;23:533. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 121.Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2 C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- 122.Maj J, Rogoz Z. Pharmacological effects of venlafaxine, a new antidepressant, given repeatedly, on the alpha 1-adrenergic, dopamine and serotonin systems. J. Neural Transm. 1999;106:197. doi: 10.1007/s007020050151. [DOI] [PubMed] [Google Scholar]
- 123.Maj J, Rogoz Z, Dlaboga D, Dziedzicka-Wasylewska M. Pharmacological effects of milnacipran, a new antidepressant, given repeatedly on the alpha1-adrenergic and serotonergic 5-HT2A systems. J. Neural Transm. 2000;107:1345. doi: 10.1007/s007020070022. [DOI] [PubMed] [Google Scholar]
- 124.Granoff MI, Ashby CR., Jr. The effect of the repeated administration of the compound 3,4-methylenedioxymethamphetamine on the response of rats to the 5-HT2A,C receptor agonist (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI). Neuropsychobiology. 1998;37:36. doi: 10.1159/000026474. [DOI] [PubMed] [Google Scholar]
- 125.Vetulani J, Bednarczyk B, Reichenberg K, Rokosz A. Head twitches induced by LSD and quipazine: similarities and differences. Neuropharmacology. 1980;19:155. doi: 10.1016/0028-3908(80)90131-8. [DOI] [PubMed] [Google Scholar]
- 126.Handley SL, Singh L. Involvement of the locus coeruleus in the potentiation of the quipazine-induced head-twitch response by diazepam and beta-adrenoceptor agonists. Neuropharmacology. 1986;25:1315. doi: 10.1016/0028-3908(86)90102-4. [DOI] [PubMed] [Google Scholar]
- 127.Trulson ME, Brandstetter JW, Crisp T, Jacobs BL. Behavioral effects of quipazine in the cat. Eur. J. Pharmacol. 1982;78:295. doi: 10.1016/0014-2999(82)90031-0. [DOI] [PubMed] [Google Scholar]
- 128.Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 2009;206:345. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 2010;334:171. doi: 10.1124/jpet.110.167346. [DOI] [PubMed] [Google Scholar]
- 130.Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: A randomized Phase 2 clinical trial. Nat. Med. 2007;13:1102. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 131.Zhang C, Marek GJ. AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog. Neuro-Psychoph. 2008;32:62. doi: 10.1016/j.pnpbp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 132.Kim HS, Park IS, Park WK. NMDA receptor antagonists enhance 5-HT2 receptor-mediated behavior, head-twitch response, in mice. Life Sci. 1998;63:2305. doi: 10.1016/s0024-3205(98)00519-0. [DOI] [PubMed] [Google Scholar]
- 133.Dall'Olio R, Gaggi R, Bonfante V, Gandolfi O. The non-competitive NMDA receptor blocker dizocilpine potentiates serotonergic function. Behav. Pharmacol. 1999;10:63. doi: 10.1097/00008877-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 134.Klodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol. Biochem. Be. 2002;73:327. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- 135.Egashira N, Shirakawa A, Okuno R, Mishima K, Iwasaki K, Oishi R, Fujiwara M. Role of endocannabinoid and glutamatergic systems in DOI-induced head-twitch response in mice. Pharmacol. Biochem. Be. 2011;99:52. doi: 10.1016/j.pbb.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 2011;493:76. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gonzalez-Maeso J, Ang RL, Yuen T, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miyata S, Hirano S, Kamei J. Involvement of diazepam-insensitive benzodiazepine receptors in the suppression of DOI-induced head-twitch responses in diabetic mice. Psychopharmacology (Berl) 2006;186:1. doi: 10.1007/s00213-006-0350-2. [DOI] [PubMed] [Google Scholar]
- 139.Wieronska JM, Kusek M, Tokarski K, Wabno J, Froestl W, Pilc A. The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioural changes related to positive syndromes of psychosis in mice. Brit. J. Pharmacol. 2011;163:1034. doi: 10.1111/j.1476-5381.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hayslett RL, Tizabi Y. Effects of donepezil, nicotine and haloperidol on the central serotonergic system in mice: Implications for Tourette's syndrome. Pharmacol. Biochem. Be. 2005;81:879. doi: 10.1016/j.pbb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 141.Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5HT2A receptors are active under physiological conditions. Psychopharmacology (Berl) 1996;128:198. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- 142.Meltzer HY, Horiguchi M, Massey BW. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology (Berl) 2011;213:289. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- 143.Yang CR, Chen L. Targeting prefrontal cortical dopamine D1 and N-methyl-D-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11:452. doi: 10.1177/1073858405279692. [DOI] [PubMed] [Google Scholar]
- 144.Darmani NA. Role of the inhibitory adrenergic alpha 2 and serotonergic 5-HT1A components of cocaine's actions on the DOI-induced head-twitch response in 5-HT2-receptor supersensitive mice. Pharmacol. Biochem. Be. 1993;45:269. doi: 10.1016/0091-3057(93)90238-o. [DOI] [PubMed] [Google Scholar]
- 145.Darmani NA. The effects of acute cocaine administration on the DOI-induced head-twitch response in reserpinized mice. Pharmacol. Biochem. Be. 1994;49:229. doi: 10.1016/0091-3057(94)90481-2. [DOI] [PubMed] [Google Scholar]
- 146.Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol. Biochem. Be. 2001;68:311. doi: 10.1016/s0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- 147.Darmani NA, Pandya DK. Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J. Neural Transm. 2000;107:931. doi: 10.1007/s007020070043. [DOI] [PubMed] [Google Scholar]
- 148.Janoyan JJ, Crim JL, Darmani NA. Reversal of SR 141716A-induced head-twitch and ear-scratch responses in mice by delta 9-THC and other cannabinoids. Pharmacol. Biochem. Be. 2002;71:155. doi: 10.1016/s0091-3057(01)00647-5. [DOI] [PubMed] [Google Scholar]
- 149.Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 150.Egashira N, Mishima K, Uchida T, Hasebe N, Nagai H, Mizuki A, Iwasaki K, Ishii H, Nishimura R, Shoyama Y, Fujiwara M. Anandamide inhibits the DOI-induced head-twitch response in mice. Psycho-pharmacology (Berl) 2004;171:382. doi: 10.1007/s00213-003-1611-y. [DOI] [PubMed] [Google Scholar]
- 151.Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J. Neurosci. 2008;28:6508. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, Witkin JM, Nomikos GG. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Brit. J. Pharmacol. 2003;138:544. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gaynor CM, Handley SL. Effects of nicotine on head-shakes and tryptophan metabolites. Psychopharmacology (Berl) 2001;153:327. doi: 10.1007/s002130000558. [DOI] [PubMed] [Google Scholar]
- 154.Tizabi Y, Russell LT, Johnson M, Darmani NA. Nicotine attenuates DOI-induced head-twitch response in mice: Implications for Tourette syndrome. Prog. Neuro-Psychoph. 2001;25:1445. doi: 10.1016/s0278-5846(01)00194-4. [DOI] [PubMed] [Google Scholar]
- 155.Handley SL, Singh L. The modulation of head-twitch behaviour by drugs acting on beta-adrenoceptors: evidence for the involvement of both beta 1- and beta 2-adrenoceptors. Psychopharmacology (Berl) 1986;88:320. doi: 10.1007/BF00180832. [DOI] [PubMed] [Google Scholar]
- 156.Singh L, Heaton JC, Rea PJ, Handley SL. Involvement of noradrenaline in potentiation of the head-twitch response by GABA-related drugs. Psychopharmacology (Berl) 1986;88:315. doi: 10.1007/BF00180831. [DOI] [PubMed] [Google Scholar]
- 157.Heal DJ, Philpot J, O'Shaughnessy KM, Davies CL. The influence of central noradrenergic function on 5-HT2-mediated head-twitch responses in mice: Possible implications for the actions of antidepressant drugs. Psychopharmacology (Berl) 1986;89:414. doi: 10.1007/BF02412113. [DOI] [PubMed] [Google Scholar]
- 158.Handley SL, Brown J. Effects on the 5-hydroxytryptamine-induced head-twitch of drugs with selective actions on alpha1 and alpha2-adrenoceptors. Neuropharmacology. 1982;21:507. doi: 10.1016/0028-3908(82)90040-5. [DOI] [PubMed] [Google Scholar]
- 159.Marek GJ. Activation of adenosine(1) (A(1)) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology. 2009;56:1082. doi: 10.1016/j.neuropharm.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brand A, Vissiennon Z, Eschke D, Nieber K. Adenosine A(1) and A (3) receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- 161.Marek GJ. Behavioral evidence for mu-opioid and 5-HT2A receptor interactions. Eur. J. Pharmacol. 2003;474:77. doi: 10.1016/s0014-2999(03)01971-x. [DOI] [PubMed] [Google Scholar]
- 162.Ogren SO, Fuxe K. Intraventricular injections of galanin counteract development of head twitches induced by the 5-HT-2 agonist 1-(2,5-dimethoxyphenyl-4-bromo)-2-aminopropane. Acta Physiol. Scand. 1989;136:297. doi: 10.1111/j.1748-1716.1989.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 163.Patel S, Hutson PH. Effects of galanin on 8-OH-DPAT induced decrease in body temperature and brain 5-hydroxytryptamine metabolism in the mouse. Eur. J. Pharmacol. 1996;317:197. doi: 10.1016/s0014-2999(96)00716-9. [DOI] [PubMed] [Google Scholar]
- 164.Wang H, Grahame-Smith DG. The effects of rubidium, caesium and quinine on 5-HT-mediated behaviour in rat and mouse-2. Caesium. Neuropharmacology. 1992;31:421. doi: 10.1016/0028-3908(92)90078-4. [DOI] [PubMed] [Google Scholar]
- 165.Wang H, Grahame-Smith DG. The effects of rubidium, caesium and quinine on 5-HT-mediated behaviour in rat and mouse-3. Quinine. Neuropharmacology. 1992;31:425. doi: 10.1016/0028-3908(92)90079-5. [DOI] [PubMed] [Google Scholar]
- 166.Wang H, Grahame-Smith DG. The effects of rubidium, caesium and quinine on 5-HT-mediated behaviour in rat and mouse-1. Rubidium. Neuropharmacology. 1992;31:413. doi: 10.1016/0028-3908(92)90077-3. [DOI] [PubMed] [Google Scholar]
- 167.Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010;11:642. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- 168.Vollenweider FX. Brain mechanisms of hallucinogens and entactogens. Dialogues Clin. Neurosci. 2001;3:265. doi: 10.31887/DCNS.2001.3.4/fxvollenweider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 170.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weber ET, Andrade R. Htr2a gene and 5-HT(2A) receptor expression in the cerebral cortex studied using genetically modified mice. Front. Neurosci. 2010;4:36. doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc. Natl Acad. Sci. USA. 1998;95:735. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbol S, Madsen K, Frokjaer V, Martiny L, Paulson OB, Knudsen GM. A database of [(18)F]-altanserin binding to 5-HT(2A) receptors in normal volunteers: Normative data and relationship to physiological and demographic variables. Neuroimage. 2004;21:1105. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 174.Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- 175.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995;351:357. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 176.Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 177.Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod Mapping of G. 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J. Comp. Neurol. 2001;429:571. doi: 10.1002/1096-9861(20010122)429:4<571::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 178.Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex. 2004;14:1100. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- 179.Celada P, Puig MV, Diaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol. Psychiat. 2008;64:392. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 180.Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl Acad. Sci. USA. 2007;104:9870. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 182.Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- 183.Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb. Cortex. 2003;13:870. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- 184.Ashby Jr CR, Jiang LH, Kasser RJ, Wang. RY. Electrophysiological characterization of 5-hydroxytryptamine2 receptors in the rat medial prefrontal cortex. J. Pharmacol. Exp. Ther. 1990;252:171. [PubMed] [Google Scholar]
- 185.Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex. 2004;14:281. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- 186.Kuroki T, Meltzer HY, Ichikawa J. 5-HT 2A receptor stimulation by DOI, a 5-HT 2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2003;972:216. doi: 10.1016/s0006-8993(03)02516-2. [DOI] [PubMed] [Google Scholar]
- 187.Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J. Neurochem. 2005;95:1597. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- 188.Nair SG, Gudelsky GA. Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse. 2004;53:202. doi: 10.1002/syn.20054. [DOI] [PubMed] [Google Scholar]
- 189.Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT(2A) antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- 190.Huang M, Dai J, Meltzer HY. 5-HT(2A) and 5-HT(2C) receptor stimulation are differentially involved in the cortical dopamine efflux-Studied in 5-HT(2A) and 5-HT(2C) genetic mutant mice. Eur. J. Pharmacol. 2010;652:40. doi: 10.1016/j.ejphar.2010.10.094. [DOI] [PubMed] [Google Scholar]
- 191.Abdallah L, Bonasera SJ, Hopf FW, et al. Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J. Neurosci. 2009;29:8156. doi: 10.1523/JNEUROSCI.3905-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J. Neurosci. 2000;20:8846. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gingrich JA, Zhou M, Sealfon SC, Hen R. Mice lacking 5-HT2A receptor are insensitive to many of the behavioral and physiological effects of hallucinogens. Soc. Neurosci. Abs. 1999;25:799. [Google Scholar]
- 194.Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 2003;23:8836. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Leysen JE. 5-HT2 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004;3:11. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- 196.Garcia EE, Smith RL, Sanders-Bush E. Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li Q, Muma NA, Battaglia G, Van de Kar LD. Fluoxetine gradually increases [125I]DOI-labelled 5-HT2A/2C receptors in the hypothalamus without changing the levels of Gq- and G11-proteins. Brain Res. 1997;775:225. doi: 10.1016/s0006-8993(97)00961-x. [DOI] [PubMed] [Google Scholar]
- 198.Canal CE, Mahautmr KC, Cao C, Sanders-Bush E, Airey DC. RNA editing of the serotonin 2C receptor and expression of Galpha(q) protein: Genetic mouse models do not support a role for regulation or compensation. J. Neurochem. 2009;108:1136. doi: 10.1111/j.1471-4159.2008.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cussac D, Newman-Tancredi A, Duqueyroix D, Pasteau V, Millan MJ. Differential activation of Gq/11 and Gi(3) proteins at 5-hydroxytryptamine(2C) receptors revealed by antibody capture assays: Influence of receptor reserve and relationship to agonist-directed trafficking. Mol. Pharmacol. 2002;62:578. doi: 10.1124/mol.62.3.578. [DOI] [PubMed] [Google Scholar]
- 200.McGrew L, Chang MS, Sanders-Bush E. Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol. Pharmacol. 2002;62:1339. doi: 10.1124/mol.62.6.1339. [DOI] [PubMed] [Google Scholar]
- 201.Bowers BJ, Miyamoto-Ditmon J, Wehner JM. Regulation of 5-HT2A/C receptors and DOI-induced behaviors by protein kinase Cgamma. Pharmacol. Biochem. Be. 2006;85:441. doi: 10.1016/j.pbb.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl Acad. Sci. USA. 2008;105:1079. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a beta-arrestin2/Src/Akt signaling complex in vivo. J. Neurosci. 2010;30:13513. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J. Neurosci. 2009;29:7124. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 1997;17:2785. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol. Biochem. Be. 2002;73:317. doi: 10.1016/s0091-3057(02)00844-4. [DOI] [PubMed] [Google Scholar]
- 207.Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat. Neurosci. 2007;10:702. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- 208.Klein AB, Santini MA, Aznar S, Knudsen GM, Rios M. Changes in 5-HT2A-mediated behavior and 5-HT2A- and 5-HT1A receptor binding and expression in conditional brain-derived neurotrophic factor knock-out mice. Neuroscience. 2010;169:1007. doi: 10.1016/j.neuroscience.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Roth BL. Irving Page Lecture: 5-HT(2A) serotonin receptor biology: interacting proteins, kinases and paradoxical regulation. Neuropharmacology. 2011;61:348. doi: 10.1016/j.neuropharm.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gertz M. BLAST Scoring Parameters. 2005 Published online: ftp://ftp.ncbi. nlm.nih.gov/blast/documents/developer/scoring.pdf.
- 211.Braden MR, Nichols DE. Assessment of the roles of serines 5.43 (239) and 5.46(242) for binding and potency of agonist ligands at the human serotonin 5-HT2A receptor. Mol. Pharmacol. 2007;72:1200. doi: 10.1124/mol.107.039255. [DOI] [PubMed] [Google Scholar]
- 212.Canal CE, Cordova-Sintjago TC, Villa NY, Fang LJ, Booth RG. Drug discovery targeting human 5-HT(2 C) receptors: Residues S3.36 and Y7.43 impact ligand-binding pocket structure via hydrogen bond formation. Eur. J. Pharmacol. 2011;673:1. doi: 10.1016/j.ejphar.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Johnson MP, Loncharich RJ, Baez M, Nelson DL. Species variations in transmembrane region V of the 5-hydroxytryptamine type 2A receptor alter the structure-activity relationship of certain ergolines and tryptamines. Mol. Pharmacol. 1994;45:277. [PubMed] [Google Scholar]
- 214.Johnson MP, Baez M, Kursar JD, Nelson DL. Species differences in 5-HT2A receptors: cloned pig and rhesus monkey 5-HT2A receptors reveal conserved transmembrane homology to the human rather than rat sequence. Acta. 1995;1236:201. doi: 10.1016/0005-2736(95)00073-c. [DOI] [PubMed] [Google Scholar]
- 215.Hemrick-Luecke SK, Evans DC. Comparison of the potency of MDL 100,907 and SB 242084 in blocking the serotonin (5-HT)(2) receptor agonist-induced increases in rat serum corticosterone concentrations: Evidence for 5-HT(2A) receptor mediation of the HPA axis. Neuropharmacology. 2002;42:162. doi: 10.1016/s0028-3908(01)00166-6. [DOI] [PubMed] [Google Scholar]
- 216.Dekeyne A, Iob L, Hautefaye P, Millan MJ. The selective serotonin (2A) receptor antagonist, MDL100,907, elicits a specific interoceptive cue in rats. Neuropsychopharmacology. 2002;26:552. doi: 10.1016/S0893-133X(01)00389-X. [DOI] [PubMed] [Google Scholar]
- 217.Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60–0175: a pharmacological analysis. Neuropharmacology. 1999;38:415. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- 218.Damjanoska KJ, Muma NA, Zhang Y, D'Souza DN, Garcia F, Carrasco GA, Kindel GH, Haskins KA, Shankaran M, Petersen BR, Van De Kar LD. Neuroendocrine evidence that (S)-2-(chloro-5-fluoro-indol- l-yl)-1-methylethylamine fumarate (Ro 60–0175) is not a selective 5-hydroxytryptamine(2C) receptor agonist. J. Pharmacol. Exp. Ther. 2003;304:1209. doi: 10.1124/jpet.102.043489. [DOI] [PubMed] [Google Scholar]
- 219.Dunlop J, Lock T, Jow B, et al. Old and new pharmacology: Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor by the 5-hydroxytryptamine(2B/C) receptor antagonist SB-206553 (3,5-dihydro-5-methyl-N-3-pyridinylbenzo[1,2-b:4,5-b’] di pyrrole-1(2H)-carboxamide). J. Pharmacol. Exp. Ther. 2009;328:766. doi: 10.1124/jpet.108.146514. [DOI] [PubMed] [Google Scholar]
- 220.Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: A combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 221.De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J. Neurosci. 2004;24:3235. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sanchez C, Arnt J. In-vivo assessment of 5-HT2A and 5-HT2C antagonistic properties of newer antipsychotics. Behav. Pharmacol. 2000;11:291. doi: 10.1097/00008877-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 223.Tsai E MJ, Lee H. Differences in the disposition and toxicity of 1-methyl-4-phenylpyridinium in cultured rat and mouse astrocytes. Glia. 1994;12:329. doi: 10.1002/glia.440120409. [DOI] [PubMed] [Google Scholar]
- 224.Ichikawa J, Dai J, Meltzer HY. DOI, a 5-HT2A/2C receptor agonist, attenuates clozapine-induced cortical dopamine release. Brain Res. 2001;907:151. doi: 10.1016/s0006-8993(01)02596-3. [DOI] [PubMed] [Google Scholar]
- 225.Kenakin T. Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol. Sci. 2003;24:346. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- 226.Kenakin T. Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 2010;336:296. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- 227.Kenakin TP. Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat. Rev. Drug Discov. 2009;8:617. doi: 10.1038/nrd2838. [DOI] [PubMed] [Google Scholar]
- 228.Grotewiel MS, Chu H, Sanders-Bush E. m-chlorophenylpiperazine and m-trifluoromethylphenylpiperazine are partial agonists at cloned 5-HT2A receptors expressed in fibroblasts. J. Pharmacol. Exp. Ther. 1994;271:1122. [PubMed] [Google Scholar]
- 229.Dalton GL, Lee MD, Kennett GA, Dourish CT, Clifton PG. mCPP-induced hyperactivity in 5-HT2C receptor mutant mice is mediated by activation of multiple 5-HT receptor subtypes. Neuropharmacology. 2004;46:663. doi: 10.1016/j.neuropharm.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 230.Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: In vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Ther. 2008;325:577. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 231.Fiorella D, Helsley S, Rabin RA, Winter JC. 5-HT2C receptor-mediated phosphoinositide turnover and the stimulus effects of m-chlorophenylpiperazine. Psychopharmacology (Berl) 1995;122:237. doi: 10.1007/BF02246545. [DOI] [PubMed] [Google Scholar]
- 232.Gonzalez-Maeso J, Sealfon SC. Agonist-trafficking and hallucinogens. Curr. Med. Chem. 2009;16:1017. doi: 10.2174/092986709787581851. [DOI] [PubMed] [Google Scholar]
- 233.Bossong MG, Brunt TM, Van Dijk JP, Rigter SM, Hoek J, Goldschmidt HM, Niesink RJ. mCPP: An undesired addition to the ecstasy market. J. Psychopharmacol. 2010;24:1395. doi: 10.1177/0269881109102541. [DOI] [PubMed] [Google Scholar]
- 234.Arena Pharmaceuticals Lorcaserin hydrochloride (APD356), NDA 22–529, Briefing Document for FDA Advisory Committee Meeting.; August 13, 2010. [Google Scholar]
- 235.Carlsson ML, Burstein ES, Kloberg A, Hansson S, Schedwin A, Nilsson M, Rung JP, Carlsson A. I. In vivo evidence for partial agonist effects of (−)-OSU6162 and (+)-OSU6162 on 5-HT2A serotonin receptors. J. Neural Transm. 2011;118:1511. doi: 10.1007/s00702-011-0704-8. [DOI] [PubMed] [Google Scholar]
- 236.Burstein ES, Carlsson ML, Owens M, Ma JN, Schiffer HH, Carlsson A, Hacksell U., II. In vitro evidence that (−)-OSU6162 and (+)-OSU6162 produce their behavioral effects through 5-HT2A serotonin and D2 dopamine receptors. J. Neural Transm. 2011;118:1523. doi: 10.1007/s00702-011-0701-y. [DOI] [PubMed] [Google Scholar]
- 237.Green AR, O'Shaughnessy K, Hammond M, Schachter M, Grahame-Smith DG. Inhibition of 5-hydroxytryptamine-mediated behaviour by the putative 5-HT2 antagonist pirenperone. Neuropharmacology. 1983;22:573. doi: 10.1016/0028-3908(83)90147-8. [DOI] [PubMed] [Google Scholar]
- 238.Glennon RA. Discriminative stimulus properties of hallucinogens and related designer drugs. NIDA Res. Monogr. 1991;25:1. [PubMed] [Google Scholar]
- 239.Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: Reassessment of LSD false positives. Psychopharmacology (Berl) 1995;121:357. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- 240.Benneyworth MA, Smith RL, Barrett RJ, Sanders-Bush E. Complex discriminative stimulus properties of (+)lysergic acid diethylamide (LSD) in C57Bl/6J mice. Psychopharmacology (Berl) 2005;179:854. doi: 10.1007/s00213-004-2108-z. [DOI] [PubMed] [Google Scholar]
- 241.Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology (Berl) 2005;182:197. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav. Brain Res. 1996;73:121. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 243.Gonzalez-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 244.Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol. Sci. 2008;29:445. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 245.Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc. Natl Acad. Sci. USA. 1954;40:228. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Arguello PA, Gogos JA. Modeling madness in mice: One piece at a time. Neuron. 2006;52:179. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 247.Arora RC, Meltzer HY. Serotonin2 (5-HT2) receptor binding in the frontal cortex of schizophrenic patients. J. Neural. Transm-Gen. 1991;85:19. doi: 10.1007/BF01244654. [DOI] [PubMed] [Google Scholar]
- 248.Offord SJ, Wong DF, Nyberg S. The role of positron emission tomography in the drug development of M100907, a putative antipsychotic with a novel mechanism of action. J. Clin. Pharmacol. 1999;(Suppl):17S. doi: 10.1002/j.1552-4604.1999.tb05933.x. [DOI] [PubMed] [Google Scholar]
- 249.Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol. Psychiatry. 2007;12:904. doi: 10.1038/sj.mp.4002062. [DOI] [PubMed] [Google Scholar]
- 250.Ionov ID. Synergistic effect of decreased opioid activity and sleep deprivation on head-twitch response in mice. Pharmacol. Biochem. Be. 2010;96:48. doi: 10.1016/j.pbb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 251.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry. 2010;167:261. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hayslett RL, Tizabi Y. Effects of donepezil on DOI-induced head twitch response in mice: Implications for Tourette syndrome. Pharmacol. Biochem. Be. 2003;76:409. doi: 10.1016/j.pbb.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 253.Rinaldi-Carmona M, Bouaboula M, Congy C, Oury-Donat F, Simiand J, Shire D, Casellas P, Soubrie P, Breliere JC, Le Fur G. Up-regulation of 5-HT2 receptors in the rat brain by repeated administration of SR 46349B, a selective 5-HT2 receptor antagonist. Eur. J. Pharmacol. 1993;246:73. doi: 10.1016/0922-4106(93)90012-x. [DOI] [PubMed] [Google Scholar]
- 254.Nagatomo T, Rashid M, Abul Muntasir H, Komiyama T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol. Ther. 2004;104:59. doi: 10.1016/j.pharmthera.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 255.Jensen NH, Cremers TI, Sotty F. Therapeutic potential of 5-HT2C receptor ligands. Scientific World Journal. 2010;10:1870. doi: 10.1100/tsw.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Monti JM. Serotonin 5-HT(2A) receptor antagonists in the treatment of insomnia: present status and future prospects. Drugs Today (Barc.) 2010;46:183. doi: 10.1358/dot.2010.46.3.1437247. [DOI] [PubMed] [Google Scholar]
- 257.Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011;22:390. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- 258.Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J. Physiol. 2009;587:49. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Carr GV, Lucki I. The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacology (Berl) 2011;213:265. doi: 10.1007/s00213-010-2097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl) 2011;218:649. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Maclean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J. Psychopharmacol. 2011;25:1453. doi: 10.1177/0269881111420188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology. 2003;28:402. doi: 10.1038/sj.npp.1300057. [DOI] [PubMed] [Google Scholar]
- 263.Rothman RB, Baumann MH. Serotonergic drugs and valvular heart disease. Expert Opin. Drug Saf. 2009;8:317. doi: 10.1517/14740330902931524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]