Abstract
Objective
Contingency management (CM) is efficacious in reducing drug use. Typically, reinforcers are provided on an individual basis to patients for submitting drug-negative samples. However, most treatment is provided in a group context, and poor attendance is a substantial concern. This study evaluated whether adding CM to group-based outpatient treatment would increase attendance and drug abstinence relative to standard care.
Methods
Substance abusing patients (N = 239) initiating outpatient treatment at two community-based clinics were randomized to standard care with frequent urine sample monitoring for 12 weeks (SC) or that same treatment with CM delivered in the context of group counseling sessions. In the CM condition, patients earned opportunities to put their names in a hat based on attendance and submission of drug-negative samples. At group counseling sessions, therapists selected names randomly from the hat, and individuals whose names were drawn won prizes ranging from $1 to $100.
Results
Patients assigned to CM earned a median of $160 in prizes, and they attended significantly more days of treatment (d = 0.25), remained in treatment for more continuous weeks (d = 0.40), and achieved longer durations of drug abstinence (d = 0.26) than patients randomized to SC. Group adherence and therapeutic alliance also improved with CM. In addition, HIV risk behaviors were significantly lower in CM relative to SC patients during early phases of treatment and at the 12-month follow-up.
Conclusions
These data demonstrate that CM delivered in the context of outpatient group counseling can increase attendance and improve drug abstinence.
Keywords: contingency management, substance abuse, treatment, group therapy
Contingency management interventions provide tangible reinforcers for objective evidence of behavior change. Typically, researchers provide vouchers exchangeable for retail goods or services (Higgins et al., 1994) or the chance to win prizes of varying magnitudes (Petry, Martin, Cooney, & Kranzler, 2000) when patients submit urine samples that indicate drug abstinence. A recent meta-analysis (Dutra et al., 2008) of psychosocial treatments for substance use disorders found that CM is the intervention with the greatest effect size. Meta-analyses of CM studies (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006) likewise reveal efficacy of CM in treating substance use disorders. However, the vast majority of studies evaluated CM when delivered entirely on an individual basis, yet most treatment is provided in a group context. An empirically established method for delivering CM in group settings would enhance the dissemination of CM.
Given providers’ concerns about the practicality of individually administered CM (Kellogg et al., 2005; Kirby, Benishek, Dugosh, & Kerwin, 2006), a handful of studies have begun examining CM when delivered in a group context (Alessi, Hanson, Tardif, & Petry, 2007; Kirby, Kerwin, Carpendo, Rosenwasser, & Gardner, 2008; Ledgerwood, Alessi, Hanson, Godley, & Petry, 2008; Petry, Martin, & Finocche, 2001; Sigmon & Stitzer, 2005). With the exception of the Alessi et al. (2007) and Kirby et al. (2008) studies, the researchers chose attendance alone, rather than drug abstinence, as the behavioral target of their intervention, primarily because of concerns about confidentiality related to urine testing; these studies did not monitor substance use, so effects of the group-based CM interventions on substance use outcomes could not be determined. Although these studies found benefits of CM on attendance, more research on group-based CM approaches is needed to evaluate its effects on substance use.
If efficacious, a CM intervention delivered in a group context could have benefits for patients, providers, and society at large. Patients may remain in treatment longer and reduce drug use (Higgins et al., 1994; Higgins, Wong, Badger, Ogden, & Dantona, 2000; Petry, Alessi, Marx, Austin, & Tardif, 2005; Petry, Peirce et al., 2005). Providers could benefit because CM can increase attendance at treatment (Higgins et al., 1994; Higgins, Wong et al., 2000; Ledgerwood et al., 2008; Petry, Alessi et al., 2005; Petry et al., 2001; Petry, Peirce et al., 2005) so that groups should be fuller, and unexcused absences and attrition reduced. Job satisfaction may increase when therapists provide CM (Kellogg et al., 2005), which in turn may reduce therapist turnover rates. Potentially, CM could also enhance the therapeutic alliance and improve group cohesion, resulting in more satisfying groups for both patients and providers. Greater attendance can increase provider reimbursement rates in non-capitated reimbursement systems (Lott & Jencius, 2009). Society may also benefit from the introduction of CM clinically. Reductions in drug use that occur with CM may reduce drug-related criminal activity and medical consequences associated with substance abuse. In particular, recent analyses (DeFulio, Donlin, Wong, & Silverman, 2009; Ghitza, Epstein, & Preston, 2008; Hanson, Alessi, & Petry, 2008) find that CM reduces HIV risk behaviors in methadone-maintained patients, and it is associated with a reduction in viral loads when administered to HIV-positive substance abusers (Petry, Weinstock, Alessi, Lewis, & Dieckhaus, 2010).
CM can be very beneficial, but a practical group-based CM approach for use in community settings must be applicable to all patients attending a group, as groups in community settings are usually heterogeneous with respect to substance use problems. To date, however, most CM studies have limited enrollment to individuals with a particular substance use disorder, and most reinforcement has been contingent on abstinence from just the primary substance of abuse (Higgins et al., 1994; Lussier et al., 2006). Meta-analyses reveal that effect sizes for CM are higher when abstinence from a single substance is reinforced versus when abstinence from multiple substances is reinforced (Griffith, Rowan-Szal, Roark, & Simpson, 2000; Lussier et al., 2006), but the vast majority of patients entering treatment programs have polysubstance use problems (Substance Abuse and Mental Health Services Administration Office of Applied Studies, 2009).
The purpose of the present study was to evaluate the efficacy of CM when delivered in a group format and applicable to patients with a range of substance use disorders. The reinforcement procedure was a novel adaptation from previous group-based CM interventions (Alessi et al., 2007; Ledgerwood et al., 2008; Petry et al., 2001). Each day CM patients attended group counseling sessions, they earned at least one chance to have their name drawn from a hat, and chances increased with sustained attendance and abstinence from four substances (cocaine, methamphetamine, opioids and alcohol) as assessed by twice-weekly testing. Whenever their names were drawn from the hat, patients won a prize ranging from $1 to $100 in value. We evaluated the efficacy of this CM treatment delivered in a group format compared to standard care. Patients with a variety of substance use disorders were included to enhance generalization to those typically treated in outpatient treatment programs, and the study was conducted at two community clinics that had never participated in CM projects. We also assessed effects of CM on the therapeutic process and HIV risk behaviors. We expected patients assigned to the CM condition would evidence greater attendance, more abstinence, stronger group cohesion and therapeutic alliances, and reduced HIV risk behaviors than patients assigned to standard care.
Methods
Participants
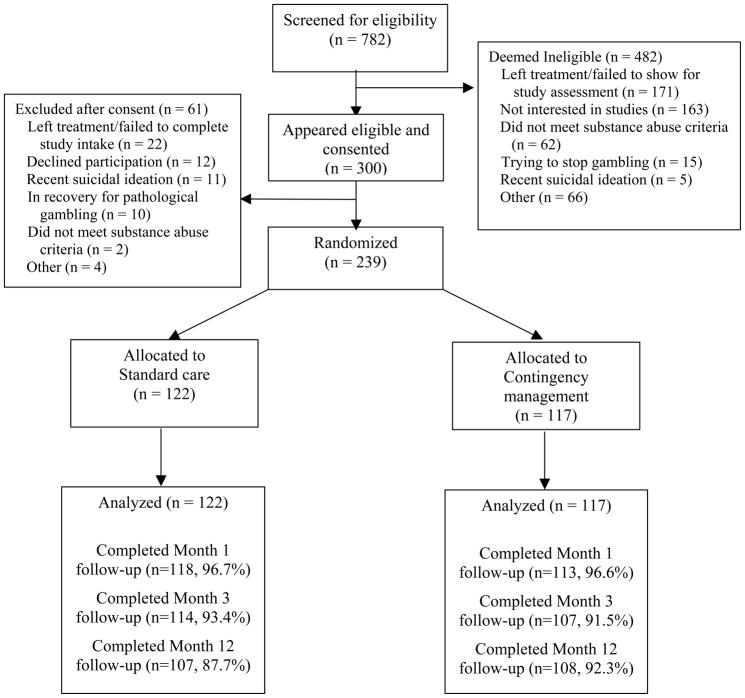
Participants were 239 outpatients initiating treatment at one of two community-based clinics located in urban areas in southern Connecticut between 2005 and 2009. The sample size of about 120 patients per condition was estimated from meta-analyses of CM interventions on attendance (Lussier et al., 2006; Prendergast et al., 2006), in conjunction with effect size estimates from prior prize CM studies (Petry, Alessi et al., 2005; Petry, Peirce et al., 2005; Petry et al., 2004). Patients were eligible for the study if they began intensive outpatient treatment at one of the clinics within 72 hours, met past-year Diagnostic and Statistical Manual of Mental Disorders-IV diagnosis of cocaine, opioid or alcohol abuse or dependence (American Psychiatric Association, 2000), were 18 years or older, and spoke English. Exclusion criteria were inability to understand the study, uncontrolled psychotic symptoms, active suicidal intentions, or in recovery for pathological gambling (because prize CM has an element of chance, but see Petry & Alessi, 2010; Petry, Kolodner et al., 2006). University and hospital Institutional Review Boards approved study procedures. All participants signed written informed consent. Figure 1 shows the flow of participants through study procedures.
Figure 1.
Flow chart of participants in study.
Procedures
After obtaining informed consent, research assistants (RAs) administered an interview consisting of modules adapted from the Structured Clinical Interview for DSM-IV for assessing substance use diagnoses (First, Spitzer, Gibbon, & Williams, 1996), the Addiction Severity Index (ASI) (McLellan et al., 1985), and the HIV Risk Behavior Scale (HRBS) (Drake, Hall, Heather, Ward, & Wodak, 1991). Follow-up evaluations were scheduled for 1, 3, and 12 months after randomization. Patients received $40 for completing each follow-up evaluation. Figure 1 shows rates of follow-up completion, which exceeded 86% in both conditions at each time point.
The ASI is a well-established instrument (Bovasso, Alterman, Cacciloa, & Cook, 2001; Kosten, Rounsaville, & Kleber, 1983; Leonhard, Mulvey, Gastfriend, & Schwartz, 2000) that evaluates severity of psychosocial problems in seven domains. Composite scores are derived in each domain and range from 0 to 1, with higher scores reflecting greater severity of problems.
The HRBS examined risk behaviors occurring over the past 1 or 3 months. The past month version was used at the Month 1 assessment, and the past 3-month version at other time points. The HRBS contains six questions about injection and five about sexual risk behaviors. Responses are coded on a six-point scale, and higher values denote greater risk. An overall summary score, and drug and sexual behavior subscale scores, are computed by summing ordinal values of responses. Total scores range from 0 to 55, and from 0 to 30 and 0 to 25 on the drug use and sexual risk subscales, respectively. Prior studies report upon psychometric properties of the HRBS (Drake et al., 1991; Kelley & Petry, 2000; Petry, 2001; Petry, Weinstock et al., 2010).
Patients who remained in treatment at the end of Week 1, Month 1 and Month 2 of study participation completed two indices of therapeutic processes. One was the Group Cohesion Questionnaire (van Andel, Erdman, Karsdorp, Appels, & Trijsburg, 2003), which assesses member-to-member alliance, and the second was the Group Helping Alliance Questionnaire (Luborsky, Barber, Siqueland, Johnson, & Najavits, 1996), which measures patients’ bond to the therapist. Each is measured on a six-point Likert scale, with higher scores reflecting greater cohesion or alliance, and ranges of 25–150 and 19–114, respectively. Test-retest reliability and predictive validity of these instruments are established in other samples (Luborsky et al., 1996; Petry & Bickel, 1999; van Andel et al., 2003).
Assignment to treatment conditions
After completing the baseline assessment, RAs randomly assigned patients to one of two treatment conditions using urn randomization programs at each clinic. Treatment conditions were balanced (Stout, Wirtz, Carbonari, & Del Boca, 1994) on past-year dependence on opioids, cocaine and alcohol. Due to the behavioral nature of the intervention, it was not possible to blind individuals to treatments. Clinic counselors, ranging in education from no degree to masters degrees, conducted group counseling sessions for patients in both study conditions.
Standard care (SC)
Standard outpatient treatment at both clinics consisted of rolling admission group counseling sessions that included life skills training, relapse prevention, and 12-step oriented treatment. Intensive care (up to four hours/day, five days/week) was available for up to six weeks, followed by reductions in intensity. Aftercare consisted of one group counseling session per week for up to 12 months.
In addition to standard clinic services, patients submitted up to 24 urine and breath samples (two per week) for the 12-week study period. Urine specimens were tested for cocaine, opioids, methamphetamine, and marijuana metabolites using OnTrak TesTstiks (Varian, Inc., Walnut Creek, CA), an onsite system, and breath samples for recent alcohol use using an Intoximeter Breathalyzer (Intoximeters, Inc., St. Louis, Mo). Research assistants screened the samples and congratulated patients whenever they tested negative, and in the case of positive samples, encouraged patients to discuss any use in group counseling sessions.
CM
Patients assigned to this condition received the same SC and sample monitoring described above. However, for the first group counseling session of the day, CM patients were separated from SC patients. In the CM group counseling session, all patients put their name into a hat at least once, and those who had their urine and breath samples tested since the last group session put their name in the hat twice if their most recent sample tested negative for cocaine, methamphetamine, opioids, and alcohol concurrently. Because patients were on different toxicology testing schedules, patients were unaware of why other patients had just a single name slip on a given day (e.g., they may not have had a sample tested since the last session, or they may have tested positive for one or more substances since the last session). Each day of the week other than Mondays (or the first group of the week if Monday was a holiday), three name slips were drawn from the hat at the start of the group counseling session, and those individuals then drew once from a standard prize bowl, which contained 200 cards. Of these cards, 174 were small prizes (patient’s choice of $1 McDonald’s coupons, food items, bus tokens, etc.). Twenty-five cards were large prizes, worth up to $20 (choice of movie tickets, CDs, phone cards, watches, pan sets, etc.), and one was a jumbo prize worth up to $100 (choice of stereo, DVD player, or television). A representative selection of prizes was available for selection immediately in group, or patients could elect to select from the full range of prizes right after the group session. Prize cards were replaced after each drawing, so that chances of winning prizes remained constant. Name slips, however, were not replaced into the hat, but patients could have their name drawn more than once if their name was in the hat twice on any given day. After each group counseling session, all the name slips were discarded.
On Mondays, names went into the hat at least once for attendance that day plus a bonus number of times based upon the number of weeks in a row they attended all scheduled group counseling sessions and submitted all negative samples. Thus, a patient who had attended all scheduled group counseling sessions six weeks in a row and submitted all twice-weekly negative samples over that time frame would put her name in the hat seven times on Monday (once for attendance that Monday and six bonus times), thereby increasing her chances of having her name selected. On Mondays, six names were selected from the hat. The first five people whose names were selected drew for one prize each, and the sixth person drew for five prizes. All draws on Mondays were from an Enhanced Prize Bowl containing 30 cards; 25 cards were for small prizes, four for larges, and one was the jumbo. Thus, the probability of winning a jumbo on Mondays was greater than on other days of the week that utilized the standard prize bowl.
If patients refused to submit a sample, provided a sample positive for any of the target drugs (cocaine, methamphetamine, opioids, or alcohol), or had an unexcused absence from one or more scheduled group counseling sessions on a treatment day (excused absences include court appearance, family emergencies, commitments cleared 24 hours in advance by the primary therapist), the string of abstinence/attendance was broken. The next week of consecutive attendance and negative samples would result in their name going into the hat twice on Monday (once for attendance that day, plus once more for one week of continuous attendance/abstinence). If patients were reset for any reason and they then attended fully and provided negative samples for two consecutive weeks, the number of times their name went into the hat increased back to the previously highest level achieved. Lateness to group (i.e., arriving after names were drawn) resulted in a forfeit of one’s name going in the hat that day, but did not reset name slips for the following Monday.
A project manager reviewed name slips earned and draw data, based on patient attendance and abstinence data, weekly initially and then at least monthly at both clinics throughout the study period. Few deviations from protocol were noted in either clinic.
Data analyses
We employed an intent-to-treat approach, including all 239 randomized patients, and primary outcomes were available for 100% of patients. Initially, t-tests and chi-square tests compared baseline characteristics between treatment conditions. Although not all continuous variables were normally distributed, these tests are robust to departures from normality when the sample size is large (Lumley, Diehr, Emerson, & Chen, 2002), and non-parametric tests yielded similar results.
Primary outcomes were total number of group counseling sessions attended and longest durations of attendance and abstinence achieved. A week of consecutive attendance was defined as a 7-day period in which all scheduled groups were attended, and a week of abstinence was counted for two consecutively scheduled samples that tested negative for all reinforced substances (cocaine, methamphetamine, opioids and alcohol). We also present supplementary analysis for each substance separately, including marijuana, which did not impact reinforcement. If a patient did not attend a scheduled group counseling session or refused to provide or missed a sample because of an unexcused absence, we coded the string of attendance or abstinence as broken. Consistent with the reinforcement schedule, excused missed sessions did not break a string of attendance or abstinence, but excused absences were rare and did not differ between treatment conditions, with means (standard deviations [SD]) of 1.3 (2.7) in the SC condition and 1.3 (1.9) in the CM condition, p > .99. Initially, we examined the impact of treatment condition, clinic, and their interaction on outcomes. Although there was a main effect of clinic on one variable (number of days attended treatment, F (1, 235) = 392.49, p = .03), there was no main effect of clinic on other outcome measures, and in no case was the clinic by treatment condition interaction effect significant (all ps > .40). For ease of interpretation, we therefore present outcome measures collapsed across clinics.
Because continuous attendance and abstinence are impacted by missed groups or samples and treatment drop-out, we also analyzed proportions of expected sessions attended and negative samples submitted. These measures are unaffected by missed sessions or samples, as the denominators consist of the total number of counseling groups in which patients were expected (i.e., prior to withdrawing from treatment) or samples submitted.
Survival analysis using the Kaplan-Meier-Breslow model evaluated differences between treatment conditions in terms of days until discharge from the clinic. Data were censored at day 84, reflecting the maximal time in study treatment.
Logistic regression identified predictors of a negative toxicology screen at the most distal 12-month follow-up evaluation. In step 1, age, gender, baseline urine toxicology result and clinic were independent variables; gender and clinic were included as categorical variables and age as a continuous variable. In step 2, treatment condition and longest duration of abstinence (LDA) were entered. Because not all patients completed the follow-up, analyses were conducted twice—initially excluding non-followed up patients, and then including them as positive.
Additional exploratory analyses assessed differences between treatment conditions with respect to group cohesion, therapeutic alliance, and HIV risk behaviors. Again, none of these scores differed by clinic, so data are collapsed across clinic. For cohesion and alliance measures, independent t-tests evaluated treatment condition differences at the three time points at which these constructs were assessed. In addition, we conducted correlations between process measures obtained at Week 1 (when most data were available) and primary attendance and drug use outcomes. HIV risk scores were highly positively skewed and could not be normalized even with transformations, so we evaluated change scores. Baseline scores were subtracted from post-baseline scores to derive change scores, such that negative scores reflect decreased risk behaviors relative to pre-treatment. Changes scores were normally distributed, and independent t-tests evaluated between differences between conditions on these indices. All analyses were performed on SPSS for Windows (version 15), and we considered two-tailed alphas < 0.05 to be significant.
Results
Demographic and baseline characteristics
Table 1 shows baseline characteristics for patients randomized to each treatment condition. No significant differences between treatment conditions occurred on any variables.
Table 1.
Demographic and baseline characteristics
| Variable | Standard care | CM | Significance test value (df) | p-value |
|---|---|---|---|---|
| 117 | ||||
| Clinic, % (n) | χ2(2)=0.14 | .70 | ||
| A | 27.9 (34) | 25.6 (30) | ||
| B | 72.1 (88) | 74.4 (87) | ||
| Age | 38.0 ± 11.4 | 37.3 ± 10.9 | t (237) = 0.50 | .62 |
| Male, % (n) | 54.1 (66) | 59.8 (70) | χ2(1) = 0.80 | .37 |
| Years of education | 12.3 ± 2.0 | 12.8 ± 2.1 | t (237) = −1.82 | .07 |
| Currently married, % (n) | 14.0 (17) | 11.2 (13) | χ2(1) = 0.43 | .51 |
| Employed full-time, % (n) | 42.6 (52) | 50.4 (59) | χ2(1) = 1.46 | .23 |
| Past year income | $16,307 ± 24,605 | $12,841± 15,532 | t (235) = 1.29 | .20 |
| Ethnicity, % (n) | χ2(3) = 1.57 | .67 | ||
| African American | 32.8 (40) | 28.2 (33) | ||
| European American | 55.7 (68) | 57.3 (67) | ||
| Hispanic American | 9.0 (11) | 9.4 (11) | ||
| Other | 2.5 (3) | 5.1 (6) | ||
| Cocaine dependent, % (n) | 62.3 (76) | 66.7 (78) | χ2(1) = 0.50 | .48 |
| Opioid dependent, % (n) | 31.1 (38) | 29.1 (34) | χ2(1) = 0.12 | .73 |
| Alcohol dependent, % (n) | 58.2 (71) | 57.3 (67) | χ2(1) = 0.02 | .88 |
| Addiction Severity Index Scores | ||||
| Medical | 0.24 ± 0.35 | 0.24 ± 0.34 | t (237) = −0.06 | .95 |
| Employment | 0.65 ± 0.30 | 0.66 ± 0.28 | t (237) = −0.28 | .78 |
| Alcohol | 0.25 ± 0.26 | 0.21 ± 0.23 | t (237) = 1.45 | .15 |
| Drug | 0.14 ± 0.11 | 0.14 ± 0.12 | t (237) = 0.22 | .82 |
| Legal | 0.16 ± 0.22 | 0.14 ± 0.22 | t (237) = 0.78 | .44 |
| Family/social | 0.25 ± 0.21 | 0.23 ± 0.18 | t (237) = 0.82 | .41 |
| Psychiatric | 0.25 ± 0.25 | 0.27 ± 0.22 | t (237) = −0.51 | .61 |
Values are means and standard deviations unless otherwise noted. CM=contingency management
Attendance and abstinence during treatment
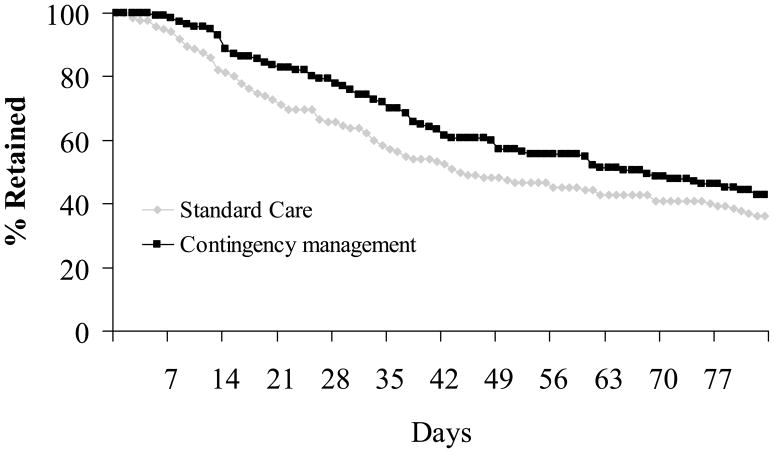
Table 2 shows attendance variables: mean days in which all group counseling sessions were attended, longest period of consecutive group attendance, and percent of expected groups attended. For all variables, patients assigned to the CM condition evidenced significantly greater attendance than those assigned to SC. Figure 2 depicts clinic retention data throughout the 84 day study treatment period. Survival analyses revealed that patients assigned to CM remained in treatment significantly longer than those assigned to SC, χ2 (2) =3.75, p = .05.
Table 2.
Attendance and abstinence outcomes
| Variable | Standard care (n = 122) | CM (n = 117) | Significance test Value (df), p-value | Effect size Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| Days attended treatment | 14.7 ± 9.6 | 17.0 ± 8.9 | t (237) = 1.93, p =.05 | 0.25 | |||
| Continuous weeks of attendance | 4.1 + 3.7 | 5.7 + 4.3 | t (237) = 3.17, p =.002 | 0.40 | |||
| Proportion of expected days attended treatment | 77.2 ± 26.7 | 83.8 ± 23.1 | t (237) = 2.04, p =.05 | 0.26 | |||
| Longest duration of abstinence from all reinforced substances (in weeks) | 4.1 ± 3.6 | 5.3 ± 4.2 | t (237) = 2.47, p =.02 | 0.31 | |||
| Cocaine | 4.3 ± 3.7 | 5.8 ± 4.3 | t (237) = 2.72, p =.01 | 0.37 | |||
| Methamphetamine | 5.1 ± 3.8 | 6.4 ± 3.9 | t (237) = 2.57, p =.02 | 0.34 | |||
| Opioids | 4.8 ± 3.8 | 6.2 ± 4.0 | t (237) = 2.72, p =.01 | 0.39 | |||
| Alcohol | 4.8 ± 3.7 | 6.4 ± 4.0 | t (237) = 3.13, p =.01 | 0.42 | |||
| Marijuanaa | 4.9 ± 3.8 | 6.1 ± 4.1 | t (237) = 2.27, p =.03 | 0.30 | |||
| Proportion of samples negative from all reinforced substances | 79.6 ± 30.0 | 83.6 ± 27.2 | t (237) = 1.06, p = .29 | 0.14 | |||
| Cocaine | 84.7 ± 27.8 | 87.0 ± 25.4 | t (237) = 0.68, p =.50 | 0.09 | |||
| Methamphetamine | 97.8 ± 8.1 | 98.7 ± 7.2 | t (237) = 0.92, p =.36 | 0.12 | |||
| Opioids | 91.8 ± 21.8 | 95.4 ± 14.4 | t (237) = 1.51, p =.13 | 0.19 | |||
| Alcohol | 96.1 ± 10.4 | 99.3 ± 3.5 | t (237) = 1.06, p =.29 | 0.41 | |||
| Marijuanaa | 94.3 ± 17.7 | 92.4 ± 23.5 | t (237) = −0.71, p =.48 | −0.09 | |||
Values represent means and standard deviations.
Marijuana abstinence was not reinforced.
CM=contingency management
Figure 2.
Survival analysis of retention in treatment. Treatment conditions differ, p = .05
Substance use outcomes were longest duration of continuous abstinence and percentages of negative samples submitted. Table 2 presents these outcomes for the four targeted substances concurrently (i.e., abstinence from cocaine, methamphetamine, opioids and alcohol) and for each substance separately. Results from marijuana testing are also presented, even though abstinence from marijuana was not reinforced. Although total number of samples collected did not differ significantly between treatment conditions (means (SD) of 13.9 (6.8) and 15.4 (7.0) for SC and CM, respectively, t (237) = 1.72, p = .09), patients assigned to CM achieved significantly longer durations of abstinence from all reinforced substances concurrently, and each substance independently, than patients assigned to SC. Proportions of negative samples submitted were high overall, and did not vary between conditions.
Post-treatment abstinence
At the Month 12 evaluation, 70.1% of patients assigned to SC provided a sample negative for cocaine, methamphetamine, opioids and alcohol, compared with 69.4% of patients assigned to CM. In evaluating predictors of abstinence at the most distal follow-up, baseline characteristics and clinic were not significant, ps > .18. Step 2 was significant, χ2 (2) = 5.68, p = 0.05, and 73.0% of cases were correctly identified. LDA achieved during treatment was a significant predictor of abstinence, Beta (B) = 0.11, Standard error (SE) = 0.05, Wald = 5.31, p = .02. The odds ratio (OR) was 1.12, 95% confidence interval (CI) = 1.02 – 1.22, indicating that each additional week of abstinence achieved during treatment was associated with a 12% increased probability of abstinence at month 12.
Results were similar when patients who failed to provide a urine sample at month 12 were included in the analysis as having relapsed. Step 1 was not significantly associated with abstinence, p > .26, but Step 2 was, χ2 (2, N = 239) = 13.69, p < 0.001, with 61.9% of cases correctly identified. Again, LDA was the only significant predictor of abstinence at month 12, with Beta (SE) = 0.13 (0.04), Wald = 11.60, p <. 001, OR (95% CI) = 1.14 (1.06 – 1.23).
Group process measures
Table 3 shows scores on the group cohesion and therapeutic alliance questionnaires. After just a single week in treatment, patients assigned to CM reported significantly greater group cohesion and alliances than patients assigned to SC. These differences between treatment conditions remained at the Month 1 assessment period. By Month 2, with less than a third of the original sample still attending treatment, neither measure differed significantly, but effect sizes for the alliance questionnaire were in the range of those obtained at earlier time points.
Table 3.
Group cohesion and helping alliance scores
| Variable | Standard care | CM | Significance test value (df), p-value | Effect size Cohen’s d |
|---|---|---|---|---|
| Week 1 | n = 89 | n = 87 | ||
| Group cohesion | 114.3 ± 17.0 | 119.5 ± 15.1 | t (174) = 2.11, p < .04 | .32 |
| Helping alliance | 88.7 ± 13.5 | 94.4 ± 12.2 | t (175) = 2.93, p < .01 | .44 |
| Month 1 | n = 73 | n = 84 | ||
| Group cohesion | 113.5 ± 14.6 | 120.6 ± 17.5 | t (155) = 2.73, p < .01 | .44 |
| Helping alliance | 87.3 ± 17.3 | 96.7 ± 13.0 | t (149) = 3.78, p < .001 | .61 |
| Month 2 | n = 34 | n = 42 | ||
| Group cohesion | 114.4 ± 18.1 | 118.7 ± 17.3 | t (73) = 1.04, p = .30 | .24 |
| Helping alliance | 89.5 ± 13.0 | 95.4 ± 15.1 | t (71) = 1.77, p = .08 | .42 |
Note: Sample sizes are based on patients remaining in group treatment session at each time point and completing the questionnaires; degrees of freedom differ at time points based on some incomplete assessments. CM=contingency management
Scores on the Group Cohesion Questionnaire at Week 1 (n = 176) correlated significantly with primary outcomes, including days attended treatment (r = 0.16, p < .05), longest period of consecutive attendance (r = 0.23, p <.01), longest duration of abstinence (r = 0.20, p < .01), and proportion of negative samples submitted (r = 0.17, p <.05). Scores on the Helping Alliance Questionnaire were not significantly associated with outcomes (ps > .05).
HIV risk behaviors
Table 4 presents actual HRBS scores at baseline and change scores at follow-up assessments relative to baseline. Patients assigned to the CM condition showed no difference from patients assigned to SC in HIV risk behaviors at baseline. The mean total score was about 4, with slightly less than half the sample reporting intravenous drug use in the 3 months before entering treatment; mean number of sexual partners in that timeframe was 1, but condoms were “never” used by nearly half the sample. At the Month 1 assessment, change scores were significantly lower in CM relative to SC patients. There were no differences in change scores at the post-treatment assessment, but differences between treatment conditions were significant at the Month 12 follow-up. SC patients, on average, increased risk behaviors in the last three months of the follow-up period relative to the three months before treatment. CM patients, in contrast, showed reductions in overall and sexual risk behaviors compared to baseline rates.
Table 4.
HIV risk behaviors
| Variable | Standard care | CM | Significance test value (df), p-value | Effect size Cohen’s d |
|---|---|---|---|---|
| Baseline Past 3-month Total Score | 3.8 ± 4.6 | 4.4 ± 4.1 | t (235) = 0.98, p =.33 | |
| Drug score | 0.5 ± 2.2 | 0.3 ± 1.8 | t (235) = 0.81, p =.42 | |
| Sexual score | 3.3 ± 3.8 | 4.0 ± 3.8 | t (235) = 1.54, p =.12 | |
| Month 1 Total Change Score | −0.1 ± 3.7 | −1.2 ± 3.9 | t (225) = 2.35, p < .02 | 0.29 |
| Drug change score | 0.1 ± 1.5 | −0.3 ± 1.5 | t (225) = 1.17, p = .24 | 0.20 |
| Sexual change score | 0.0 ± 3.3 | −1.0 ± 3.7 | t (225) = 2.07, p < .04 | 0.29 |
| Month 3 Total Change Score | 0.1 ± 5.2 | 0.2 ± 5.0 | t (217) = −0.08, p = .94 | −0.02 |
| Drug change score | 0.1 ± 2.6 | −0.1 ± 2.1 | t (217) = 0.63, p = .53 | 0.08 |
| Sexual change score | 0.1 ± 3.9 | 0.3 ± 4.4 | t (217) = −0.46, p = .65 | −0.05 |
| Month 12 Total Change Score | 1.6± 6.3 | −0.3 ± 5.0 | t (211) = 2.49, p < .02 | |
| Drug change score | 0.3± 3.4 | −0.1 ± 2.4 | t (211) = 0.96, p = .34 | 0.13 |
| Sexual change score | 1.3 ± 4.4 | −0.3 ± 4.3 | t (211) = 2.55, p < .01 | 0.36 |
Notes: Change score values represent means and standard deviations of change scores, calculated by subtracting baseline past 3-month scores from during or post-treatment scores, such that negative scores represent reductions and positive scores reflect increases in risk behaviors. Note that Month 1 change scores represent scores reflecting risk behaviors from the past 1 month minus baseline risk behaviors assessed over the past 3 months. CM=contingency management.
Evaluation of specific HRBS items revealed change scores on one item of the HRBS differed significantly between conditions at the Month 1 evaluation. Patients in the CM condition were more likely to increase use of condoms with regular partners relative to patients in SC, t (225) = 2.02, p = .04, with 24.5% of CM patients versus 16.2% of SC patients reporting more condom use with regular partners at Month 1 relative to baseline. There were no statistically significant differences on change scores of individual HRBS items at Month 12. However, between months 9 and 12 of follow-up relative to the three-month period before baseline, 19.7% of CM patients reported reductions in number of sexual partners while 36.9% of standard care patients reported increases in number of sexual partners, with t (211) = 1.81, p = .07 for change scores on this item. In terms of frequency of condom use, 25.5% and 15.4% of patients assigned to CM reported greater use of condoms with regular and casual sexual partners, respectively, in the follow-up relative to baseline period. On the other hand, 33.0% and 23.3% of patients assigned to SC reported reductions in condom usage with regular and casual partners, respectively, with change scores of t (211) = 1.81 and 1.85, ps < .07, respectively.
Reinforcement earned and adverse events
Patients assigned to the CM condition had their names drawn enough times to earn a median (interquartile range) of 31.0 (46.5) prize drawings over the course of the study, resulting in a median (interquartile range) cost of $160 ($272) per CM patient. There were no study-related adverse events reported, and no patients experienced increases in gambling.
Discussion
This CM treatment delivered in a group context engendered statistically significant benefits for group counseling attendance and drug use outcomes. In terms of attendance, patients randomly assigned to CM attended about two more group counseling sessions on average, which resulted in an additional 1.6 weeks of continuous attendance, and they participated in 6.6% more days of assigned groups than their counterparts assigned to SC. Survival analyses likewise revealed greater retention in the CM compared to SC condition. Further, durations of abstinence from all reinforced substances together, as well as each substance individually, was higher in the CM condition relative to the SC condition. Other CM studies that reinforce patients individually tend to have mixed effects with respect to improving attendance (Petry, Alessi et al., 2006; Petry, Alessi et al., 2005; Petry, Peirce et al., 2005; Petry et al., 2004). In part, differences may relate to targets of reinforcement. In individually administered CM, submission of negative samples is reinforced, and hence it is not surprising that a non-targeted behavior (therapy attendance) does not always increase. This CM treatment reinforced abstinence and attendance, and benefits were observed for both outcomes. Results for abstinence outcomes mirror those from CM studies that deliver reinforcement on an individual basis (Petry, Alessi, et al., 2006; Petry, Alessi et al., 2005; Petry, Peirce et al., 2005; Petry et al., 2004), and they demonstrate that reinforcement provided in the context of group therapy appears to be comparably beneficial to individually-based CM approaches, with a similar overall effect size as noted in Lussier et al.’s (2006) meta-analysis.
Although when delivered in the context of group therapy CM had significant benefits for increasing durations of abstinence achieved, it did not impact proportions of negative samples submitted. These results are similar to those noted in individually-based CM interventions conducted in outpatient, non-methadone settings (Petry, Alessi, Carroll, Hanson, McKinnon et al., 2006; Petry, Alessi et al., 2005; Petry, Peirce et al., 2005). High rates of abstinence while patients attend outpatient treatment (>80%) may result in a ceiling effect.
As in other studies of CM (Carpenedo, Kirby, Dugosh, Rosenwasser, & Thompson, 2010; Higgins, Badger, & Budney, 2000; Petry, Alessi, et al., 2006; Petry, Alessi, Hanson, & Sierra, 2007; Petry, Alessi et al., 2005; Petry, Martin, & Simcic, 2005; Petry, Weinstock et al., 2010), the longest duration of abstinence achieved during treatment was significantly and consistently related to long-term abstinence. Each week of abstinence achieved during treatment was associated with a 12% increased probability of abstinence at the one-year follow-up even after controlling for other variables that may impact long-term abstinence. These data point to the importance of encouraging uninterrupted strings of abstinence during treatment (Roll, Higgins, & Badger, 1996), and effective CM reinforcement schedules such as that used in this study provide increased reinforcement for sustained behavior changes.
Nevertheless, treatment condition was not significantly associated with long-term abstinence. Some studies find long-term effects of CM on abstinence (Higgins et al., 2007; Higgins et al., 2003; Iguchi, Belding, Morral, & Lamb, 1997; Kosten et al., 2003; Petry & Martin, 2002; Petry, Martin et al., 2005), while others do not (Petry, Alessi et al., 2005; Rawson et al., 2002; Rawson et al., 2006). Improved methods are needed to extend the benefits of CM or increase durations of abstinence achieved during treatment, as LDA is a consistent predictor of long-term abstinence (Higgins, Badger et al., 2000; Petry et al., 2007; Petry, Alessi et al., 2005; Petry, Martin et al., 2005; Petry, Weinstock et al., 2010). Cognitive-behavioral therapy alone or in combination with CM may result in persisting benefits (Carroll et al., 1994; Epstein, Hawkins, Covi, Umbricht, & Preston, 2003; McKay et al., 2010; Rawson et al., 2002; Rawson et al., 2006). More research is needed on interventions that extend CM’s benefits and investigate mechanisms by which LDA is associated with long-term abstinence.
This is the first CM study to examine therapeutic processes, and it shows that group cohesion and alliance improve during CM relative to SC treatment, and these benefits were noted very early. Further, group cohesion was significantly correlated with during treatment attendance and substance use outcomes. Although treatment condition differences in process indices were no longer statistically significant at the most distal assessment period, the lack of significance may relate to decreasing sample sizes, as cohesion and alliance were measured only among patients who remained in treatment. Future studies should attempt to assess cohesion and alliance more regularly and soon after drop-out occurs to ascertain whether such indices mediate attendance and drug use outcomes. The present analyses, while indicating significant differences at some time points, should be interpreted with caution as corrections for multiple testing were not made. Moreover, this study did not evaluate therapists’ assessment of alliance or job satisfaction indices, but inclusion of such measures may reveal additional benefits of CM delivered in the context of group therapy, especially from providers’ perspectives. This study did not assess differences in patients’ alliances or cohesion across therapists, which may impact outcomes (e.g., Hovarth & Symonds, 1991). Therapists and research staff did note informally that patients in the CM condition enjoyed the reinforcement procedures, and camaraderie appeared to develop in CM group counseling sessions. More systematic evaluation of CM and its impact on therapeutic processes may lead to further improvements on abstinence outcomes.
This study, similarly to those conducted in methadone maintenance samples (DeFulio et al., 2009; Ghitza et al., 2008; Hanson et al., 2008), revealed potential benefits of CM on reducing HIV risk behaviors, primarily sexual risk behaviors. Risk behaviors decreased early in treatment and at the most distal follow-up in patients receiving CM relative to those receiving SC, but not at the Month 3 assessment period. The reasons for inconsistent effects over time are unclear. CM does not explicitly address HIV sexual risk reduction, but perhaps greater psychosocial stability associated with more participation in treatment and reduced drug use results in less impulsive decision making with respect to the number of sexual partners and use of protection during sexual activity (Black, Serowik, & Rosen, 2009; Semple, Zians, Grant, & Patterson, 2006). Although the mechanisms by which CM reduces sexual risk behaviors are unknown and only one significant difference was noted with respect to change scores on specific items, these data suggest a public health benefit of CM, especially because substance abusers are a population at high risk for contracting and spreading HIV and other infectious diseases (Metzger et al., 1993).
Results from this study should be interpreted in the context of some issues related to the study design. The study was conducted in community clinics, but only two New England clinics were represented. These effects may not generalize to other areas of the country where access to and services provided in outpatient substance abuse clinics may differ from those herein. In addition, data were not collected with respect to individual therapist effects or changes in group composition over time, which may have impacted outcomes. Although rates of follow-up were high, a conservative approach was taken for handling missing toxicology data, and results were similar between analyses. Nevertheless, the follow-up data may be biased due to non-completion, and even longer term follow-up results would be preferable.
Despite these limitations, this study is important for moving CM closer into the hands of clinicians. It not only confirmed and extended benefits of CM treatments, but it also directly addressed issues of central importance to the dissemination and use of CM in practice settings (Kirby et al., 2006). Many clinicians express concern about reinforcing abstinence from only a single drug at a time, yet most CM studies have reinforced abstinence from just one substance. Effect size estimates from meta-analyses (Lussier et al., 2006) confirm that CM is more efficacious when one drug at a time is reinforced. Nevertheless, this study demonstrated that abstinence from multiple substances can be simultaneously reinforced using this CM approach and beneficial effects achieved. A related concern about CM is that reinforcing abstinence from particular substances may result in symptom substitution and increases in use of non-reinforced substances (Kadden, Litt, Kabela-Cormier, & Petry, 2009). Because of the long half-life of marijuana metabolites, the CM condition in this study did not reinforce abstinence from marijuana, yet no evidence for increases in marijuana usage was noted. To the contrary, longest duration of abstinence from marijuana significantly increased with this CM treatment that did not reinforce marijuana abstinence. These data provide further evidence that CM does not have adverse effects in terms of increasing other drug use behavior (Kadden et al., 2009). Further, application of CM among patients with a wide range of substance use problems with broad inclusion and few exclusion criteria enhances generalization of this CM intervention to patients most often treated in community-based settings.
Importantly, this study is one of the first randomized studies to evaluate CM when delivered entirely in the context of group counseling sessions. As such, it represents an important step toward bringing CM into the hands of providers, who rarely provide individual treatment to patients in community settings. Effect sizes were similar to those noted with individually-delivered CM approaches (Lussier et al., 2006; Prendergast et al., 2006), suggesting that an approach that applies CM during group counseling sessions may be equally efficacious.
The magnitude of reinforcement earned in this study was similar to that used in individually-based prize CM protocols, but it may be too high for clinics to consider supporting given resource constraints and lack of insurer coverage for CM. In addition, the time required to collect and screen samples from patients is a barrier to CM administration (Kirby et al., 2006), and in the present study, research assistants, rather than clinical staff, collected urine samples and monitored protocol adherence. Costs of reinforcement and onsite toxicology screening, as well as clinician time, are therefore two obstacles to CM delivery in practice.
Recent data (Petry, Barry, Alessi, Rounsaville, & Carroll, under review) reveal that reinforcing attendance alone (on an individual basis) can be equally efficacious to reinforcing abstinence among patients who initiate treatment while abstinent. Thus, subsequent studies should evaluate effects of CM delivered solely in the contexts of groups on the basis of counseling attendance, as this procedure would eliminate the need to frequently collect and screen urine samples. Interestingly, other CM studies that reinforced attendance in group counseling sessions (Ledgerwood et al., 2008; Petry et al., 2001) were able to realize benefits at much lower reinforcement magnitudes than those provided herein. For example, using the name-in-the-hat prize CM procedure reinforcing group attendance (Ledgerwood et al., 2008; Petry et al., 2001), overall costs can be as low as $20 per group counseling session per week, a cost that is divided among all the patients in a group. If such an approach is efficacious in enhancing attendance at treatment using fully randomized designs, and at the same time improves substance use outcomes, two primary barriers to CM administration (costs of reinforcers and time and direct costs of frequent sample screening) will be overcome.
In summary, this study found that a CM approach delivered in the context of group counseling is efficacious in improving attendance at group therapy sessions and lengthening abstinence. This CM treatment also enhanced group cohesion and the therapeutic alliance, which can have benefits for both patients and providers, and it reduced HIV risk behaviors, an effect that speaks to potential societal benefits of CM. Economic analyses are beginning to address conditions under which CM is most cost-effective (Olmstead & Petry, 2009; Olmstead, Sindelar, & Petry, 2007a, 2007b; Sindelar, Elbel, & Petry, 2007; Sindelar, Olmstead, & Peirce, 2007). Future large-scale analyses may ultimately demonstrate the cost-effectiveness and cost-benefits of CM, which should further stimulate interest in adoption of CM. Importantly, with appropriate training and oversight, community-based providers can successfully administer CM (Ledgerwood et al., 2008; Petry, Alessi, Ledgerwood, & Sierra, 2010). Additional research and dissemination of this efficacious intervention is warranted.
Acknowledgments
We thank the staff and patients at participating clinics for their support of and participation in this project. This research and preparation of this report were funded by NIH grant R01-DA018883, P30-DA023918, R01-DA027615, R01-DA022739, R01-DA13444, R01-DA016855, R01-DA14618, P50-DA09241, P60-AA03510, R01-DA024667, and General Clinical Research Center Grant M01-RR06192.
References
- Alessi SM, Hanson T, Tardif M, Petry NM. Low-cost contingency management in community substance abuse treatment settings: A transition to delivering incentives in group therapy. Experimental and Clinical Psychopharmacology. 2007;15:293–300. doi: 10.1037/1064-1297.15.3.293. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Barlow DH. Healthcare policy, psychotherapy research, and the future of psychotherapy. American Psychologist. 1996;51:1050–1058. doi: 10.1037//0003-066x.51.10.1050. [DOI] [PubMed] [Google Scholar]
- Black RA, Serowik KL, Rosen MI. Associations between impulsivity and high risk sexual behaviors in dually diagnosed outpatients. American Journal of Drug and Alcohol Abuse. 2009;35:325–328. doi: 10.1080/00952990903075034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovasso GB, Alterman AI, Cacciloa JS, Cook TG. Predictive validity of the Addiction Severity Index's composite scores in the assessment of 2-year outcomes in a methadone maintenance population. Psychology of Addictive Behaviors. 2001;15:171–176. [PubMed] [Google Scholar]
- Carpenedo CM, Kirby KC, Dugosh KL, Rosenwasser BJ, Thompson DL. Extended voucher based reinforcement therapy for long-term drug abstinence. American Journal of Health Behavior. 2010;34:776–787. doi: 10.5993/ajhb.34.6.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon L, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- DeFulio A, Donlin WD, Wong CJ, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: A randomized controlled trial. Addiction. 2009;104:1530–1528. doi: 10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behavior among intravenous drug users. AIDS. 1991;5:181–185. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Publishing; 1996. [Google Scholar]
- Ghitza UE, Epstein DH, Preston KL. Contingency management reduces injection-related HIV risk behaviors in heroin and cocaine using outpatients. Addictive Behaviors. 2008;33:593–604. doi: 10.1016/j.addbeh.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug and Alcohol Dependence. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Hanson T, Alessi SM, Petry NM. Contingency management reduces drug-related human immunodeficiency virus risk behaviors in cocaine-abusing methadone patients. Addiction. 2008;103:1187–1197. doi: 10.1111/j.1360-0443.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger G, Budney A. Initial abstinence and success in achieving longer termcocaineabstinence. Experimental &Clinical Psychopharmacology. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona RL, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, et al. Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increase cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Horvath AO, Symonds BD. Relation between working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38:139–149. [Google Scholar]
- Iguchi M, Belding M, Morral A, Lamb R. Reinforcing operants other than abstinence in drug abuse treatment. Journal of Consulting and Clinical Psychology. 1997;65:421–428. doi: 10.1037//0022-006x.65.3.421. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Increased drinking in a trial of treatments for marijuana dependence: Substance substitution? Drug and Alcohol Dependence. 2009;105:168–171. doi: 10.1016/j.drugalcdep.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JL, Petry NM. HIV risk behaviors in male substance abusers with and without antisocial personality disorder. Journal of Substance Abuse Treatment. 2000;19:59–66. doi: 10.1016/s0740-5472(99)00100-2. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, Burns M, Coleman P, Stitzer M, Wale JB, Kreek MJ. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. Journal of Substance Abuse Treatment. 2005;28:57–65. doi: 10.1016/j.jsat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers' beliefs and objections regarding contingency management: Implications for dissemination. Drug and Alcohol Dependence. 2006;85:19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Kerwin ME, Carpendo CM, Rosenwasser BJ, Gardner RS. Interdependent contingency management for cocaine-dependent methadone maintenance patients. Journal of Applied Behavior Analysis. 2008;41:579–595. doi: 10.1901/jaba.2008.41-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug and Alcohol Dependence. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Concurrent validity of the Addiction Severity Index. Journal of Nervous and Mental Disease. 1983;171:606–610. doi: 10.1097/00005053-198310000-00003. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Hanson T, Godley M, Petry NM. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. Journal of Applied Behavior Analysis. 2008;41:617–622. doi: 10.1901/jaba.2008.41-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C, Mulvey K, Gastfriend DR, Schwartz M. Addiction Severity Index: A field study of internal consistency and validity. Journal of Substance Abuse Treatment. 2000;18:129–135. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug and Alcohol Dependence. 2009;102:162–165. doi: 10.1016/j.drugalcdep.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Luborsky L, Barber JP, Siqueland L, Johnson S, Najavits L. The revised helping alliance questionnaire (HAq-II): Psychometric properties. Journal of Psychotherapeutic Practice and Research. 1996;5:260–271. [PMC free article] [PubMed] [Google Scholar]
- Lumley T, Diehr P, Emerson S, Chen L. The importance of normality assumption in large public health datasets. Annual Review of Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McKay JR, Lynch KG, Coviello D, Morrison R, Cary MS, Skalina L, et al. Randomized trial of continuing care enhancements for cocaine-dependent patients following initial engagement. Journal of Consulting & Clinical Psychology. 2010;78:111–120. doi: 10.1037/a0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciloa J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Metzger D, Woody GE, McLellan AT, O'Brien CP, Druley P, Navaline H, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: An 18-month prospective follow-up. Journal of Acquired Immune Deficiency Syndromes. 1993;6:1049–1056. [PubMed] [Google Scholar]
- Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine- or opioid-dependent outpatients. Drug and Alcohol Dependence. 2009;102:108–115. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Petry NM. Clinic variation in the cost-effectiveness of contingency management. American Journal on Addictions. 2007a;16:457–460. doi: 10.1080/10550490701643062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Petry NM. Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug and Alcohol Dependence. 2007b;87:175–182. doi: 10.1016/j.drugalcdep.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Reliability of drug users' self-reported HIV risk behaviors using a brief, 11-item scale. Substance Use and Misuse. 2001;36:1731–1747. doi: 10.1081/ja-100107576. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology. 2006;74:592–601. doi: 10.1037/0022-006X.74.3.592. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, McKinnon S, Rounsaville B, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology. 2006;74:592–601. doi: 10.1037/0022-006X.74.3.592. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology. 2007;75:983–991. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the contingency management competence scale. Drug and Alcohol Dependence. 2010;109:167–174. doi: 10.1016/j.drugalcdep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. doi: 10.1037/a0026883. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Therapeutic alliance and psychiatric severity as predictors of completion of treatment for opioid dependence. Psychiatric Services. 1999;50:219–227. doi: 10.1176/ps.50.2.219. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, et al. Prize-based contingency management does not increase gambling: Results of the National Drug Abuse Treatment Clinical Trials Network multi-site study. Drug and Alcohol Dependence. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler H. Give them prizes and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Finocche C. Contingency management in a group treatment: A demonstration project in an HIV drop-in program. Journal of Substance Abuse Treatment. 2001;21:80–96. doi: 10.1016/s0740-5472(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F. Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Weinstock J, Alessi SM, Lewis MW, Dieckhaus K. Group-based randomized trial of contingencies for health and abstinence in HIV patients. Journal of Consulting and Clinical Psychology. 2010;78:89–97. doi: 10.1037/a0016778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann MJ, Shoptaw S, Farabee D, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Archives of General Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, et al. Comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:309–316. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Grant I, Patterson TL. Methamphetamine use, impulsivity and sexual risk behavior among HIV-positive mean who have sex with men. Journal of Addictive Diseases. 2006;25:105–114. doi: 10.1300/J069v25n04_10. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone maintenance patients. Journal of Substance Abuse Treatment. 2005;29:253–258. doi: 10.1016/j.jsat.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction. 2007;102:309–316. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Olmstead TA, Peirce JM. Cost-effectiveness of prize-based contingency management in methadone maintenance treatment programs. Addiction. 2007;102:1463–1471. doi: 10.1111/j.1360-0443.2007.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balance distributions of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Office of Applied Studies. Treatment Episode Data Set (TEDS) Rockville, MD: DHHS; 2009. Highlights - 2007. National Admissions to Substance Abuse Treatment Services. (Vol. DHHS Publication No. (SMA) 09-4360 ) [Google Scholar]
- van Andel P, Erdman RA, Karsdorp PA, Appels A, Trijsburg RW. Group cohesion and working alliance: Prediction of treatment outcome in cardiac patients receiving cognitive behavioral group psychotherapy. Psychotherapy and Psychosomatics. 2003;72:141–149. doi: 10.1159/000069733. [DOI] [PubMed] [Google Scholar]