Abstract
Using a well-established model of binge-like ethanol treatment of rat pups on postnatal days (PD) 4–9, we found that maturation of GABAA receptor (GABAAR) miniature postsynaptic currents (mPSCs) was substantially blunted for medial septum/diagonal band (MS/DB) neurons in brain slices on PD 11–16. Ethanol reduced mPSC amplitude, frequency, and decay kinetics, while attenuating or exaggerating allosteric actions of zolpidem and allopregnanolone, respectively. The impact of ethanol in vivo was long lasting as most changes in MS/DB GABAAR mPSCs were still observed as late as PD 60–85. Maturing MS/DB neurons in naïve brain slices PD 4–16 showed increasing mPSC frequency, decay kinetics, and zolpidem sensitivity that were nearly identical to our earlier findings in cultured septal neurons [17, 18]. These rapidly developing mPSC parameters continued to mature through the first month of life then stabilized throughout the remainder of the lifespan. Finally, equivalent ethanol-induced alterations in GABAAR mPSC signaling were present in MS/DB neurons from both male and female animals. Previously, we showed ethanol treatment of cultured embryonic day 20 septal neurons distorts the maturation of GABAAR mPSCs predicting that early stages of GABAergic transmission in MS/DB neurons are vulnerable to intoxication injury [17, 18]. Since the overall character, timing, and magnitude of GABAergic mPSC developmental- and ethanol-induced changes in the in vivo model so closely mirror chronologically equivalent adaptations in cultured septal neurons, this suggests that such parallel models of ethanol impairment of GABAergic synaptic development in vivo and in vitro should be useful for translational studies exploring the efficacy and mechanism of action of potential therapeutic interventions from the cellular to whole animal level.
Keywords: Fetal alcohol spectrum disorder, GABAA receptor, Miniature postsynaptic current, Medial septum/diagonal band, Development, Zolpidem, Allopregnanolone
1. INTRODUCTION
The medial septum/diagonal band (MS/DB) is a significant component of the limbic system and plays an important role in theta rhythm as well as regulation of hippocampal spatial learning and memory mechanisms through the cholinergic, GABAergic, and glutamatergic projections of the septo-hippocampal pathway [1, 11, 20, 29, 69]. Ethanol is a teratogen that can cause embryonic death, or in those surviving to term, a range of varying phenotypes including retarded growth, various physical malformations, and/or cognitive deficits now collectively recognized as fetal alcohol spectrum disorders (FASD) [45, 56]. The wide range in predicted prevalence and degree of injury suggest that many offspring who have had some degree of injurious ethanol exposure in utero likely go unidentified [56]. Injury to developing septo-hippocampal neurocircuits may contribute to deficits in spatial learning and memory found both in children with FASD [27] as well as in animal models [24, 48, 58]. How ethanol impairs learning and memory mechanisms requiring the MS/DB and hippocampus is unknown, but in the absence of gross damage to these structures, subtle changes in the timing or strength of synaptic signaling in septo-hippocampal neurocircuits could contribute to cognitive impairment.
Previously we have shown that surface receptors and synapses for the inhibitory neurotransmitter, GABA, on cultured septal or acutely isolated MS/DB neurons show functional abnormalities after daily cycles of intoxication intended to simulate in utero ethanol exposure when the mother is binge drinking late in gestation [17, 18, 34, 35]. Earlier studies have shown that exposure to a brief high peak blood ethanol level during a single episode of drinking can significantly injure the developing brain [23, 25, 41]. In septal cultures, six brief cycles of intoxication distort kinetic parameters and pharmacological sensitivity of GABAAR-mediated miniature postsynaptic currents (mPSCs) days after ethanol exposure ends, suggesting that synapses formed during intoxication are abnormal in this in vitro developmental model [17, 18]. Here, we examined whether GABAergic synaptic injury also occurs in intact rat brain with cyclic intoxication during the brain growth spurt equivalent to that occurring in the human third trimester [15] and which is associated with impaired spatial learning and memory performance [2]. Cortical neurons show remarkably parallel patterns of GABAAR expression and synaptic maturation, whether developing in vivo in intact brain or in vitro in dispersed cultures [19]. This suggests intrinsic, cellular level mechanisms alone may be sufficient to drive normal receptor expression and inhibitory synapse formation at the local level. Previously, we found ethanol exposure of primary septal cultures 6–11 days in vitro (DIV) distorted developing GABAAR-mediated miniature postsynaptic currents (mPSCs) [17] suggesting cellular level mechanisms guiding synapse formation are vulnerable to ethanol. The present results confirm the vulnerability of newly forming GABAergic synapses to intoxication injury in the developing brain as well as demonstrate that these synaptic modifications last into adulthood and are equivalent both in males and females. Furthermore, we establish the remarkable parallels between in vivo MS/DB GABAergic synaptic development and sensitivity to ethanol-induced damage and that modeled in vitro in dispersed septal cultures [17, 18].
2. RESULTS
2.1 Kinetic changes in GABA mPSCs of MS/DB neurons after postnatal ethanol intoxication
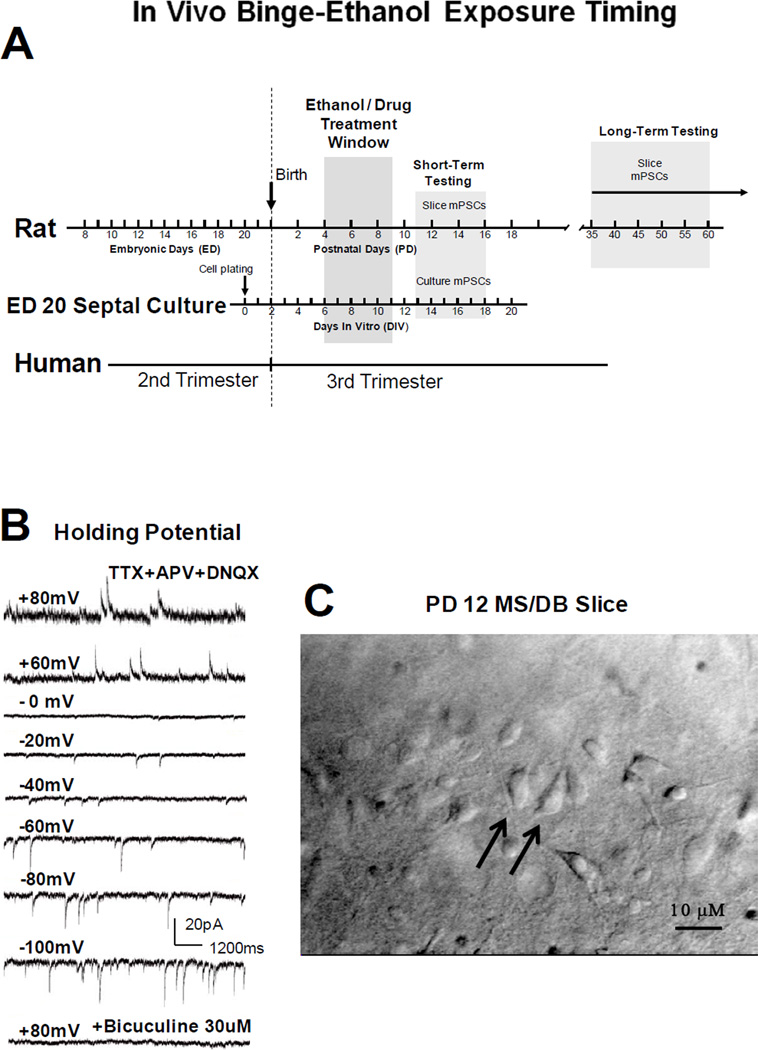
Figure 1A shows the protocol used to determine whether ethanol intoxication distorts GABAergic transmission in the intact animal. GABAAR-mediated mPSCs, representing selective activation of postsynaptic receptors by asynchronous release of neurotransmitter [13, 14], were studied in acutely prepared MS/DB slices on PD 11–16 after treating pups with ethanol on PD 4–9. This ethanol intubation protocol in rat pups chronologically matched the septal cultures treated after DIV 6–11 and tested on DIV 13–18 [17, 18]. Dobbing and Sands, [15] have suggested this period in the rat represents brain development resembling early 3rd trimester in humans. In rodents, this time likely includes a period following generation of MS/DB neurons when septo-hippocampal pathway formation is underway [38, 44, 52, 53]. Figure 1B shows reversal of mPSCs from inward to outward currents near 0 mV as holding potentials were stepped from +80 mV to −100 mV and blockade of currents was achieved using bicuculline (30 µM), consistent with pharmacologically isolated GABAAR-mediated Cl− currents under the recording conditions used. Electrophysiological recordings were performed from MS/DB neurons like those in Figure 1C which were relatively easy to visualize and similar to septal neurons recorded previously in culture [17, 35]. Septal neurons were not identified as cholinergic or non-cholinergic for the purposes of this study. Investigation of septal cell type specific responses to neonatal binge ethanol exposure will be the focus of future studies.
Figure 1. Schematic of binge-like ethanol intoxication timing, voltage-dependent whole cell GABAAR mPSC recordings, and a representative MS/DB rat brain slice.
(A) Relative human and rat brain development based on the ‘brain growth spurt’ concept of Dobbing and Sands [15]. Rats were treated during brain development equivalent to human 3rd trimester and when septo-hippocampal pathway formation is underway. GABAAR mPSCs were recorded after ‘binge ethanol’ in vivo (ethanol on PD 4–9, then slice recording PD 11–16). (B) GABAAR mPSCs reverse near 0 mV and are blocked by bicuculline as expected for a GABAAR-mediated chloride conductance under our recording conditions. (C) PD 12 coronal slice showing diagonal band neurons (40X water immersion, differential interference contrast optics, Olympus BX50WI microscope). Larger, bipolar neurons are indicated by arrows.
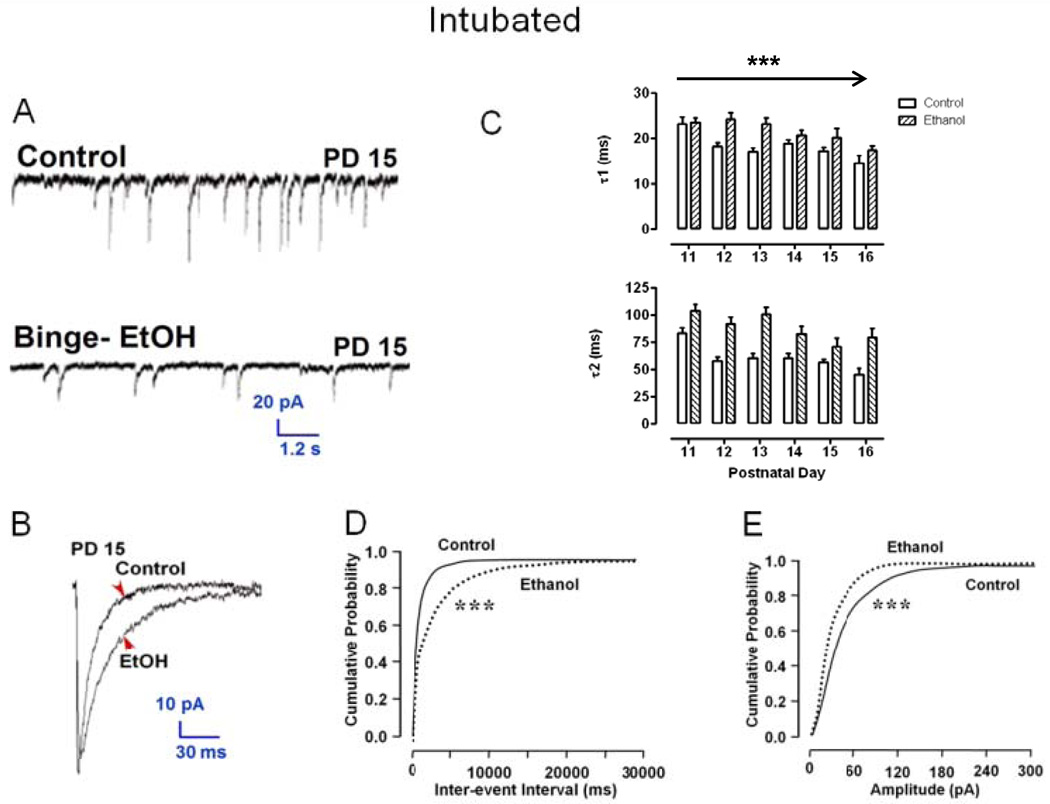
Figure 2A shows GABAAR mPSCs recorded from two representative MS/DB neurons on PD 15 where ethanol exposure blunted synaptic development in vivo. Apparent mPSC frequency and event amplitude were substantially decreased in the Ethanol treated neuron and the decay phase of the ensemble average of all events was slowed relative to Control (Figure 2B). Overall, PD 4–9 intoxication significantly changed kinetic parameters of GABAAR mPSCs recorded in MS/DB neurons on PD 11–16, shifting decay phase bi-exponential time constants, τ1 and τ2, to longer times (Figure 2C), increasing mPSC inter-event intervals (Figure 2D), and shifting mPSC amplitudes toward smaller events (Figure 2E). These changes closely mirror those reported previously in cultured septal neurons [17] suggesting that ethanol may act on the local cellular level to distort GABAergic transmission both in vitro and in vivo.
Figure 2. Postnatal day 4–9 Ethanol exposure reduces GABAAR mPSC amplitude and frequency but prolongs decay in MS/DB neurons recorded on PD 11–16 in brain slices.
(A) Recording from a Control and Ethanol neurons (Binge-EtOH) on PD 15; (B) ensemble averaged mPSCs from neurons in A. (C) Bars represent mean ± S.E.M. time constants, τ1 and τ2 (ms), estimated from the decay phase of ensemble averages for Ethanol (n = 9–30) and Control cells (n = 11–32) across PD 11–16 (ANOVA, ***P <0.0001); (D or E) Represent the range of intervals between mPSCs (ms; D) or the peak mPSC amplitude (pA; E) for events recorded in randomly sampled groups of 20 Ethanol and 20 Control neurons on PD 11–16, respectively (K-S, ***p <0.001).
2.2 Postnatal intoxication in vivo takes place during early synapse formation and refinement
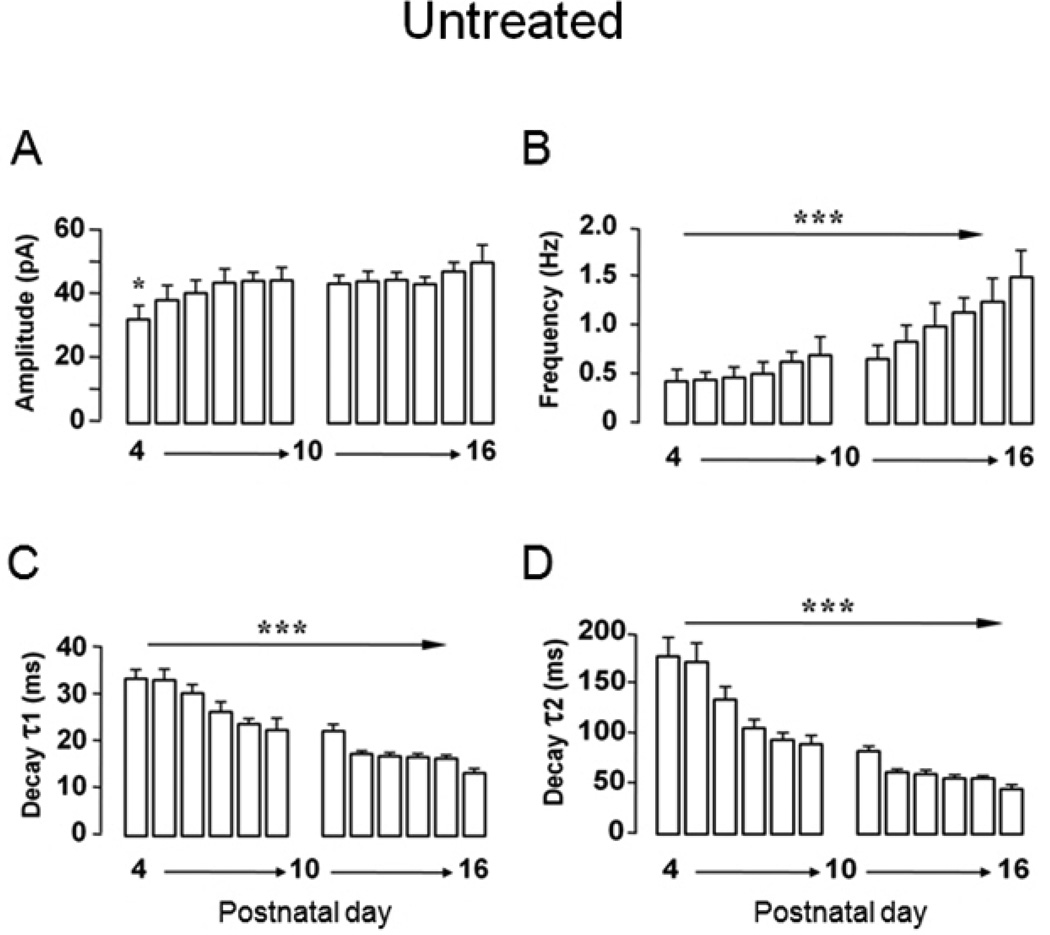
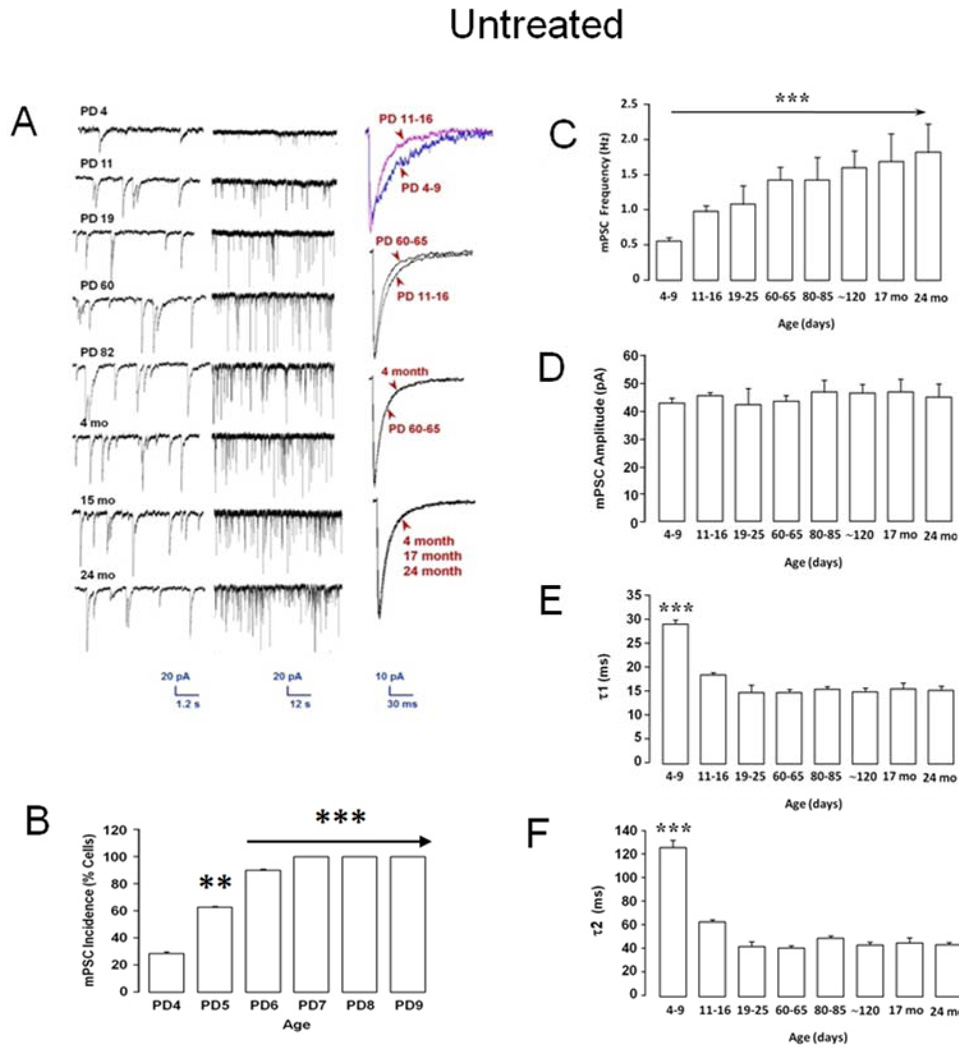
Our septal culture model involves plating and growing embryonic day 20 neurons for 6 days in vitro (DIV 6) before ethanol treatment (DIV 6–11) [17, 35]. This timing intentionally parallels chronologically the in vivo model where rat pups born ∼ED 22 (e.g., PD 0), are intubated with ethanol on PD 4–9 (Figure 1A). Whole cell recordings are also made over an equivalent interval (e.g., DIV 13–18 in cultures or PD 11–16 in MS/DB slices). In septal cultures, GABAAR mPSCs are first detected in 25% of neurons between DIV 0–6, increasing to 100% of cells DIV 7–13 during ethanol exposure (see Figure 1C) [17]. All septal neurons showed robust Cl− currents with exogenously applied GABA indicating abundant receptors on the cell surface as early as DIV 2. In untreated cultures, GABAAR mPSC kinetic parameters substantially accelerated over 28 days (see Figure 4 A&B; and Figure 5) [17], consistent with increasing synapse formation and maturation. Interestingly, early GABAAR mPSCs in MS/DB neurons from untreated rats developed and matured in much the same pattern as in septal cultures (Figure 3A–F). On PD 4, ∼30% of neurons exhibited GABAAR mPSCs (Figure 3B). By PD 7, essentially 100% had GABAAR-mediated mPSCs. Substantial changes in GABAAR mPSC kinetic parameters were evident between the earliest recordings over PD 4–9 and those from MS/DB neurons of aged animals ∼ 24 months (Figure 3A). Mean mPSC frequency, an index related to functional synapse number as well as the rate of presynaptic asynchronous transmitter release [10, 19, 39], rapidly increased 2 fold between PD 4–9 and PD 60–65, but was statistically unchanged thereafter (Figure 3C). Surprisingly, mean mPSC amplitude (∼ 43–45 pA), which is influenced by postsynaptic receptor density, synaptic vesicle GABA content, and dendrite cable properties [10, 50, 54, 55], was consistent across rat lifespan (Figure 3D). Decay time constants, τ1 and τ2, accelerated substantially between PD 4–9 and 19–25, but then stabilized after adulthood through 24 months of age (Figure 3E & F). Since GABAAR mPSC decay reflects various functional states of postsynaptic GABAARs as they respond to the initial impact and subsequent dissipation of the trans-synaptic neurotransmitter wave [3, 10, 30, 50], this suggests that GABA signaling on average, rapidly matures shortly after birth in MS/DB neurons. This conclusion is further strengthened when mPSC kinetics for individual days are examined in Untreated cells (Figure 4). Amplitude showed a small but significant increase initially on PD 4 before stabilizing while a marked increase in event frequency (∼ 2 fold) is delayed until PD 11–16. Acceleration of decay kinetics over PD 4–9 decreases time constants ∼30–45% but the swift decline stabilizes by PD 16 (Figure 4). In absolute terms, GABAAR mPSC development in cultured septal neurons between DIV 0–28 and MS/DB neurons PD 4–25 are surprisingly similar [17]. The most striking exception is the ∼4 fold greater mPSC frequency in cultured septal neurons [17] vs MS/DB neurons (DIV 21–28 = 4.7 ± 2.0 Hz vs PD 19–25 = 1.1 ± 0.3 Hz). For DIV 0–6 septal neurons relative to PD 4–9 MS/DB neurons, mean GABAAR mPSC amplitudes were ∼18% smaller, while decay kinetics (τ1 & τ2) were ∼22% and 5% faster for MS/DB mPSCs. For DIV 21–28 septal neurons relative to PD 19–25 MS/DB neurons, mean GABAAR mPSC amplitudes were ∼16% smaller, while decay kinetics (τ1 & τ2) were ∼7% and 88% faster for MS/DB mPSCs. Taken together, these results indicate that PD 4–9 ethanol exposure occurs during a critical 'synaptogenesis spurt' which establishes life-long parameters for basic mPSC related GABA synaptic function in MS/DB neurons. Furthermore, this initial maturational period in MS/DB neurons is generally well reproduced in cultured septal neurons with the exception of mPSC frequency [17] suggesting maturation is primarily dependent on local cellular mechanisms.
Figure 4. Daily changes in MS/DB GABAAR mPSCs between PD 4 and 16.
GABAAR mPSC parameters are grouped by day for amplitude (A), frequency (B), and ensemble average bi-exponential time constant τ1(C), and τ2(D). Bars represent mean ± S.E.M for n = 12–31 neurons per day (ANOVA, ***P <0.001).
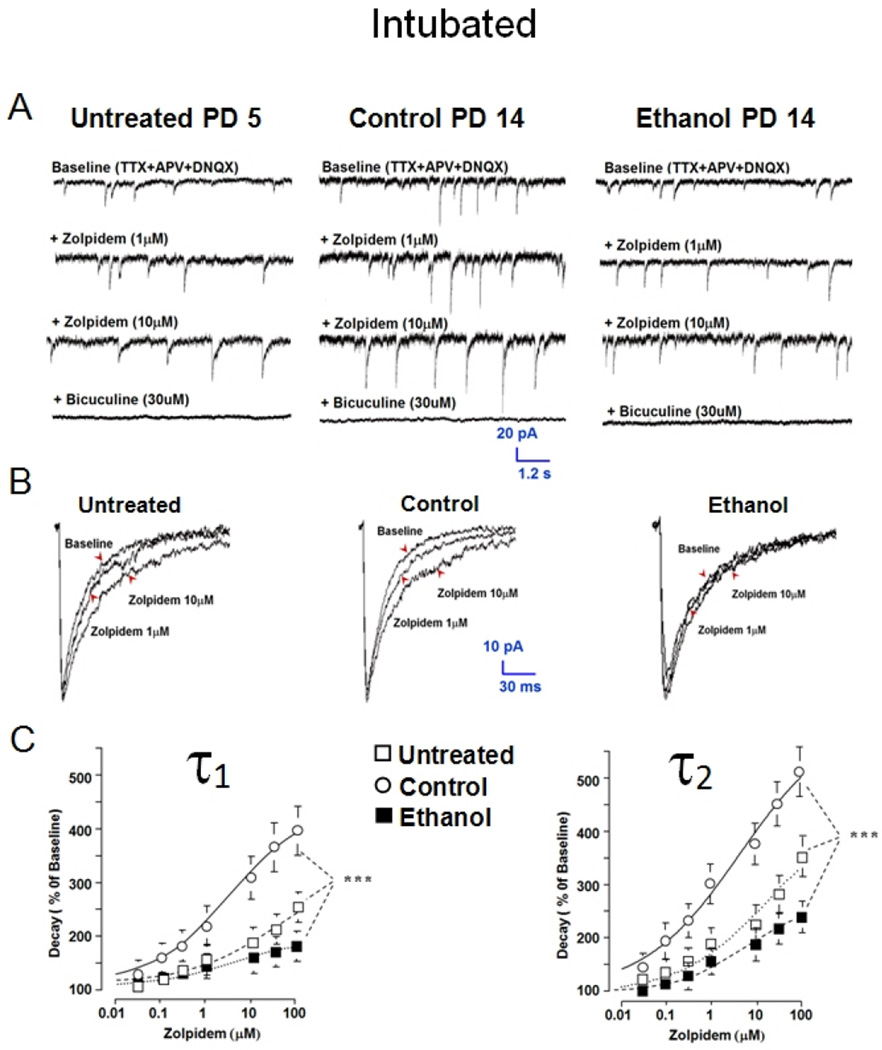
Figure 5. Postnatal day 4–9 ethanol intoxication decreases sensitivity of GABAAR mPSCs to zolpidem on PD 11–16.
GABAAR mPSCs were recorded in MS/DB slices from Untreated (PD 4–9), and (PD 11–16) Control or Ethanol intubated rats. (A) Representative traces for a cell from each group before and after adding zolpidem or bicuculline to the tissue bath. (B) Ensemble averages of events for the neurons in ‘A’ are shown in presence and absence of zolpidem. (C) & (D) Points represent mean ± S.E.M. of estimates of ensemble average decay phase bi-exponential time constants τ1 and τ2 (n=12–31; ANOVA *** P<0.001).
Figure 3. GABAAR mPSCs in MS/DB neurons mature and stabilize during the first postnatal month.
(A) Shows representative traces (left; middle, NOTE time scale change) with ensemble averages (right) from 8 MS/DB neurons ranging in age from 4 days to 24 months. (B) Percent of all neurons recorded with GABAAR mPSCs in MS/DB slices on PD 4–9 (n=12–24 cells across groups); differences vs PD 4; ANOVA, **P <0.01, ***<0.001). (C–F) GABAAR mPSC parameters are grouped by age-related intervals for amplitude (C), frequency (D), and ensemble average bi-exponential time constant τ1 (E), and τ2(F) (n=9–137 cells across all groups; values represent Mean ± S.E.M; comparison based on difference vs PD 4–9 group; ANOVA, ***P <0.001).
2.3 Postnatal intoxication in vivo blocks development of enhanced sensitivity to zolpidem
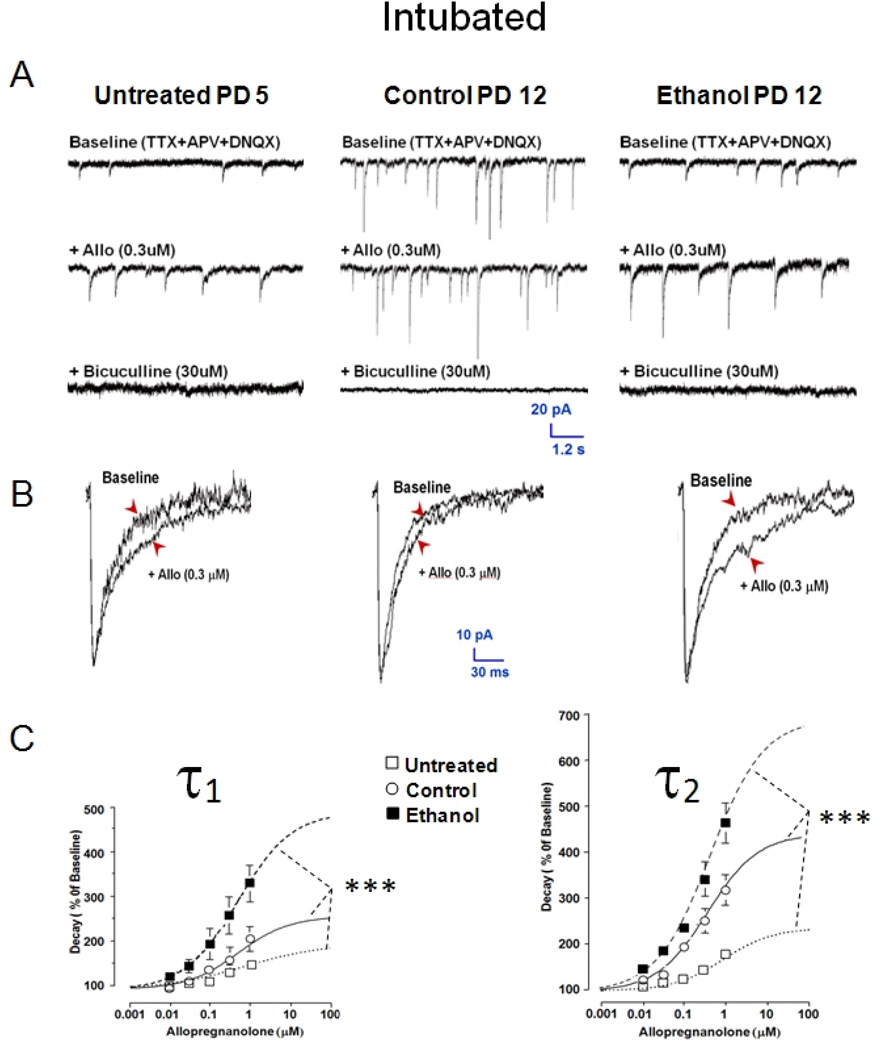
Following ethanol intoxication, GABAAR mPSCs in septal cultures show low sensitivity to zolpidem (0.03–100 µM), an α1 subtype GABAAR benzodiazepine site agonist. Zolpidem sensitivity normally increases with age along with expression of α1 protein [18, 19, 65]. In Figure 5 A & B, zolpidem, 1 & 10 µM, prolongs representative mPSC traces and ensemble averaged GABAAR mPSC decay recorded from a PD 5 Untreated and PD 14 Control MS/DB neuron. Zolpidem sensitivity in Untreated controls was assessed during the first postnatal week to illustrate developmental changes when compared to Intubated Controls; however, a role for stress from intubation cannot be ruled out here since PD 11–16 Untreated animals were not tested. Slowing of event decay by zolpidem is more prominent in both Untreated and Control neurons, but less evident in a PD 14 Ethanol neuron. Mean values for Untreated PD 4–9 neurons show concentration-dependent (0.3–100 µM) increases in time constants τ1 and τ2 relative to baseline records. An even greater increase in decay time constants is present in PD 11–16 Control neurons after zolpidem (Figure 5C), consistent with increased sensitivity with age as occurs in septal cultures [18]. Potentiation of decay time constants by zolpidem (0.3–100 µM) diminishes on PD 11–16 after binge Ethanol exposure (Figure 5C) relative to Control and is even slightly less than that for Untreated cells (Figure 5C). Untreated neurons recorded during the more immature PD 4–9 window were examined specifically to demonstrate that immature neuron decay kinetic profiles and zolpidem sensitivity were very similar to profiles induced by binge ethanol exposure. Zolpidem does not consistently change GABAAR mPSC rise time, peak amplitude, or event frequency in any group of MS/DB neurons (data not shown). Overall, the similarity of Ethanol and Untreated group results would seem consistent with the concept that some form of arrested development is occurring after in vivo cyclic ethanol intoxication.
2.4 Postnatal intoxication in vivo amplifies sensitivity to allopregnanolone
Following ethanol treatment, GABA mPSCs in septal cultures show enhanced sensitivity to allopregnanolone (3–1000 nM), a 5α-reduced neurosteroid site agonist at GABAARs [32], but unlike zolpidem, allopregnanolone sensitivity did not change with age in untreated cultured neurons [18]. Figure 6 shows that GABAAR mPSCs in MS/DB neurons show a concentration-dependent increase in sensitivity to allopregnanolone (0.01–1 µM) with age (Figure 6C). Allopregnanolone (0.3 µM) appears to prolong the decay phase and increase event amplitude for individual traces (Figure 6A) from a PD 12 Control neuron relative to a PD 5 Untreated cell. Allopregnanolone sensitivity in Untreated controls was assessed during the first postnatal week to illustrate developmental changes when compared to Intubated Controls; however, a role for stress from intubation cannot be ruled out here since PD 11–16 Untreated animals were not tested. Mean amplitude (data not shown) and decay time constant increase in ensemble averages is only marginal from these neurons (Figure 6B). Slowing of event decay by allopregnanolone in Untreated and Control neurons is further exaggerated for the PD 12 Ethanol cell. Mean values for Untreated PD 4–9 neurons (Figure 6C) show concentration-dependent increases in the biexponential time constants τ1 and τ2 relative to baseline recordings. A significantly larger increase in decay time constants is present in PD 11–16 Control neurons vs PD 4–9 Untreated cells (Figure 6C), suggesting an age-dependent increase in neurosteroid sensitivity from weeks one to two during the early postnatal period. Finally, there is significant exaggeration of decay time constants on PD 11–16 after Ethanol exposure (PD 4–9) in MS/DB neurons relative to Control (Figure 6C), which also occurs for septal cultures [18]. Like zolpidem, allopregnanolone did not consistently change GABAAR mPSC rise time, peak amplitude, or event frequency in any group (data not shown). In sharp contrast to the results with zolpidem (Figure 5), the greater sensitivity to allopregnanolone for the Ethanol versus the Untreated group is not consistent with simple arrest of expected developmental maturation by in vivo ethanol intoxication, but suggests an abnormal plastic change in postsynaptic GABA signaling is likely evoked by ethanol.
Figure 6. Postnatal day 4–9 ethanol intoxication and withdrawal increases sensitivity of GABAAR mPSCs to allopregnanolone on PD 11–16.
GABAAR mPSCs were recorded in MS/DB slices from Untreated (PD 4–9), and (PD 11–16) Control or Ethanol intubated rats. (A) Representative traces for a cell from each group before and after adding allopregnanolone (allo) or bicuculline to the tissue bath. (B) Ensemble averages of events for the neurons in ‘A’ in presence and absence of allo. (C) & (D) Points represent mean ± S.E.M. of estimates of ensemble average decay phase bi-exponential time constants τ1 and τ2 (n=10–14; ANOVA *** P<0.001).
2.5 Distortions of synaptic function are long-lasting and similar in males and females
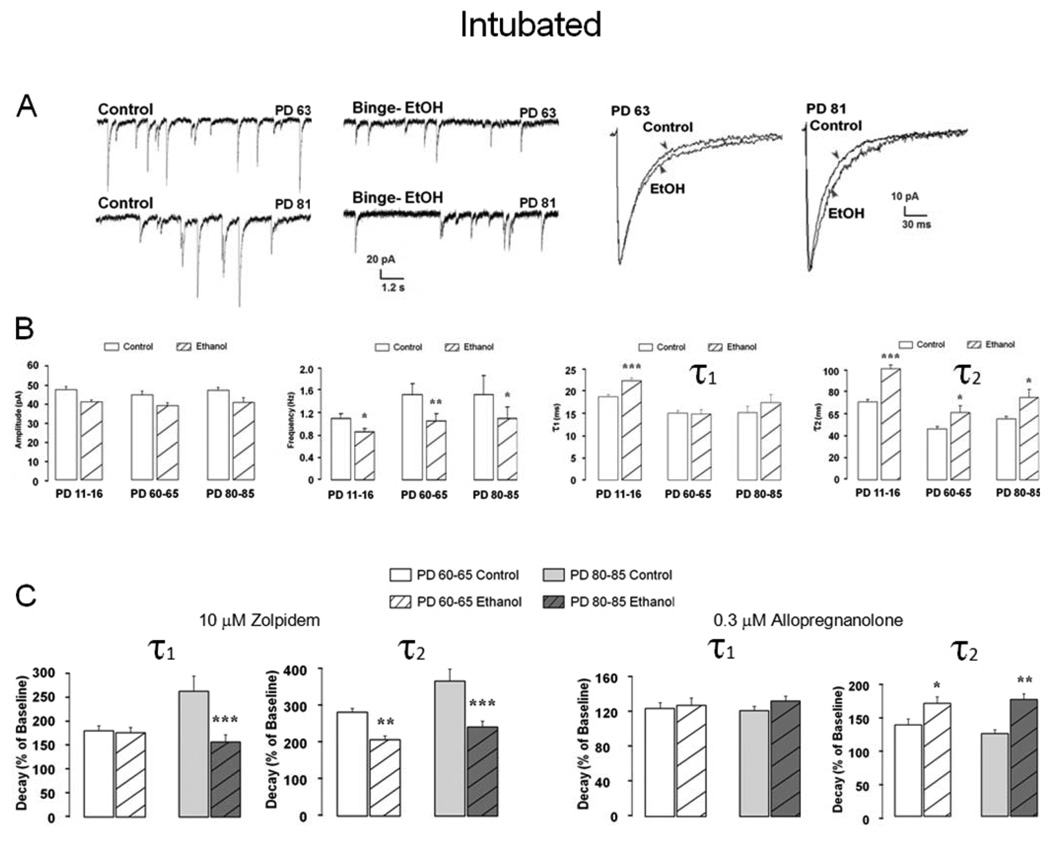
If PD 4–9 Ethanol exposure arrests normal GABAAR mPSC development as some findings in septal cultures and MS/DB slices suggest, the deficit might be expected to reverse, once developmental maturation progresses to completion. For example, whole cell GABA evoked currents in acutely isolated MS/DB neurons are blunted on PD 11–16 after PD 4–9 ethanol intoxication, but recover to normal values by PD 25–35 [36]. However, figure 7 shows GABAAR mPSCs still exhibit detectible changes weeks after ethanol treatment. Representative traces and superimposed ensemble averages from PD 63 or 81 Control and Ethanol neurons show a slower mPSC decay phase is still present. Although mean ± S.E.M. mPSC amplitudes are not significantly different in Ethanol neurons relative to age appropriate PD 11–16, 60–65 or 80–85 Controls (Figure 7B), yet event frequency is reduced by intoxication in all groups. Decay time constants, τ1, which are increased by Ethanol on PD 11–16, are no longer different from Control by PD 60–65 or 80–85, but τ2 which also increases on PD 11–16 remains elevated (figure 7B). The extent to which 10 µM zolpidem increases decay time constants τ1 and τ2 remains reduced on PD 80–85, although only τ2 is reduced in the PD 60–65 group. Decreased zolpidem sensitivity also is present for a lower concentration (1 µM) in the PD 80–85 group, but not in PD 60–65 (data not shown) suggesting that synaptic changes after ethanol remain, but are potentially masked by plasticity that may reshape GABAAR mPSCs kinetics. Consistent with this assumption, allopregnanolone potentiation of decay time constants remains for Ethanol PD 60–65 and PD 80–85 groups where τ2 is increased, but is no longer detected for τ1 in either group.
Figure 7. Changes in GABAAR mPSC kinetics and zolpidem or allopregnanolone sensitivity persist well after PD 4–9 ethanol intoxication and withdrawal.
(A) On LEFT are representative traces for neurons recorded in MS/DB slices from a PD 63 or PD 81 Control or Ethanol rat previously intubated on PD 4–9. On RIGHT are ensemble averages for these cells. (B) Bars represent mean ± S.E.M values for GABAAR mPSC amplitude, frequency, or decay time constants τ1 or τ2 for PD 11–16, 60–95 or 80–85 Control or Ethanol groups (n = 8–21; ANOVA * P < 0.05; ** P < 0.01; *** P < 0.001 vs Control). (C) Bars represent mean ± S.E.M. percent change above baseline (e.g. 100%) for decay time constants τ1 and τ2 in presence of zolpidem (LEFT) or allopregnanolone (RIGHT). Differences within group, t-test; difference between group of treatments ANOVA; n=8–18; ANOVA *** P <0.001 vs Control).
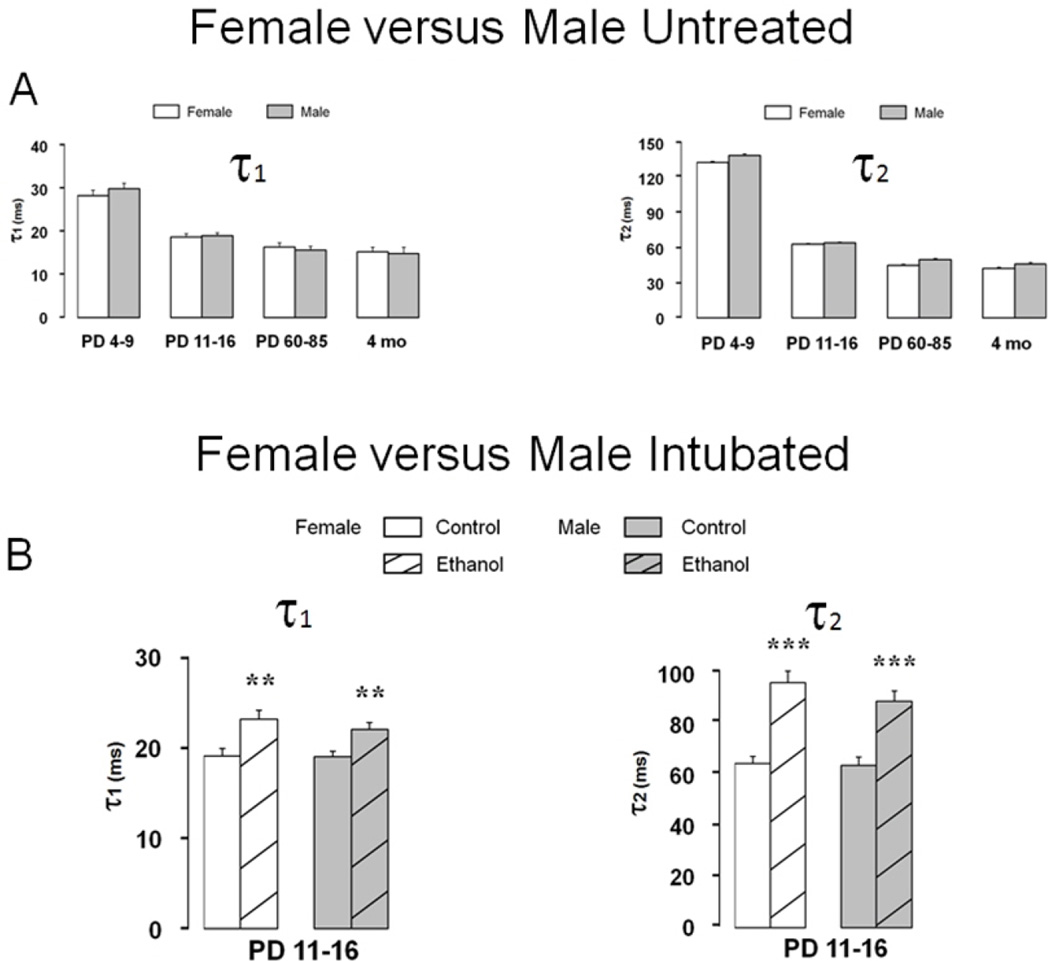
Finally, evidence suggests that vulnerability to ethanol-induced spatial learning deficits may be sex-specific [2, 27, 57, 59], however, synaptic injury in MS/DB neurons does not show major differences between male versus female rats. Figure 8A shows developmental changes in mean ± S.E.M. GABAAR mPSC decay time constants between PD 4–9 and 4 months of age are essentially identical in male and female MS/DB. Developmental changes in mPSC amplitude, frequency, and rise time also do not differ between sexes (data not shown) but closely resemble those in figure 3. Mean ± S.E.M. decay time constants are increased similarly after ethanol intoxication both in τ1 and τ2 in PD 11–16 males and females Figure 8B, as are changes in mPSC amplitude, frequency, and rise time after Ethanol (data not shown). The presence of parallel changes in GABAAR mPSCs after Ethanol in males and females is consistent with a local cellular level target for ethanol as a basis for synaptic injury, that is likely not dependent on influences from outside the immediate brain area, such as peripherally produced hormones. However, no attempts were made to determine estrus cycle status in adult females.
Fig. 8. Developmental changes GABAAR mPSC kinetics and vulnerability to ethanol injury are equivalent in females and males.
(A) Bars represent mean ± S.E.M. decay time constants τ1 and τ2 for Untreated MS/DB neurons from males or females on PD 4–9, 11–16, 60– 85 or 4 months of age (n=15–76 across groups). (B) Bars represent mean ± S.E.M. decay time constants τ1 and τ2 for MS/DB neurons from male or female animals in Control or Ethanol groups on PD 11–16. (n=52–79 across groups; t-test, ** P<0.01, *** P<0.001).
3. DISCUSSION
3.1 Ethanol-induced injuries are long lasting but not sex specific
In vivo evidence reported here from electrophysiological recordings of GABAAR mPSCs indicate that after initial maturation during the first postnatal month of life kinetic parameters remain remarkably stable across the complete life span of rats despite ongoing behavioral and physiological changes during aging. Equally interesting, ethanol-induced distortions of GABAAR-mediated synaptic function during the early maturational period show surprising evidence of stability in that they appear to last well into adulthood. One might expect ethanol-induced distortions in early life may be obscured by developing synaptic plasticity changes with age, or the postsynaptic receptor complement to gradually revert to normal in the absence of ethanol intoxication, yet observed changes appear to persist for at least two months into adulthood suggesting relatively permanent disruptions have taken place. In a similar early postnatal ethanol treatment model, Green et al. found residual deficits in eye-blink conditioning behavior in adult rats that correlated with deficits in firing of nucleus interpositus neurons in cerebellum [33]. Because synaptic deficits can still be measured in early adulthood with our in vivo binge ethanol exposure model, this may provide an advantage for using animal behavioral testing to further explore the likely correlation between the extent of synaptic injury at a cellular level and the degree of cognitive impairment as reflected in behavioral performance [2]. Another interesting result of the current study also demonstrates that vulnerability to injury from ethanol in the in vivo model of exposure was equivalent whether MS/DB neurons were derived from males or females despite observations made in other various animal models suggesting FAS related mental defects may be gender specific [2, 27, 57, 59, 63, 67]. The ability to determine sex differences across ethanol exposed rats provides an added benefit to the in vivo model relative to our primary culture model where animals are not sexed prior to the culturing process. Since data from female animals were randomly sampled throughout life, the effects of the estrus cycle on results from adult females were not determined. Di-estrus can increase extrasynaptic but not synaptic ‘phasic’ GABA-mediated currents in mice [51], but investigation of GABAergic ‘tonic’ extrasynaptic currents across the sexes was beyond the scope of the present study.
3.2 Septal cultures reliably model ethanol injury of developing GABA synapses in MS/DB
The vulnerability of new synapses to injury by ethanol intoxication is remarkably parallel in developing MS/DB neurons in vivo and in vitro in dispersed septal cultures [17, 18]. Ethanol-induced injury, characterized by altered GABAAR-mediated mPSC kinetics and by changes in zolpidem or allopregnanolone allosteric modulation, takes place during rapid maturational refinement of GABAergic postsynaptic signaling in both models. Such a consistent maturational pattern of synaptic GABAARs in both models verifies the use of in vitro cultured septal neurons as a reliable tool for investigating the development of synaptic GABAAR function during a period equivalent to the human brain growth spurt. Unfortunately, septal neuronal cultures are only viable for approximately one month making them limited in determining long-term changes caused by exogenous factors like ethanol over the lifetime of the animal. Overall, the striking similarities in cultures and slices during maturation- and intoxication-related distortion of GABAAR mPSC kinetic and pharmacological properties, suggests ethanol injury largely involves local cellular level mechanisms. Furthermore, these results support the value of ED 20 septal cultures as a cost effective model to screen potential interventions for offsetting ethanol-mediated injury to developing synapses before confirming efficacy in more costly whole animal studies.
3.3 Ethanol-induced changes in mPSC kinetic parameters suggest synaptic injury
The frequency of mPSCs likely reflects relative numbers of synaptic inputs to a given neuron, assuming similar asynchronous vesicular release rates across synapses and equivalent recording conditions [10, 19, 39]. Synapses giving rise to events far from the recording electrode may be minimized by cable effects or incomplete clamping of membrane potential, and smaller events can be lost in background noise [61]. In our study, the rapid increase in mPSC frequency and synaptotagmin levels in slices [66] and cultures [17] suggests synapse number rapidly escalates during initial synaptogenesis, but then stabilizes. The higher mPSC frequency in untreated cultures versus slices could be due to higher relative synaptic density or perhaps formation of more electrically compact neurons in this simplified two dimensional model. Synapse numbers may continue to increase even when the apparent frequency stabilizes with age in slices, because new synapses may form well out on expanding dendritic arbors away from the somatic recording electrode and be lost due to cable effects and baseline noise [61]. Although a mechanism for the alteration in developing GABA synapses after binge ethanol exposure remains to be identified, one interpretation of the reduced frequency after ethanol intoxication is that new synapse formation is arrested, at least temporarily. A similar reduction in GABAAR mPSC frequency was recently observed in CA3 hippocampus yet mPSC amplitude was increased after 3rd trimester-equivalent ethanol exposure [47]. Interestingly, similar reductions in GABAAR mPSC frequency and amplitude were noted in primary septal cultures after chronic picrotoxin treatment [17] and in primary cortex cultures after two day TTX exposure [9]. These findings suggest that binge ethanol treatment may be acting in a picrotoxin/TTX-like mechanism to chronically disrupt developing GABAARs and/or neuronal excitability. Nevertheless, frequency remains reduced relative to Control long after Ethanol injury (figure 7B), perhaps suggesting continuing synapse formation is relatively limited in older animals or approaches a steady state balanced by synaptic loss.
The amplitude of mPSCs is also a complex measure to interpret and can be influenced by the synaptic vesicle transmitter loading, the concentration of transmitter in the trans-synaptic wave, the number and density of postsynaptic receptors and the extent of receptor exposure to transmitter [10, 50, 54, 55]. Mean mPSC amplitude is surprisingly stable in untreated septal cultures and MS/DB slices. . How ethanol intoxication reduces mPSC amplitude is not clear, but possibly involves less GABA loaded into vesicles or a postsynaptic GABAAR receptor density below that of Controls[10, 54]. Allosteric actions of zolpidem and allopregnanolone increase GABAAR mPSC amplitudes if postsynaptic receptors are not fully saturated by the peak of the trans-synaptic GABA wave [5, 10], so the lack of consistent amplitude change with these agents between Control and Ethanol neurons in MS/DB slices (data not shown) or septal cultures [18] suggests receptor density or GABA saturation are not different. Western analysis of the vesicular inhibitory amino acid transporter, VGAT, shows expression in PD 11–16 MS/DB tissue is reduced by ethanol intoxication [66], while synaptotagmin and α1 GABAAR subunit are unchanged both in MS/DB [66] and in septal cultures [17, 18]. Since ethanol does not change synaptotagmin, reduced VGAT supports incomplete GABA loading from fewer transporters per vesicle, rather than fewer GABAergic terminals.
3.4 Ethanol-induced changes in mPSC decay kinetics suggest postsynaptic injury
The mean bi-exponential decay time constants, τ1 and τ2 derived from ensemble averaged GABAAR mPSCs likely reflect changing functional states of postsynaptic GABA receptors as they respond to the rapid rise and fall of the trans-synaptic GABA wave. Simulations of synaptic events suggest GABA concentrations at the peak of the wave likely exceed 1 mM [50, 62]. Modeling studies suggest that at this high concentration, receptors initially bind transmitter, enter an open state, may undergo transition to a desensitized state, then return to a re-sensitized conformation before finally unbinding transmitter, about the time the synaptic transmitter levels have dissipated toward ambient extra-synaptic levels [13, 14]. A slowing of mPSC decay time constants by ethanol is likely the result of changes in the subunit composition of GABAARs anchored in the region of the postsynaptic density most likely to be impacted by the GABA wave during asynchronous release. Conceivably, ethanol injury might involve disruptions in the architecture of the synaptic cleft thus altering the dynamics of the GABA wave [10]. However, this seems an unlikely explanation since the magnitude of allosteric actions of exogenously applied zolpidem and allopregnanolone that slow mPSC decay kinetics are shown to change in opposite directions after ethanol. At the concentrations tested, both agents act as positive allosteric agonists at unique sites on postsynaptic GABAARs to increase receptor sensitivity to ambient GABA and thereby prolong the probability of receptor activation as synaptic GABA levels fall towards the ambient baseline [7, 10, 46, 64, 70]. After ethanol injury, enhancement with zolpidem is reduced, but with allopregnanolone it is increased, suggesting GABAARs with unique kinetic properties and allosteric regulatory sites replaced the 'normal' postsynaptic receptor complement. For example, across various ethanol exposure models, ethanol exposure consistently upregulates GABAAR α4 subunit expression in rats while downregulating α1 subunit expression and zolpidem sensitivity [6, 8, 12, 22, 31, 68]. In comparison, accelerated GABAAR mPSC decay kinetics during development and differential pharmacological sensitivity to various allosteric modulators have been attributed to the switching of the GABAAR subunit composition [18, 19, 28, 40, 42, 65]. However, the exact configuration of synaptic GABAARs after binge ethanol injury is still unclear. Withdrawal from chronic ethanol exposure during adolescence or adulthood also alters zolpidem and neurosteroid sensitivity in the hippocampus [43] and amygdala [21] due to changes in GABAAR subunit composition. Although we cannot rule out the possibility that similar mechanisms due to withdrawal from binge ethanol exposure may be playing a role in the animals tested during the second postnatal week (PD 11–16), it is unlikely that withdrawal plays a role in the more persistent changes in synaptic function due to binge ethanol exposure seen in animals tested during adulthood (see figure 7; PD 60–65; 80–85). Finally, differences in zolpidem and allopregnanolone sensitivity between Untreated and Intubated Controls (figures 5 & 6) may be explained by stress from the intubation procedure. Stress and stress-related neurosteroids can have a profound impact on GABAAR physiology [4, 60], and it is hypothesized that the interactions between stress, ethanol, and neuroactive steroids may underly adaptations in GABAAR physiology seen in ethanol dependence and withdrawal [4, 49]. Thus, although intubation stress cannot be ruled out as a possible mechanism for GABAAR adaptations, the exact mechanism of ethanol-induced adaptations in GABAAR physiology in developing neurons is complex and should be the focus of future studies.
3.5 Conclusions
In summary, in vivo ethanol intoxication on PD 4–9 results in equivalent, long lasting distortion of asynchronous GABAergic mPSCs in MS/DB neurons in rat brain slices that closely parallel changes observed previously in cultured rat septal neurons given comparable ethanol exposure in vitro. Additionally, ethanol-induced GABAAR mPSC deficits were not sex specific. In ethanol naïve animals, mPSCs showed marked changes in kinetics during the first postnatal month that then approached steady state conditions that remained in place throughout the rest of life. An essentially identical pattern of reduced mPSC amplitude, frequency, and slowed decay kinetics in addition to reduced zolpidem or enhanced allopregnanolone modulation of GABAergic mPSCs in MS/DB slice and cultured septal neurons provides striking evidence that ethanol injury likely results from distortion of some intrinsic cellular level mechanism early in development that is faithfully replicated in both in vitro and in vivo models of synaptogenesis.
4. EXPERIMENTAL PROCEDURES
4.1 Experimental Animals and Postnatal Binge Ethanol Treatment
Timed-pregnant Sprague–Dawley rats (Harlan) and Fischer 344 male rats (National Institute of Aging colony) were maintained in a AAALAC-accredited facility under controlled conditions (22–25 °C; lights 0700–1900 h; rat chow and water ad lib) in accordance with policies of the Texas A&M University Laboratory Animal Care Committee and NIH guidelines. Fischer 344 male rats were only used for experiments performed on naïve aged (17–24 month) neurons (see figure 3) due to the availability of these animals from the Griffith laboratory. Postnatal ethanol intubation of Sprague-Dawley rat pups only was performed as previously described [34, 35]. Gestational day 22 was designated as PD 0. Twenty two litters were culled to equal numbers of vigorous male and female pups (8 total per litter when available) on PD 2. Pups remained with the dam except for ∼10 min periods when placed on a cotton towel covered heating pad for treatment. For postnatal ethanol treatment on PD 4, both male and female pups were randomly assigned to Ethanol, Control, or Untreated groups. Ethanol (5.25 g/kg/day, ethanol 11.9% v/v-95% w/v in Enfamil with iron; Mead Johnson, to offset reduced nursing) was given by oral gastric intubation over 2 daily treatments [34, 35]. Sham intubation without milk was given to Control animals on the same schedule since their ability to nurse was unimpaired by ethanol intoxication. Untreated animals were handled only during routine care and not intubated. On PD 9, all pups were tattooed with non-toxic permanent black India ink for future identification. Pups stayed with the dam until used or weaned on PD 25 when each animal received a subcutaneous microchip (Avid Identification Systems). Blood samples (20 µl from the tip of the tail) were collected in heparinized Unipets (Beckon Dickinson) on PD 6 from all pups. Samples were collected 90 min after the second ethanol treatment, a time point previously shown to represent peak BEC level in the artificial rearing (pup-in-a-cup) and intragastric intubation models [24]. Blood ethanol concentrations (BEC) were determined using gas chromatography [16]. For this model, blood ethanol levels have been shown to peak 90 min after treatment at ∼325–359 mg% [2, 34, 35, 37] and then decline to zero by 24hr [26]. Mean blood ethanol levels sampled across animals (n = 43) tested for the purposes of this study were 319 ± 6 mg%.
4.2 MS/DB Slices
For whole cell recordings, brain tissue, from both male and female pups, was harvested after lethal isoflurane inhalation and cooled by immersion in 0–4°C ‘low sodium-sucrose’ artificial cerebral spinal fluid (ACSF) solution containing (mM): KCl, 2; MgCl2, 1; MgSO4, 2; CaCl2, 1; NaH2PO4, 1.25; sucrose, 206; D-(+)-glucose, 30; NaHCO3, 26; kynurenic acid, 1 (Sigma); bubbled with 95/5% O2/CO2; pH 7.4; 290–310 mosM. For animals > 30 days of age, brain tissue was cooled initially in situ by cardiac perfusion with ACSF before removal and preparation of 200–300 µm coronal slices using a Vibroslice (Campden Instr.) in the same solution. Slices were transferred to a holding chamber of oxygenated (95/5% O2/CO2), ‘standard’ ACSF solution containing, (mM): NaCl, 124; KCl, 3; MgSO4, 1.5; CaCl2, 2.4; NaH2PO4, 1.25; D-(+)-glucose, 10; NaHCO3, 26; pH 7.4; 290–310 mosM, gradually warmed to ∼32° C and incubated for ∼30 min then allowed to cool to ∼22° C prior to experimentation. Individual slices were transferred to a recording chamber, submerged using a slice anchor (Warner Instruments Corp.), and continuously perfused with oxygenated ACSF solution during recordings and drug applications.
4.3 Whole Cell Recording and Acute Drug Application
Conventional patch-clamp recording techniques were used as described previously [17, 35]. Briefly, patch pipettes were pulled from glass capillary tubing (KG-33, 1.5mm OD, Garner Glass Co.) on a Brown and Flaming P-97 pipette puller (Sutter Instr.) with resistance at ∼2–8 MΩ when filled with a pipette solution (mM): CsCl, 130; ethylene glycol-bis (β-aminoethyl ether) N,N,N',N'-tetraacetic acid (EGTA), 10; MgCl2, 2; HEPES, 10; Mg-ATP, 4; GTP, 0.1; pH 7.2 with CsOH; 295–300 mosM. Individual neurons were visualized in the MS/DB region of brain slices using water immersion, differential interference contrast, video-enhanced optics on the stage of an upright Olympus BX50WI microscope (Figure 1C). Voltage-clamp current recordings were collected / digitized with an AxonClamp 200 (Axon Inst.), Digidata 1200 interface and pClamp 7/8/10 software (Molecular Devices). Capacitance (pF) was obtained from the potentiometer used to zero capacitance transients. Traces were recorded under voltage clamp at −60mV, low-pass filtered at 2 kHz and digitized at 20 kHz uncompensated. GABAAR-mediated mPSCs were isolated as previously reported by recording in the presence of tetrodotoxin (TTX, 0.5 µM, Calbiochem), D(−)2-amino-5-phosphonovaleric acid (APV, 40 µM, Sigma), and 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 µM, Sigma). Acute applications of drugs, including zolpidem (Sigma) and 3α-hydroxy-5α-pregnan-20-one (allopregnanolone, Allo; Sigma), were made by applying drug solutions to the slice with a dual barrel perfusion system (760 µm i.d., PEEK tubing #1533). The upstream barrel continually flushed the environment above the recorded neurons with standard ACSF until displaced manually with the adjacent barrel which delivered drug solution directly over the recorded region of the slice.
4.4 Miniature Postsynaptic Current Analysis and Statistics
Off-line analysis of GABAergic mPSC kinetic parameters was performed using Mini-Analysis 6.0 (Synaptosoft Inc., Decatur, GA) and Prism 4 (GraphPad, San Diego, CA) as previously described [17, 18, 35]. Generally, individual currents >10 pA could be clearly distinguished above baseline noise in the 2–3 min current traces collected from individual neurons. Event frequency was determined from the mean inter-event interval, while event peak amplitude was estimated as the absolute difference between the preceding baseline and maxima of the current. For mPSC decay analysis, low noise traces and non-overlapping events were used to generate an ensemble averaged mPSC by aligning currents on the rising phase. The 10–90% decay phase of this average for each neuron was fitted with a biexponential function:
| (1) |
where A1 and A2 were the fraction of the fast and slow decay components, respectively, As was the steady-state current, and τ1 and τ2 were the fast and slow decay time constants, respectively. Previously we found that mPSC ensemble decay data for cultured septal neurons under the present conditions gave a significantly better fit with two time constants relative to a fit with a single time constant [17]. Data are expressed as mean ± SEM for mPSC kinetic parameters such as frequency, amplitude, rise time, and decay time constants. Two-way ANOVA was used to test significance of differences within and across groups, while specific pairs of means were compared with a Bonferroni post-test. Comparisons of cumulative probability distributions were made using the Kolmogorov-Smirnov (K-S) test. Unless stated otherwise, data are reported as mean ± SEM and significance is assumed for P values < 0.05 from two-tailed tests.
Highlights.
Binge-like ethanol treatment blunts GABAAR-mediated mPSC development and maturation.
Ethanol exposure attenuates zolpidem action while exaggerating allopregnanolone’s.
Binge-like ethanol-induced disruptions are long lasting and not sex specific.
In vivo model of ethanol injury reliably models results from cultured septal neurons.
Acknowledgements
Supported by NIH-NIAAA project AA012386 (GDF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arjona A, Boyadjieva N, Kuhn P, Sarkar DK. Fetal ethanol exposure disrupts the daily rhythms of splenic granzyme B, IFN-gamma, and NK cell cytotoxicity in adulthood. Alcohol Clin Exp Res. 2006;30(6):1039–1044. doi: 10.1111/j.1530-0277.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 2.Banuelos C, Gilbert RJ, Montgomery KS, Fincher AS, Wang H, Frye GD, Setlow B, Bizon JL. Altered spatial learning and delay discounting in a rat model of human third trimester binge ethanol exposure. Behav Pharmacol. 2012;23(1):54–65. doi: 10.1097/FBP.0b013e32834eb07d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barberis A, Lu C, Vicini S, Mozrzymas JW. Developmental changes of GABA synaptic transient in cerebellar granule cells. Mol Pharmacol. 2005;67(4):1221–1228. doi: 10.1124/mol.104.006437. [DOI] [PubMed] [Google Scholar]
- 4.Biggio G, Concas A, Follesa P, Sanna E, Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116(1):140–171. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein AH, Clark DJ, O’Gorman M, Willavize SA, Brayman TG, Grover GS, Walsky RL, Obach RS, Faessel HM. Lack of pharmacokinetic and pharmacodynamic interactions between a smoking cessation therapy, varenicline, and warfarin: an in vivo and in vitro study. J Clin Pharmacol. 2007;47(11):1421–1429. doi: 10.1177/0091270007307574. [DOI] [PubMed] [Google Scholar]
- 6.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63(1):53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 7.Camarillo C, Miranda RC. Ethanol exposure during neurogenesis induces persistent effects on neural maturation: evidence from an ex vivo model of fetal cerebral cortical neuroepithelial progenitor maturation. Gene Expr. 2008;14(3):159–171. [PMC free article] [PubMed] [Google Scholar]
- 8.Charpier S, Behrends JC, Triller A, Faber DS, Korn H. "Latent" inhibitory connections become functional during activity-dependent plasticity. Proc Natl Acad Sci U S A. 1995;92(1):117–120. doi: 10.1073/pnas.92.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WJ, Maier SE, West JR. Prenatal alcohol treatment attenuated postnatal cocaine-induced elevation of dopamine concentration in nucleus accumbens: a preliminary study. Neurotoxicol Teratol. 1997;19(1):39–46. doi: 10.1016/s0892-0362(96)00188-2. [DOI] [PubMed] [Google Scholar]
- 10.Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24(3):155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- 11.Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse. 2005;58(3):151–164. doi: 10.1002/syn.20184. [DOI] [PubMed] [Google Scholar]
- 12.Devaud LL, Alele P. Differential effects of chronic ethanol administration and withdrawal on gamma-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol Clin Exp Res. 2004;28(6):957–965. doi: 10.1097/01.alc.0000128225.83916.40. [DOI] [PubMed] [Google Scholar]
- 13.Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15(5):1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 14.Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17(12):4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23(17):6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBois DW, Parrish AR, Trzeciakowski JP, Frye GD. Binge ethanol exposure delays development of GABAergic miniature postsynaptic currents in septal neurons. Brain Res Dev Brain Res. 2004;152(2):199–212. doi: 10.1016/j.devbrainres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 18.DuBois DW, Trzeciakowski JP, Parrish AR, Frye GD. GABAergic miniature postsynaptic currents in septal neurons show differential allosteric sensitivity after binge-like ethanol exposure. Brain Res. 2006;1089(1):101–115. doi: 10.1016/j.brainres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Dunning DD, Hoover CL, Soltesz I, Smith MA, O’Dowd DK. GABA(A) receptor-mediated miniature postsynaptic currents and alpha-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82(6):3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer TA, Servatius RJ, Pang KC. Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J Neurosci. 2007;27(2):299–303. doi: 10.1523/JNEUROSCI.4189-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faber DS, Fetcho JR, Korn H. Neuronal networks underlying the escape response in goldfish. General implications for motor control. Ann N Y Acad Sci. 1989;563:11–33. doi: 10.1111/j.1749-6632.1989.tb42187.x. [DOI] [PubMed] [Google Scholar]
- 22.Faber MD. The monster in the bone-house: Beowulf. Psychoanal Rev. 1989;76(2):263–280. [PubMed] [Google Scholar]
- 23.Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21(4):738–744. [PubMed] [Google Scholar]
- 24.Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19(6):435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 25.Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7(2):107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- 26.Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20(3):285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 28.Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 2002;309(1):73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- 29.Hindson C, Lawlor F, Taylor AE. Liquid nitrogen cryotherapy of basal cell carcinomata. Ir Med J. 1982;75(11):418. [PubMed] [Google Scholar]
- 30.Hollrigel GS, Soltesz I. Slow kinetics of miniature IPSCs during early postnatal development in granule cells of the dentate gyrus. J Neurosci. 1997;17(13):5119–5128. doi: 10.1523/JNEUROSCI.17-13-05119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hording M, Hording U, Daugaard S, Norrild B, Faber V. Human papilloma virus type 11 in a fatal case of esophageal and bronchial papillomatosis. Scand J Infect Dis. 1989;21(2):229–231. doi: 10.3109/00365548909039974. [DOI] [PubMed] [Google Scholar]
- 32.Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther. 2007;116(1):7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiao SH, Acevedo JL, DuBois DW, Smith KR, West JR, Frye GD. Early postnatal ethanol intubation blunts GABA(A) receptor up-regulation and modifies 3alpha-hydroxy-5alpha-pregnan-20-one sensitivity in rat MS/DB neurons. Brain Res Dev Brain Res. 2001;130(1):25–40. doi: 10.1016/s0165-3806(01)00194-8. [DOI] [PubMed] [Google Scholar]
- 35.Hsiao SH, DuBois DW, Miranda RC, Frye GD. Critically timed ethanol exposure reduces GABAAR function on septal neurons developing in vivo but not in vitro. Brain Res. 2004;1008(1):69–80. doi: 10.1016/j.brainres.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao SH, Mahoney JC, West JR, Frye GD. Development of GABAA receptors on medial septum/diagonal band (MS/DB) neurons after postnatal ethanol exposure. Brain Res. 1998;810(1–2):100–113. doi: 10.1016/s0006-8993(98)00891-9. [DOI] [PubMed] [Google Scholar]
- 37.Hsiao SH, Parrish AR, Nahm SS, Abbott LC, McCool BA, Frye GD. Effects of early postnatal ethanol intubation on GABAergic synaptic proteins. Brain Res Dev Brain Res. 2002;138(2):177–185. doi: 10.1016/s0165-3806(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 38.Ibata Y, Takahashi Y, Okamura H, Kawakami F, Terubayashi H, Kubo T, Yanaihara N. Vasoactive intestinal peptide (VIP)-like immunoreactive neurons located in the rat suprachiasmatic nucleus receive a direct retinal projection. Neurosci Lett. 1989;97(1–2):1–5. doi: 10.1016/0304-3940(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 39.Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27(27):7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaGasse LL, Messinger D, Lester BM, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Finnegan LP, Maza PL, Liu J. Prenatal drug exposure and maternal and infant feeding behaviour. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F391–F399. doi: 10.1136/fn.88.5.F391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y, Rowe J, Eskue K, West JR, Maier SE. Alcohol exposure on postnatal day 5 induces Purkinje cell loss and evidence of Purkinje cell degradation in lobule I of rat cerebellum. Alcohol. 2008;42(4):295–302. doi: 10.1016/j.alcohol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Lester BM, Lagasse L, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J, Finnegan LP, Maza PL. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr. 2003;142(3):279–285. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- 43.Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310(3):1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- 44.Linke R, Frotscher M. Development of the rat septohippocampal projection: tracing with DiI and electron microscopy of identified growth cones. J Comp Neurol. 1993;332(1):69–88. doi: 10.1002/cne.903320106. [DOI] [PubMed] [Google Scholar]
- 45.Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 2004;127C(1):42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- 46.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 47.Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatr Res. 2005;58(6):1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell JJ, Paiva M, Heaton MB. Effect of neonatal ethanol exposure on parvalbumin-expressing GABAergic neurons of the rat medial septum and cingulate cortex. Alcohol. 2000;21(1):49–57. doi: 10.1016/s0741-8329(99)00101-9. [DOI] [PubMed] [Google Scholar]
- 49.Mody I. Extrasynaptic GABAA receptors in the crosshairs of hormones and ethanol. Neurochem Int. 2008;52(1–2):60–64. doi: 10.1016/j.neuint.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17(12):517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 51.Molkhou P, Billardon M, Jouan AM, Fondarai J. Allerg Immunol. 8. Vol. 27. Paris: 1995. [Multicenter hospital study on the efficacy of Zyrtec (R) (cetirizine) compared to a placebo, in the treatment of perennial allergic rhinitis in 254 adolescents aged 12–15] pp. 300–306. [PubMed] [Google Scholar]
- 52.Moore DC. If ventricular conduction and rhythm disorders are caused by bupivacaine, it is doubtful that intraoperative hyponatremia and hyperkalemia enhance them. Anesthesiology. 1991;74(2):383–385. doi: 10.1097/00000542-199102000-00036. [DOI] [PubMed] [Google Scholar]
- 53.Moore LJ, Boehnlein JK. Treating psychiatric disorders among Mien refugees from highland Laos. Soc Sci Med. 1991;32(9):1029–1036. doi: 10.1016/0277-9536(91)90160-e. [DOI] [PubMed] [Google Scholar]
- 54.Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19(3):697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- 55.Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49(1):13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 56.Riley EP, McGee CL. Exp Biol Med. 6. Vol. 230. Maywood: 2005. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior; pp. 357–365. [DOI] [PubMed] [Google Scholar]
- 57.Sakata-Haga H, Dominguez HD, Sei H, Fukui Y, Riley EP, Thomas JD. Alterations in circadian rhythm phase shifting ability in rats following ethanol exposure during the third trimester brain growth spurt. Alcohol Clin Exp Res. 2006;30(5):899–907. doi: 10.1111/j.1530-0277.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- 58.Savage DD, Queen SA, Sanchez CF, Paxton LL, Mahoney JC, Goodlett CR, West JR. Prenatal ethanol exposure during the last third of gestation in rat reduces hippocampal NMDA agonist binding site density in 45-day-old offspring. Alcohol. 1992;9(1):37–41. doi: 10.1016/0741-8329(92)90007-w. [DOI] [PubMed] [Google Scholar]
- 59.Sei H, Sakata-Haga H, Ohta K, Sawada K, Morita Y, Fukui Y. Prenatal exposure to alcohol alters the light response in postnatal circadian rhythm. Brain Res. 2003;987(1):131–134. doi: 10.1016/s0006-8993(03)03329-8. [DOI] [PubMed] [Google Scholar]
- 60.Serra M, Sanna E, Mostallino MC, Biggio G. Social isolation stress and neuroactive steroids. Eur Neuropsychopharmacol. 2007;17(1):1–11. doi: 10.1016/j.euroneuro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Smith CN, Neptun DA, Irons RD. Effect of sampling site and collection method on variations in baseline clinical pathology parameters in Fischer-344 rats. II. Clinical hematology. Fundam Appl Toxicol. 1986;7(4):658–63. doi: 10.1016/0272-0590(86)90115-6. [DOI] [PubMed] [Google Scholar]
- 62.Spangler JG, Williams KE, Reeves KR, Billing CB, Jr, Pennington AM, Gong J. Comment and reply: A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin. 2008;24(2):577–578. doi: 10.1185/030079908x261140. author reply 578–9. [DOI] [PubMed] [Google Scholar]
- 63.Steinhausen HC, Meier M, Angst J. The Zurich long-term outcome study of child and adolescent psychiatric disorders in males. Psychol Med. 1998;28(2):375–383. doi: 10.1017/s0033291797005989. [DOI] [PubMed] [Google Scholar]
- 64.Turner DA, Shribman AJ, Smith G, Achola KJ. Effect of halothane on cardiovascular and plasma catecholamine responses to tracheal intubation. Br J Anaesth. 1986;58(12):1365–1370. doi: 10.1093/bja/58.12.1365. [DOI] [PubMed] [Google Scholar]
- 65.Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21(9):3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Frye GD. Binge-ethanol treatment alters maturation and pharmacological properties of postsynaptic GABAergic receptors in medial septum / diagonal band brain slices. SfN. Abstr. 2007;570:16. [Google Scholar]
- 67.West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1(3):213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- 68.Wilson MJ, Garcia B, Woodson M, Sinha AA. Gelatinolytic and caseinolytic proteinase activities in the secretions of the ventral, lateral, and dorsal lobes of the rat prostate. Biol Reprod. 1993;48(5):1174–1184. doi: 10.1095/biolreprod48.5.1174. [DOI] [PubMed] [Google Scholar]
- 69.Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15(3):381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- 70.Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17(11):4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]