Abstract
BACKGROUND
Vascular smooth muscle cell (VSMC) proliferation is regulated by numerous hormones and humoral factors. Our previous study found that stimulation of D1-like dopamine receptors inhibited insulin receptor expression and function in VSMCs. We hypothesize that there is also an interaction between D3 dopamine and insulin receptors, i.e., stimulation of the D3 receptor inhibits insulin receptor expression and function.
METHODS
Receptor expression was determined by immunoblotting, immunohistochemisty, and reverse transcriptase-PCR; VSMC proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-diphenyl-tetrazolium bromide (MTT) assay and cell number.
RESULTS
Insulin receptor protein is increased in the aorta of D3 receptor deficient mice. Stimulation of the D3 receptor inhibited insulin receptor mRNA and protein expression and insulin-mediated VSMC proliferation, and increased protein kinase A (PKA) activity, insulin receptor phosphorylation, and degradation in immortalized aortic VSMCs (A10 cells). These effects were blocked by a PKA inhibitor, indicating that the D3 receptor-mediated decrease in insulin receptor expression was related to a decrease in transcription/post-transcription and increased degradation, involving PKA signaling.
CONCLUSIONS
D3 receptor stimulation may be a target to reduce the adverse effect of insulin in hypertension by inhibition of insulin receptor expression and function in arterial VSMCs.
Keywords: blood pressure, dopamine receptor, hypertension, insulin receptor, proliferation, vascular smooth muscle cells
Abnormal vascular smooth muscle cell (VSMC) proliferation, which is central to the development of vascular diseases, including hypertension, is regulated by numerous hormones and humoral factors.1–3 Epidemiological evidence supports a link between insulin resistance and hypertension.2,3 Insulin and its receptor stimulate VSMC proliferation and insulin levels are increased in patients with essential hypertension. The high levels of insulin may play an important role in the risk factors and pathogenesis of hypertension by increasing conduit vessel stiffness and peripheral vascular resistance by stimulating VSMC proliferation.2,3
Dopamine is an endogenous catecholamine that regulates or modulates many cellular and physiological processes, including behavior, hormone synthesis and release, blood pressure, and transmembrane ion transport.4 Dopamine receptors are classified into the D1- and D2-like subtypes based on their structure and pharmacology. D1-like receptors, comprised of D1 and D5 receptors, stimulate adenylyl cyclase activity, whereas D2-like receptors, comprised of D2, D3, and D4 receptors, inhibit adenylyl cyclase activity and regulate/modulate the activity of several ion channels.4 Our previous studies found that stimulation of the D3 receptor causes vasodilation, which is mediated by activation of small- and/or large-conductance calcium-activated potassium channels.5 Stimulation of α1-adrenergic receptor increases VSMC proliferation; in the presence of the D3 receptor agonist, PD128907, the α1-adrenergic receptor-mediated proliferative effect is inhibited.1
There is increasing evidence for an interaction between insulin and dopamine receptors. Hyperinsulinemic animals and patients have a defective renal dopaminergic system; activation of the D2-like dopamine receptor decreases insulin levels in obese women6 and activation of D1-like receptors improves peripheral insulin sensitivity and renal function in rats with streptozotocin-induced type 2 diabetes.7 The D3 receptor, as with the insulin receptor, is expressed in VSMCs.5 We hypothesize that the D3 receptor, as with D1-like receptors, may have an inhibitory effect on insulin receptor expression and function, which is involved in the regulation of blood pressure. Therefore, this study was designed to investigate the role of the D3 receptor on insulin receptor expression and function, and to determine the underlying mechanisms by which the D3 receptor regulates the insulin receptor in A10 cell, a VSMC line from embryonic thoracic aorta of normotensive Berlin-Druckrey IX rats. In addition, insulin receptor expression was quantified in aorta of D3 receptor deficient mice.
METHODS
Cell culture
Embryonic thoracic aortic smooth muscle cells (passage 10–20) from normotensive Berlin-Druckrey IX rats (A10; CRL 1476, ATCC, Manassas, VA) were cultured at 37 °C in 95% air/5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium.1
D3 receptor deficient mice
Heterozygous D3 receptor deficient mice (D3−/+) and control (D3+/+) mice in C57BL/6J background (>7 generations) were purchased from Jackson Laboratories (Bar Harbor, ME).8 The studies were conducted in accordance with the National Institutes of Health guidelines for the ethical treatment and handling of animals in research and approved by the Georgetown University Animal and Use Committee. After the blood pressures of D3−/+ and control (D3+/+) mice (4-month-old, mixed gender) were obtained by direct femoral artery cannulation under pentobarbital anesthesia, the aorta and kidneys were removed immediately for homogenization. Thereafter, the mice were killed by an overdose of pentobarbital cervical dislocation.9 Kidney and the aortic tunica media from D3−/− and D3+/+ mice were homogenized in ice-cold lysis buffer. All samples were stored at −70 °C until use.
Immunoblotting
The insulin receptor antibody is a polyclonal rabbit antihuman antipeptide (Santa Cruz Biotechnology, Santa Cruz, CA). A10 cells were treated with vehicle (dH2O), a D3 receptor agonist (PD128907) (Tocris Cookson, Bristol, UK)10 or a D3 receptor antagonist (U99194A) (Sigma, St Louis, MO), at the indicated concentrations and times. The transblots were probed with the insulin receptor antibody (1:400), or phosphorylated insulin receptor antibody (Santa Cruz Biotechnology, 1:800). The amount of protein transferred onto the membranes was determined by immunoblotting for α-actin. The immunoblotting density was normalized by α-actin density, each expressed as a fraction of 100%, as reported.5
To study the potential post-translational mechanisms we measured insulin protein expression in presence of 10 μg/ml cycloheximide to inhibit de novo protein synthesis.11,12
Determination of the second messenger(s) involved in the effect of D3 receptor on insulin receptor expression and function
To determine the second messenger(s) involved in the effect of the D3 receptor, several inhibitors or agonists were used: protein kinase C (PKC) inhibitor (PKC inhibitor 19–31, 10−6 mol/l),10 protein kinase A (PKA) inhibitor (PKA inhibitor 14–22, 10−6 mol/l),13 PKC activator (phorbol 12-myristate 13-acetate, PMA, 10−7 mol/l), PKA activator (Sp-cAMP-S, 10−7 mol/l), calcium channel blocker (nicardipine, 10−6 mol/l),14 and calcium channel agonist (BAY-K8644, 10−6 mol/l).15 PKC inhibitor 19–31, PMA, Sp-cAMP-S, nicardipine, BAY-K8644 were purchased from Sigma, PKA inhibitor 14–22 was purchased from Calbiochem (Darmstadt, Germany).
Measurement of PKA activity
PKA activity was measured using SignaTECT cAMP-dependent PKA assay (Promega, Southampton, UK), which utilizes biotinylated kemptide (LRRASLG), a peptide substrate derived from the in vivo substrate pyruvate kinase. A10 cells (5 × 106 cells) were preincubated with control buffer or the D3 receptor agonist, PD128907 (10−7 mol/l), for 30 min at 37 °C, then the cells were washed with ice-cold phosphate-buffered saline one time, followed by complete removal of the buffer. PKA activity was measured by scintillation counting.16,17
Reverse transcriptase-PCR of insulin receptors
A total of 2–3 μg of total RNA, extracted from A10 cells, was used to synthesize complementary DNA, which served as template for the amplification of the insulin receptor and β-actin (as housekeeping gene). For β-actin, the forward primer was 5′-GTGGGTATGGGTCAGAAGGA-3′ and the reverse primer was 5′-AGCGCGTAACCCTCATAGAT-3′ (GenBank Accession No. NM031144). The amplification was performed with the following conditions: denaturation at 94 °C for 30 s, annealing for 30 s at 60 °C, and extension for 45 s at 72 °C for 30 cycles. For the insulin receptor, the forward primer was 5′-GGA CTG AAG GTA TGA ATG GAG-3′ and the reverse primer was 5′-TAA CAC AAG CCA AGG AAG GG-3′. (GenBank Accession No. d12rat56). The amplification was performed with the following conditions: denaturation at 94 °C for 30 s, annealing for 30 s at 60 °C, and extension for 45 s at 72 °C for 30 cycles. The insulin receptor mRNA expression was normalized for β-actin mRNA.18,19
Immunohistochemistry
Cells grown in 96-well plates were fixed for 30 min in phosphate-buffered saline containing 4% paraformaldehyde. The fixed cells were incubated with anti-insulin receptor antibody (1:200) at 4 °C overnight. After incubation with the primary antibodies, the cells were rinsed three times with phosphate-buffered saline and incubated for 60 min at 37 °C with 10 μg/ml of biotin-conjugated goat anti-rabbit immunoglobulin G (Jackson ImmunoResearch Laboratories), for 20 min at room temperature with an avidin–biotin–peroxidase complex (Elite ABC kit; Vector Laboratories, Burlingame, CA) separately. The peroxidase label was then developed for 10 min using 3-amino-9-ethylcarbazole and peroxide with a kit from Calbiochem. Staining distribution and intensity were evaluated and scored by two independent investigators unaware of the treatments.8
MTT assay
The number of viable cells in each well was estimated by the uptake of the tetrazolium salt, 3-(4,5-dimethyl-thiazol-2-yl)-diphenyl-tetrazolium bromide (MTT). After the induction of quiescence in 96-well plastic culture dishes at a density of 1 × 103 cells/well, the cells were incubated with the indicated drugs for 24 h. Subsequently, 20 μl of MTT (2.5 g/l) were added to each well and the incubation continued for an additional 4 h at 37 °C. Thereafter, 150 μl dimethyl sulfoxide were added to each well and absorbance at 490 nm was read on a microplate reader (Model 680, Bio-Rad, Hercules, CA).20
Estimation of cell number
VSMCs were seeded in six-well plastic culture dishes at a density of 1 × 104/well in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and cultured for 24 h. After induction of quiescence, the medium was replaced with serum-free medium with the indicated drug concentrations (insulin 10−7 mol/l; PD128907 10−7 mol/l) and incubated for another 24 h. Viable cells, determined by the uptake of 0.4% Trypan blue after 5 min of mixing (Invitrogen Life Technologies, Carlsbad, CA), were counted using a hemocytometer (Trypan blue uptake was observed in <10% of the cells). Counting was performed in triplicate.1
Statistical analysis
The data are expressed as mean ± s.e.m. Comparison within groups was made by repeated measures ANOVA, and comparison among groups was made by factorial ANOVA and Duncan’s test (t-test when only two groups were compared). A value of P < 0.05 was considered significant.
RESULTS
Activation of the D3 receptor decreases insulin receptor expression in A10 cells
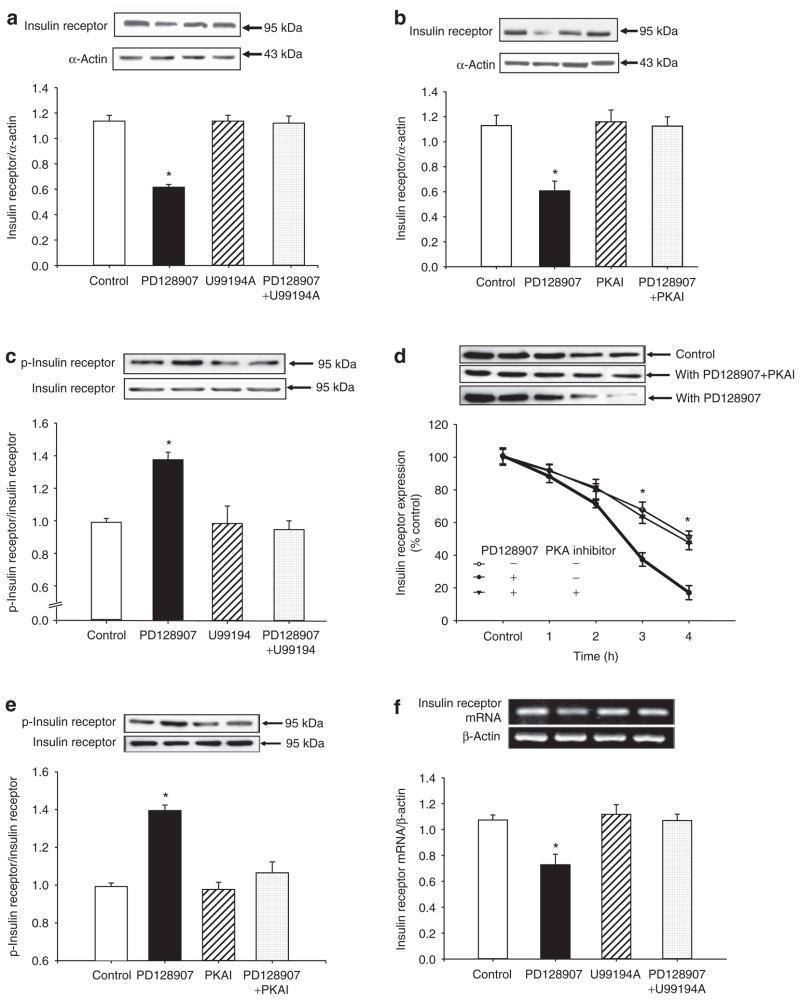
The D3 receptor agonist, PD128907, decreased insulin receptor expression in a concentration- and time-dependent manner. The inhibitory effect was evident at 10−8 mol/l. The inhibitory effect of PD128907 (10−7 mol/l) was noted as early as 2 h and maintained for at least 30 h (data not shown).
The specificity of PD128907 as a D3 receptor agonist was also determined by studying the effect of the D3 receptor antagonist, U99194A. PD128907 (10−7 mol/l/24 h), decreased insulin receptor expression. The D3 receptor antagonist, U99194A (10−6 mol/l), by itself, had no effect on insulin receptor expression, but reversed the inhibitory effect of PD128907 on insulin receptor expression (Figure 1a).
Figure 1.
Effect of D3 receptor stimulation on insulin receptor expression in A10 cells. (a) Effect of a D3 receptor agonist (PD128907) and a D3 receptor antagonist (U99194A) on insulin receptor protein expression in A10 cells. The cells were incubated with the indicated reagents (PD128907, 10−7 mol/l; U99194A, 10−7 mol/l) for 24 h. The immunoblotting density was normalized by α-actin density, each expressed as a fraction of 100%, as reported5 (n = 6, *P < 0.05 vs. others, ANOVA, Duncan’s test). (b) Effect of a protein kinase A (PKA) inhibitor on the inhibitory effect of PD128907 on insulin receptor expression in A10 cells. A10 cells were treated with a D3 receptor agonist (PD128907, 10−7 mol/l) or/and a PKA inhibitor 14–22 (PKAI, 10−7 mol/l) for 24 h. The immunoblotting density was normalized by α-actin density, each expressed as a fraction of 100% as reported5 (n = 5, *P < 0.05 vs. others, ANOVA, Duncan’s test). (c) Effect of a D3 receptor agonist (PD128907) and a D3 receptor antagonist (U99194A) on insulin receptor phosphorylation in A10 cells. The cells were incubated with the indicated reagents (PD128907, 10−7 mol/l; U99194A, 10−7 mol/l) for 30 mins. The phosphorylated insulin receptor density was normalized by total insulin receptor density, each expressed as a fraction of 100%, as reported5 (n = 4, *P < 0.05 vs. others, ANOVA, Duncan’s test). (d) Effect of a D3 receptor agonist (PD128907) on insulin receptor degradation in A10 cells. The cells were incubated with cycloheximide (20 μg/ml) with or without PD128907 (10−7 mol/l) and PKA inhibitor 14–22 (PKAI, 10−7 mol/l) for the indicated times. Results are expressed as percent change of control (0 time-point) (n = 5, *P < 0.05 vs. with PD128907, ANOVA, Duncan’s test). (e) Effect of a D3 receptor agonist (PD128907) and a PKA inhibitor 14–22 on insulin receptor phosphorylation in A10 cells. The cells were incubated with the indicated reagents (PD128907, 10−7 mol/l; PKAI, 10−7 mol/l) for 30 mins. The phosphorylated insulin receptor density was normalized by total insulin receptor density, each expressed as a fraction of 100%, as reported5 (n = 4, *P < 0.05 vs. others, ANOVA, Duncan’s test). (f) Effect of a D3 receptor agonist (PD128907) and a D3 receptor antagonist (U99194A) on insulin receptor mRNA expression in A10 cells. The cells were incubated with the indicated reagents (PD128907, 10−7 mol/l; U99194A, 10−7 mol/l) for 24 h. The immunoblotting density was normalized by β-actin density, each expressed as a fraction of 100% (n = 5, *P < 0.05 vs. control (0 time), ANOVA, Duncan’s test).
The inhibitory effect of the D3 receptor on insulin receptor expression was tissue-specific because in immortalized renal proximal tubule cells from Wistar-Kyoto rats, stimulation of the D3 receptor had no effect on insulin receptor expression (control = 1.04 ± 0.03, 10−7 mol/l PD128907 = 1.02 ± 0.04; 10−7 mol/l U99194A = 1.01 ± 0.04, PD128907+U99194A = 1.03 ± 0.04 density unit, n = 6). Moreover, the D3 receptor effect on insulin receptor expression was receptor-specific because stimulation of the D3 receptor had no effect on insulin growth factor-1 receptor expression in A10 cells (data not shown).
The PKA inhibitor 14–22 (10−7 mol/l), which by itself, had no effect on insulin receptor expression, blocked the inhibitory effect of PD128907 on insulin receptor expression in A10 cells (Figure 1b), indicating that PKA was involved into the inhibitory action of PD128907. The stimulatory effect of D3 receptor on PKA activity was confirmed in A10 cells; stimulation of the D3 receptor increased PKA activity (control = 1.0 ± 0.02, control = 1.18 ± 0.06, n = 6, P < 0.01). We also used PKC inhibitor or agonist, and calcium channel blocker or activator in this experiment. However, none of those reagents could block the effect of D3 receptor activation on insulin receptor expression (data not shown). Activation of the D3 receptor (PD128907, 10−7 mol/l) increased insulin receptor phosphorylation (Figure 1c), and increased insulin receptor protein degradation in A10 cells (Figure 1d), while in the presence of PKA inhibitor (PKA inhibitor 14–22), the stimulatory effects of D3 receptor on insulin receptor phosphorylation and degradation were blocked (Figure 1d,e), suggesting that PKA was involved in those signal pathways.
We also studied additional mechanisms by which the D3 receptor decreases insulin receptor expression (e.g., transcriptional or post-transcriptional level). We found that stimulation of the D3 receptor also decreased insulin receptor mRNA expression in A10 cells, an effect that was also blocked by D3 receptor antagonist, U99194A (10−6 mol/l) (Figure 1f). This effect was also in a time-dependent manner, the inhibitory effect was noted after stimulation for 4 h (data not shown), indicating that short-term regulation of D3 receptor on insulin receptor expression may be due to accelerated degradation, while the long-term regulation might involve both accelerated degradation and post-transcriptional levels.
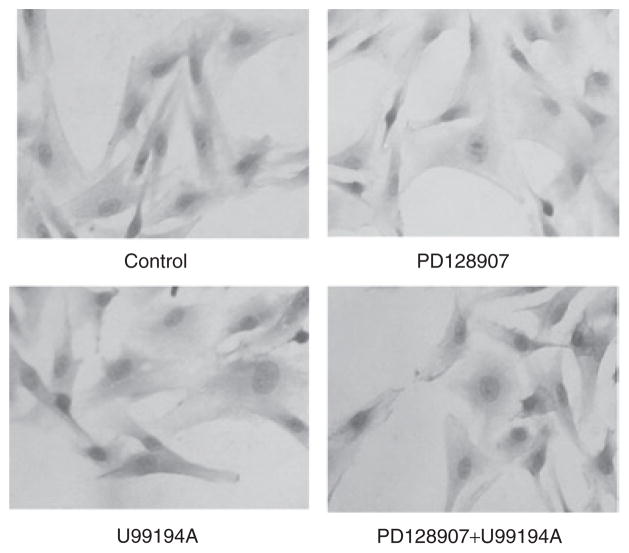
To confirm, further, the effect of the D3 receptor on insulin receptor expression, we studied insulin receptor protein abundance by immunohistochemistry. Consistent with the results in Figure 1a, stimulation of A10 cells with PD128907 (10−7 mol/l/24 h) decreased insulin receptor staining in A10 cells, which was blocked by U99194A (10−6 mol/l/24 h) (Figure 2).
Figure 2.
Effect of D3 receptor stimulation on insulin receptor staining in cells, determined by immunohistochemisty. The A10 cells were treated with PD128907 (10−7 mol/l/24 h) with or without a D3 receptor antagonist, U99194A (10−6 mol/l/24 h).
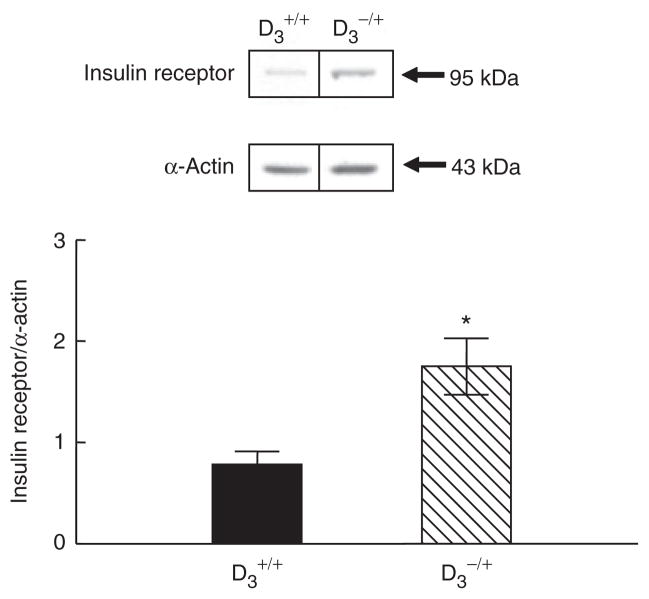
Insulin receptor expression is increased in the aorta of D3 receptor null mice
To determine whether or not the apparent D3 receptor regulation of insulin receptors in vitro occurs in vivo, we quantified insulin receptor expression in D3 receptor deficient mice. We have reported that arterial blood pressure was increased in D3 receptor null mice in mixed B129 and C57BL/6 background.8 The ability of the homozygous D3 receptor null (D3−/−) mice to excrete an acute sodium load was also impaired. In this study, D3−/+ mice were chosen because their blood pressures are elevated to the same extent as D3−/− mice and higher than their wild-type littermates, but the ability to excrete a sodium load is slightly but not significantly less than wild-type mice (systolic blood pressure, under pentobarbital anesthesia: D3+/+ = 102 ± 1; D3−/+ = 128 ± 9 mm Hg, P < 0.05, n = 5–7/group). Insulin receptor expression was increased in the aorta from D3−/+ mice relative to their D3+/+ littermates. (Figure 3). There was tissue specificity because similar to the in vitro studies, renal insulin receptor expression was not different between D3−/+ mice and D3+/+ littermates (D3+/+ = 1.04 ± 0.11; D3−/+ = 0.95 ± 0.12, P > 0.05, n = 5–7/group). These studies indicate that the results obtained in the in vitro studies have relevance in vivo.
Figure 3.
Insulin receptor protein expression in aorta from D3 receptor deficient mice (D3−/+) and their D3+/+ littermates. Results are expressed as the ratio of insulin receptor and α-actin densities (*P < 0.05 vs. D3+/+ mice, n = 5–7/group, t-test).
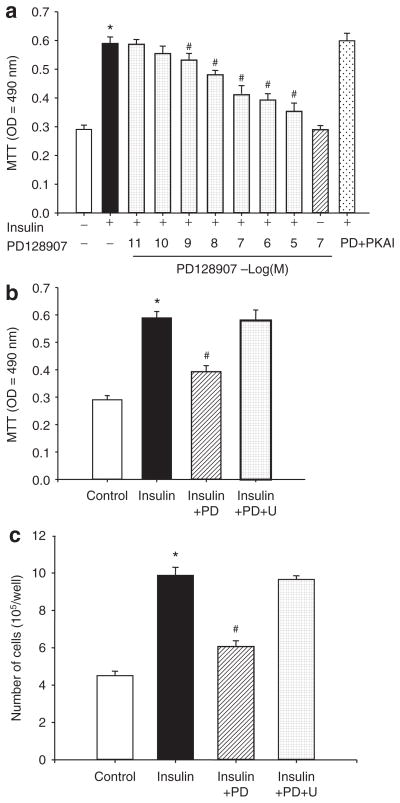
The insulin-mediated proliferation of A10 cells is attenuated by a D3 receptor agonist
Treatment of A10 cells with varying concentrations of insulin (10−10–10−6 mol/l) for 24 h increased cell proliferation, determined by MTT assay, in a concentration-dependent manner (data not shown). PD128907 by itself had no effect, but reduced the stimulatory effect of insulin (10−7 mol/l) (Figure 4a) on the proliferation of A10 cells in a concentration-dependent manner, which was reversed by U99194A (10−7 mol/l/24 h), a D3 receptor antagonist (Figure 4b) and by PKA inhibitor 14–22 (10−7 mol/l) (Figure 4a), indicating that PKA was involved in the process. In agreement with the MTT assay, insulin increased the number of VSMCs, which was reduced in the presence of the D3 receptor agonist, PD128907 (10−7 mol/l). The D3 receptor antagonist, U99194A, reversed the inhibitory effect of the D3 receptor on insulin-mediated proliferation (Figure 4c).
Figure 4.
Effect of D3 receptor on insulin-mediated proliferation of A10 cells. (a) Concentration-response of D3 receptor stimulation on the proliferation of A10 cells induced by insulin. The inhibitory effect of D3 receptor agonist, PD128907 (PD, 10−7 mol/l) on insulin (10−7 mol/l)-mediated vascular smooth muscle cell (VSMC) proliferation was abolished in the presence of protein kinase A (PKA) inhibitor 14–22 (PKAI, 10−7 mol/l). VSMC proliferation was determined by the 3-(4,5-dimethylthiazol-2-yl)-diphenyl-tetrazolium bromide (MTT) method after incubation with the indicated concentrations of insulin (10−7 mol/l) with or without PD128907 (10−11–10−5 mol/l) and/or PKA inhibitor (PKA inhibitor 14–22, 10−7 mol/l) in A10 cells. Results are expressed as MTT optical density (n = 9, *P < 0.01 vs. control; #P < 0.05 vs. insulin alone, ANOVA, Duncan’s test). (b) Effect of a D3 receptor agonist (PD128907) and a D3 receptor antagonist (U99194A) on insulin receptor-mediated proliferation in A10 cells. The cells were incubated with the indicated reagents (PD, PD128907, 10−7 mol/l; U, U99194A, 10−7 mol/l) for 24 h. Results are expressed as MTT optical density (n = 6, *P < 0.01 vs. control; #P < 0.05 vs. insulin alone, ANOVA, Duncan’s test). (c) VSMC proliferation was determined by cell number after incubation with the indicated concentrations of insulin (10−7 mol/l) with or without PD128907 (PD, 10−7 mol/l), U99194A (U, 10−7 mol/l) for 24 h. Results are expressed as cell number/well (n = 6, *P < 0.01 vs. control; #P < 0.05 vs. insulin alone, ANOVA, Duncan’s test).
DISCUSSION
There is increasing evidence for an interaction between insulin and dopamine receptors. Dopamine D2-like receptors in pancreatic β cells inhibit insulin secretion.21 In obese Zucker rats, a model of type 2 diabetes, renal D1 receptor expression is decreased and dopamine fails to increase urine flow and renal sodium excretion.22 Chronic exposure of renal proximal tubule cells to insulin reduces D1 receptor abundance, uncouples it from G proteins, and diminishes its inhibitory effect on Na+-K+ ATPase activity.23 Treatment of obese Zucker rats with insulin sensitizer rosiglitazone restores renal D1 receptor expression and function.24 The D1-like receptor agonist, fenoldopam, also improves peripheral insulin sensitivity and renal function in streptozotocin-induced type 2 diabetes in rats.7
D2-like receptors may also interact with insulin. Bromocriptine, a D2-like receptor agonist with equal affinity for D2 and D3 receptor, inhibits the insulin-like growth factor-mediated proliferation in rat VSMCs (A7r5) and human aortic smooth muscle cells.25 Bromocriptine also reduces insulin resistance, glucose intolerance, and hyperlipidemia in type 2 diabetes.26 A recent study also showed that bromocriptine ameliorates various metabolic features of obese women, including plasma insulin concentration and fasting glucose concentration; systolic blood pressure is also decreased.6 Although bromocriptine has the same affinity for D2 and D3 receptors, the above-mentioned effects may be via the D3 receptor because in male Sprague–Dawley rats, a selective D3 receptor agonist, 7-OH-DPAT, decreases plasma insulin levels.27 However, although high fat diet increases body fat and plasma leptin levels to greater extent in D3−/− than in D3+/+ mice, insulin levels are not different between the two strains,28 suggesting an effect occurs at the receptor level.
The counter regulatory actions of insulin and dopamine receptors extend to effects on vascular proliferation. Our previous study showed that the D1-like receptor has an inhibitory effect on insulin receptor-mediated VSMC proliferation.18 Whether or not D2-like receptors interact with the insulin receptor is not known. Our present study found an interaction between the D3 receptor and the insulin receptor; stimulation of the D3 receptor decreases insulin receptor mRNA and protein expression. The D3 receptor-mediated decrease in insulin receptor protein expression is also due to an increase its degradation. The D3 receptor also inhibits the proliferative effects of insulin in immortalized aortic VSMCs. The effect is tissue-specific because stimulation of the D3 receptor does not affect insulin receptor expression in renal proximal tubule cells. Moreover, insulin receptor expression is increased in the aorta but not in the kidneys from mice lacking one D3 receptor allele relative to their wild-type littermates. The D3 receptor effect is also receptor-specific because stimulation of the D3 receptor does not affect insulin growth factor-1 receptor or AT1 receptor expression in VSMCs.19 This occurs in spite of the insulin receptor sharing similar pathways with AT1 and insulin growth factor-1 receptors with activation of mitogenic-activated-protein kinase, phosphatidyl inositol phosphoinositide 3-kinase/protein kinase B/Akt in the stimulation of cell growth and protein synthesis.29,30 The mechanism(s) responsible for the tissue- and receptor- specific effect of the D3 receptor on insulin receptor expression and function remains to be determined.
The current study identifies a cellular mechanism by which the D3 receptor negatively regulates insulin receptor expression and function. Because the D3 receptor agonist, PD128907, does not inhibit the proliferation or cell viability of VSMCs, it is unlikely that the inhibitory effect of PD128907 on VSMC proliferation is due to any cytotoxic effect. Previous studies have shown that PKA phosphorylation at serine and threonine residues reduces the tyrosine kinase activity of the insulin receptor,31,32 increases insulin receptor degradation.33 Although the D3 receptor, being a member of the D2-like receptor family, normally couples to inhibitory G proteins; in renal proximal tubule cells,34 D3 receptor can also couple to the stimulatory G protein, GSα, resulting in stimulation of cAMP production.35 The linkage of the D3 receptor to other effectors, such as inhibition of K+ and Ca2+ channels, may be more sensitive than its weak linkage to G proteins.36 In the current study, we found that a PKA inhibitor prevents the inhibitory effect of the D3 receptor on insulin receptor protein expression and function suggesting involvement of PKA. It is possible that a D3 receptor agonist-mediated reduction in insulin receptor expression and function in VSMCs may be a compensatory mechanism to reduce insulin-mediated VSMC proliferation, increased stiffness of conductance vessels, and elevated peripheral resistance in hypertension.
Perspectives
VSMC proliferation plays an important role in the pathogenesis of hypertension. Insulin increases VSMC proliferation, while D3 receptor reduces insulin-mediated proliferative effects. Because dopamine receptor function in nonrenal VSMCs in hypertension is preserved,18 D1-like and D3 receptors, may be targets to reduce the insulin-mediated hyperplasia of VSMC in hypertension or diabetes. However, it remains to be determined whether or not activation of D1 or D3 receptor can reduce VSMC proliferation in vivo.
Acknowledgments
These studies were supported in part by grants from the National Institutes of Health (HL023081, HL074940, DK039308, HL068686, HL092196), the National Natural Science Foundation of China (30470728, 30672199), Natural Science Foundation Project of CQ CSTC (CSTC,2009BA5044), and the grants for Distinguished Young Scholars of China from the National Natural Science Foundation of China (30925018).
Footnotes
The first two authors contributed equally to this work and they are considered as the first co-authors.
Disclosure: The authors declared no conflict of interest.
References
- 1.Li Z, Yu C, Han Y, Ren H, Shi W, Fu C, He D, Huang L, Yang C, Wang X, Zhou L, Asico LD, Zeng C, Jose PA. Inhibitory effect of D1-like and D3 dopamine receptors on norepinephrine-induced proliferation in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2008;294:H2761–H2768. doi: 10.1152/ajpheart.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242–259. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 3.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286:H1597–H1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 4.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–H569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA. Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–679. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]
- 6.Kok P, Roelfsema F, Frölich M, van Pelt J, Stokkel MP, Meinders AE, Pijl H. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol Endocrinol Metab. 2006;291:E1038–E1043. doi: 10.1152/ajpendo.00567.2005. [DOI] [PubMed] [Google Scholar]
- 7.Umrani DN, Goyal RK. Fenoldopam treatment improves peripheral insulin sensitivity and renal function in STZ-induced type 2 diabetic rats. Clin Exp Hypertens. 2003;25:221–233. doi: 10.1081/ceh-120020392. [DOI] [PubMed] [Google Scholar]
- 8.Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Luo Y, Escano CS, Yang Z, Asico L, Li H, Jones JE, Armando I, Lu Q, Sibley DR, Eisner GM, Jose PA. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension. 2010;55:1431–1437. doi: 10.1161/HYPERTENSIONAHA.109.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 11.Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, Thöne-Reineke C, Unger T, Kintscher U. PPARγ-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 12.Sonalker PA, Jackson EK. Norepinephrine, via beta-adrenoceptors, regulates bumetanide-sensitive cotransporter type 1 expression in thick ascending limb cells. Hypertension. 2007;49:1351–1357. doi: 10.1161/HYPERTENSIONAHA.107.088732. [DOI] [PubMed] [Google Scholar]
- 13.Bobalova J, Mutafova-Yambolieva VN. Activation of the adenylyl cyclase/protein kinase A pathway facilitates neural release of beta-nicotinamide adenine dinucleotide in canine mesenteric artery. Eur J Pharmacol. 2006;536:128–132. doi: 10.1016/j.ejphar.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 14.Curran MP, Robinson DM, Keating GM. Intravenous nicardipine: its use in the short-term treatment of hypertension and various other indications. Drugs. 2006;66:1755–1782. doi: 10.2165/00003495-200666130-00010. [DOI] [PubMed] [Google Scholar]
- 15.Inui T, Mori Y, Watanabe M, Takamaki A, Yamaji J, Sohma Y, Yoshida R, Takenaka H, Kubota T. Physiological role of L-type Ca2+ channels in marginal cells in the stria vascularis of guinea pigs. J Physiol Sci. 2007;57:287–298. doi: 10.2170/physiolsci.RP006807. [DOI] [PubMed] [Google Scholar]
- 16.Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J, Rashid S, Mancino MG, LeSage G, Alpini G. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284:G683–G694. doi: 10.1152/ajpgi.00302.2002. [DOI] [PubMed] [Google Scholar]
- 17.Fetalvero KM, Shyu M, Nomikos AP, Chiu YF, Wagner RJ, Powell RJ, Hwa J, Martin KA. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol. 2006;290:H1337–H1346. doi: 10.1152/ajpheart.00936.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zeng C, Han Y, Huang H, Yu C, Ren H, Shi W, He D, Huang L, Yang C, Wang X, Zhou L, Jose PA. D1-like receptors inhibit insulin-induced vascular smooth muscle cell proliferation via down-regulation of insulin receptor expression. J Hypertens. 2009;27:1033–1041. doi: 10.1097/HJH.0b013e3283293c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Lu SY, Zhu MZ, Wang DS, Chen SY, Zhang WD, Dong H, Yu J, Guo HT. Inhibition of the proliferation of smooth muscle cells from human coronary bypass vessels by vasonatrin peptide. Physiol Res. 2004;53:387–393. [PubMed] [Google Scholar]
- 21.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- 22.Marwaha A, Lokhandwala MF. Diminished natriuretic response to dopamine D1 receptor agonist, SKF-38393 in obese Zucker rats. Clin Exp Hypertens. 2003;25:509–515. doi: 10.1081/ceh-120025334. [DOI] [PubMed] [Google Scholar]
- 23.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine-mediated inhibition of renal Na,K-ATPase is reduced by insulin. Hypertension. 2003;41:1353–1358. doi: 10.1161/01.HYP.0000069260.11830.CD. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi M, Lokhandwala MF. Rosiglitazone restores renal D1A receptor-Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol. 2005;289:F298–F304. doi: 10.1152/ajprenal.00362.2004. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Cincotta AH. Inhibitory effects of bromocriptine on vascular smooth muscle cell proliferation. Atherosclerosis. 1997;133:37–44. doi: 10.1016/s0021-9150(97)00113-5. [DOI] [PubMed] [Google Scholar]
- 26.Cincotta AH, Meier AH, Cincotta M., Jr Bromocriptine improves glycaemic control and serum lipid profile in obese Type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8:1683–1707. doi: 10.1517/13543784.8.10.1683. [DOI] [PubMed] [Google Scholar]
- 27.Uvnäs-Moberg K, Ahlenius S, Alster P, Hillegaart V. Effects of selective serotonin and dopamine agonists on plasma levels of glucose, insulin and glucagon in the rat. Neuroendocrinology. 1996;63:269–274. doi: 10.1159/000126970. [DOI] [PubMed] [Google Scholar]
- 28.McQuade JA, Benoit SC, Xu M, Woods SC, Seeley RJ. High-fat diet induced adiposity in mice with targeted disruption of the dopamine-3 receptor gene. Behav Brain Res. 2004;151:313–319. doi: 10.1016/j.bbr.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Krook A, Zierath JR. Specificity of insulin signalling in human skeletal muscle as revealed by small interfering RNA. Diabetologia. 2009;52:1231–1239. doi: 10.1007/s00125-009-1330-y. [DOI] [PubMed] [Google Scholar]
- 30.Muretta JM, Mastick CC. How insulin regulates glucose transport in adipocytes. Vitam Horm. 2009;80:245–286. doi: 10.1016/S0083-6729(08)00610-9. [DOI] [PubMed] [Google Scholar]
- 31.Roth RA, Beaudoin J. Phosphorylation of purified insulin receptor by cAMP kinase. Diabetes. 1987;36:123–126. doi: 10.2337/diab.36.1.123. [DOI] [PubMed] [Google Scholar]
- 32.Stadtmauer L, Rosen OM. Increasing the cAMP content of IM-9 cells alters the phosphorylation state and protein kinase activity of the insulin receptor. J Biol Chem. 1986;261:3402–3407. [PubMed] [Google Scholar]
- 33.Sugita H, Kaneki M, Furuhashi S, Hirota M, Takamori H, Baba H. Nitric oxide inhibits the proliferation and invasion of pancreatic cancer cells through degradation of insulin receptor substrate-1 protein. Mol Cancer Res. 2010;8:1152–1163. doi: 10.1158/1541-7786.MCR-09-0472. [DOI] [PubMed] [Google Scholar]
- 34.Pedrosa R, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Gialpha3 protein-coupled dopamine D3 receptor-mediated inhibition of renal NHE3 activity in SHR proximal tubular cells is a PLC-PKC-mediated event. Am J Physiol Renal Physiol. 2004;287:F1059–F1066. doi: 10.1152/ajprenal.00139.2004. [DOI] [PubMed] [Google Scholar]
- 35.Obadiah J, Avidor-Reiss T, Fishburn CS, Carmon S, Bayewitch M, Vogel Z, Fuchs S, Levavi-Sivan B. Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell Mol Neurobiol. 1999;19:653–664. doi: 10.1023/a:1006988603199. [DOI] [PubMed] [Google Scholar]
- 36.Robinson SW, Caron MG. Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Mol Pharmacol. 1997;52:508–514. doi: 10.1124/mol.52.3.508. [DOI] [PubMed] [Google Scholar]