Abstract
A major portion of the primary somatosensory cortex of rodents is characterized by the discrete and patterned distribution of thalamocortical axons and layer IV granule cells (‘barrels’), which correspond to the spatial distribution of whiskers and sinus hairs on the snout. In recent years several mutant mouse models began unveiling the cellular and molecular mechanisms by which these patterns emerge presynaptically and are reflected postsynaptically. Neural activity plays a crucial role in conferring presynaptic patterns to postsynaptic cells via neurotransmitter receptor-mediated intracellular signals. Here we review recent evidence that is finally opening the doors to understanding the cellular and molecular mechanisms of pattern formation in the neocortex.
Topographic maps of the sensory periphery, and patterning within these maps are characteristic features of primary sensory neocortical areas. In normal laboratory rodents, layer IV of the primary somatosensory (SI) cortex contains a body map proportionate to the sensory receptor density in the periphery. Within this cortical map, thalamocortical axon terminals (TCAs) from the ventroposteromedial nucleus (VPM) and layer IV granule cells form discrete modules (‘barrels’) that replicate the patterned array of whiskers and sinus hairs on the contralateral snout (Fig. 1). Since the coining of the term ‘barrels’1, lesion studies noted that when the sensory periphery is damaged during perinatal times, TCAs fail to develop their normal patterning. Consequently, layer IV granule cells do not form or maintain barrels as cytoarchitectonic modules2–4. Aberrant numbers of whiskers on the snout are also reflected by corresponding patterns in the SI barrel cortex5. Functional alterations in the barrel cortex of adult animals that have undergone whisker lesions or trimming have also been noted6–8. Several lines of evidence indicate that somatosensory periphery-related neural maps and patterns are conveyed by the afferents at each synaptic relay station9–12. However, the mechanisms by which TCAs develop patterns and how their postsynaptic partners detect them are not well understood. Recent observations from mutant mouse models have opened new venues for understanding the mechanisms of barrel differentiation13,14 (Fig. 2).
Fig. 1.
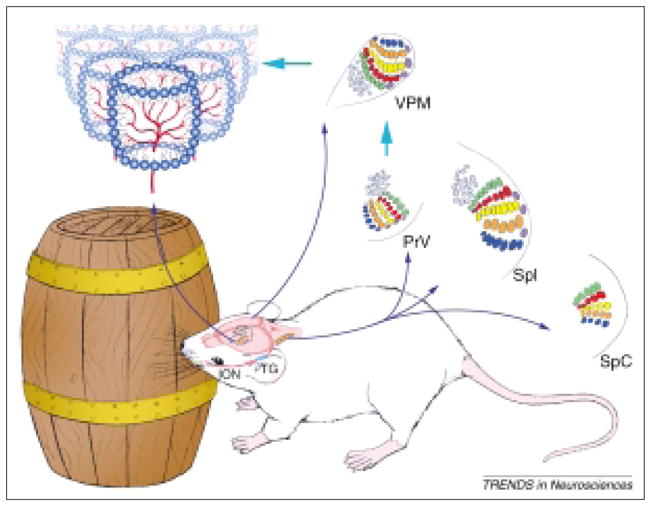
The whisker-barrel pathway of mice. The infraorbital (ION) branch of the trigeminal ganglion (TG) innervates the whiskerpad on the snout. Central counterparts of these axons deliver the patterns to the brainstem trigeminal complex, where whisker-related patches are called ‘barrelettes’.
Abbreviations: PrV, principal sensory nucleus of the trigeminal nerve; SpI, SpC, subnuclei interpolaris and caudalis of the spinal trigeminal nucleus. Barrelette neurons of the PrV carry these patterns to the ventroposteromedial nucleus (VPM) of the contralateral dorsal thalamus. In VPM, whisker-related patterns are referred to as ‘barreloids’. Barreloid or VPM neurons form the final relay to the layer IV somatosensory, ‘barrel’ cortex. At each level there is a topographically aligned map of the face (and the body) and whisker-specific patterning of axonal and cellular elements within the face map. In mice, a mature cortical barrel consists of a cell-dense barrel wall with asymmetrically projecting dendrites oriented into cell-sparse barrel hollows where incoming thalamocortical axon terminals (TCAs) segregate. These patterns are established during a sensitive period in development under the guidance of signals coming from the sensory periphery1–4.
Fig. 2.
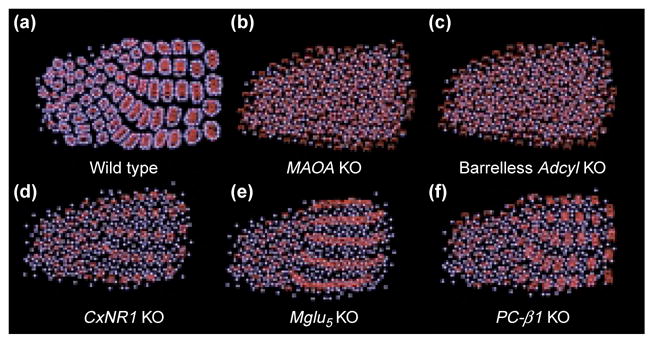
Thalamocortical axon terminals (TCAs) and barrel defects of several mutants discussed in this review. Barrel cells are depicted as blue spheres and TCAs as red arbors. In wild-type mice, TCAs form discrete patches and the distribution of these patches reflects the patterned array of whisker and sinus hair follicles on the contralateral snout. Layer IV granule cells organize around these patches forming the ‘barrels’. In Maoa and Adcyl knockout (KO) mice TCA distribution is not patterned and there are no barrels. In CxNR1 KO mice the TCAs representing the large whiskers mainly form rudimentary patches, but there are no barrels, even in this region of TCA patterning. In mglu5 KO mice, TCAs form continuous bands corresponding to five rows of whiskers, and there are no patches or barrels. In PLC-β1 KO mice, TCAs form whisker-specific patches but there are no barrels.
Gene deletion studies and in vitro assays are now revealing the molecular signals that direct topographic thalamocortical connections. For example, absence of several transcription factors prevents the normal pattern of gene expression in the thalamus and pathfinding of TCAs (Refs 15–17). Similar results have been found for the guidance molecule, semaphorin 6A (Ref. 18). Although these mutations prevent the ability of TCAs to reach appropriate cortical regions, other classes of molecules are involved in thalamocortical mapping13,14,19–21. For example, EphrinA5 knockout (KO) mice display both a distorted face map and distorted alignment of TCAs in this region, yet distinct barrel patterns are present19. Developing TCAs transiently express growth-associated phosphoprotein GAP43 (Ref. 20). In Gap43 KO mice, thalamocortical topography is severely disrupted and aberrant patches formed by TCAs no longer reflect the spatial organization of whiskers and sinus hairs21. Several neurotransmitters have also been shown to affect barrel patterning. To date, research on transgenic mice lacking a particular gene or combination of genes suggests two gross subdivisions of neurotransmitter receptor activity that are crucial for normal barrel development: presynaptic serotonergic receptor activity and postsynaptic glutamatergic receptor activity (Fig. 3).
Fig. 3.
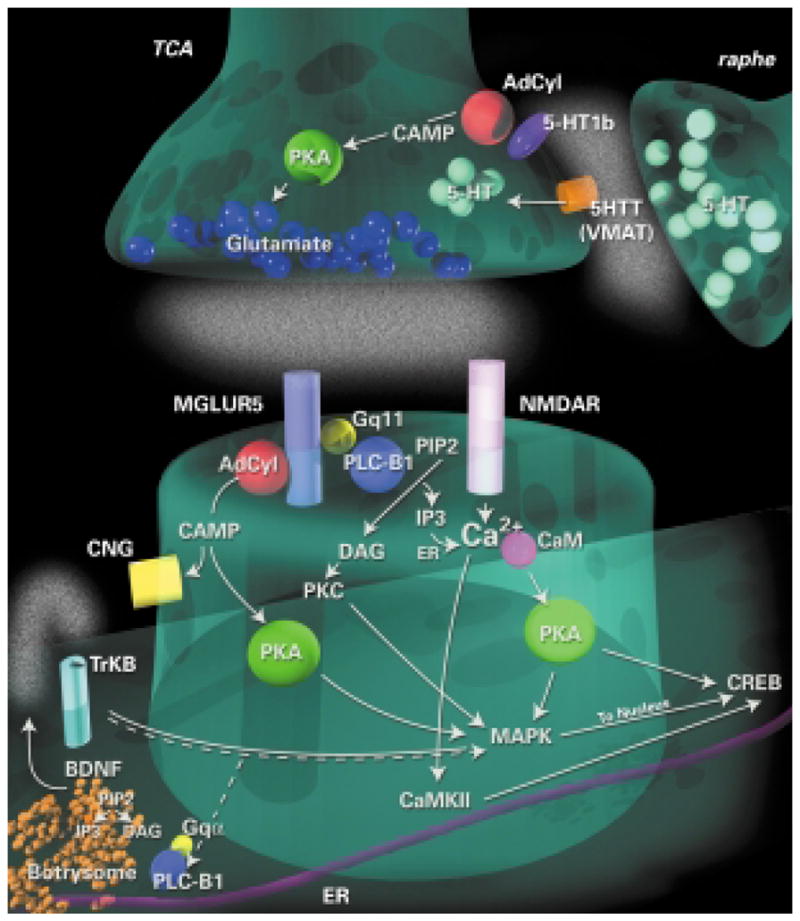
Putative intracellular signaling pathways involved in barrel differentiation. Research on transgenic mice suggests two gross subdivisions of neurotransmitter systems that are crucial for normal barrel development: presynaptic serotonergic receptor activity and postsynaptic glutamatergic receptor activity. Furthermore, the intracellular pathways downstream of these receptors are beginning to be elucidated. Abbreviations: AdCyl, adenylyl cyclase; BDNF, brain-derived neurotrophic factor; CaMKII, Ca2+–calmodulin kinase type II; CREB, cAMP response element binding protein; DAG, diacylglycerol; IP3, inositol (1,4,5)-trisphospate; MAPK, mitogen-activated protein kinase; MGLUR5; metabotropic glutamate receptor 5; NMDAR, NMDA receptor; PIP2, phosphatidylinositol (4,5)-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLC-B1, phospholipase C β1; TCA, thalamocortical axon terminals; VMAT, vesicular monoamine transporter.
Serotonergic autoregulation of thalamocortical patterning
In the late 1980s whisker-specific patterning of serotonergic fibers was noted in the developing rodent barrel cortex22,23. Combined immunohistochemistry and labeling of TCAs with lipophilic tracers, revealed that TCAs form periphery-related patterns first, followed by serotonin (5-HT)-positive fiber patterns24,25. Initially, it was thought that 5-HT-positive raphecortical projections mimicked TCA patterns22–27. A more recent study revealed that these 5-HT patterns actually reflect TCAs rather than raphecortical terminals28. Intriguingly, developing TCAs transiently express 5-HT1B receptor 29 and the serotonin transporter (SERT/5-HTT), and thalamic neurons express the genes encoding 5-HTT and the vesicular monoamine transporter (VMAT2)28,30 (Fig. 3). Although thalamic neurons do not synthesize 5-HT, their axons in the barrel cortex take up exogenous 5-HT via 5-HTT (Ref. 28). Internalized 5-HT is then stored at the nerve terminals or transported back to VPM cell bodies. In vitro recordings from thalamocortical slices indicated that 5-HT has a presynaptic inhibitory effect on glutamatergic transmission between VPM axons and barrel cortex cells31. Thus, 5-HT signaling could act as a regulator of glutamatergic transmission during patterning of TCAs. Presently, the precise role of transient 5-HT uptake and transport by TCAs during the sensitive period for pattern formation is not known.
A series of elegant studies in mice with mutations that led to increased 5-HT levels or abolished 5-HTT or 5-HT1B function now offer insights into mechanisms of axonal pattern formation in the barrel cortex32–35. Disruption of monoamine degradation enzyme, monoamine oxidase A (MAOA), either by genetic deletion or by pharmacological blockade led to a large increase in 5-HT, a permanent expansion of thalamocortical projection fields, disrupted segregation of TCAs, and lack of barrels as cytoarchitectonic entities32,34. These cortical deficits were seen even though subcortical patterns were normal. The disruption of TCA patterning was caused by elevated 5-HT levels, and not other amines, because the cortical defects in MAOA KO mice could be rescued by pharmacological blockade of 5-HT synthesis32. The same group recently showed that the deleterious effects of excess 5-HT were mediated via the 5-HT1B receptors and not 5-HT uptake via 5-HTT (Ref. 35). Although barrel patterns appear normal in 5-HT1B KO mice, genetic removal of 5-HT1B receptors in MAOA KO mice restores normal patterning of TCA terminals and near normal segregation of cortical barrels. Furthermore, 5-HTT KO mice showed similar deficits to those of the MAOA KO mice indicating a crucial role for 5-HTT in regulating extracellular 5-HT levels. 5-HT1B removal in 5-HTT KO mice or in MAOA/5-HTT double KO mice also restored barrel patterning in the cortex. In conjunction with previous findings that removal of 5-HT results in shrinkage but near normal patterning of the barrels, these data indicate that TCAs autoregulate their glutamatergic transmission via presynaptic mechanisms using 5-HT that is present in their target field. Thus, 5-HT1B receptors could act as a key switch in gating co-active, patterned inputs traveling through the thalamocortical pathway, ensuring that the activity levels are matched by the distribution of individual thalamocortical fiber terminals (Fig. 3). 5-HT involvement in cortical pattern formation is largely at the presynaptic level, and most of the deficits seen in postsynaptic elements are probably a reflection of pattern defects in their presynaptic inputs. However, the incomplete segregation of cortical barrels leaves open the possibility of a role for postsynaptic 5-HT receptors in cortical pattern formation35.
Glutamatergic neurotransmission and cortical patterning
N-methyl-D-aspartate receptors (NMDARs) are widely expressed along the developing whisker-barrel neuraxis, including all layers of the neocortex36,37. Early studies examining the role of activity during early postnatal development reported that blockade of action potentials, or NMDARs along either the trigeminal nerve or at the cortical level did not affect cortical patterning38–40. Subsequent experiments using pharmacological blockade, however, resulted in abnormalities in functional and morphological integrity of the barrels and related cortical columns41,42. The negative results obtained in the initial studies might be attributed to the timing and effectiveness of the drug applications in a system where patterns emerge at late fetal stages in the brainstem and are established shortly after birth in the neocortex43,44. Furthermore, morphological analyses were carried out using either acetylcholinesterase histochemistry (an enzyme transiently expressed by developing rat TCAs), or by cytochrome oxidase histochemistry, a ubiquitous marker for cortical barrel fields. In these studies, the distribution of layer IV granule cells and their dendritic orientation were not examined.
By contrast, targeted deletion of specific genes related to neural activity consistently showed defects in whisker-related patterning along the mouse somatosensory pathway. To date, several mutant mice have been described with deficits in axonal and/or cellular patterning. Many of these mutations involve aspects of glutamatergic synaptic communication via ionotropic and/or metabotropic receptors. Deletion of intracellular signaling molecules downstream from neural activity also show defects in barrel formation45–47 allowing, for the first time, a molecular dissection of the signaling pathways involved in cortical pattern formation and plasticity.
NMDARs and barrel development and plasticity
A role for neural activity and NMDAR-mediated activity has been underscored in the vertebrate visual system, hippocampus and neocortex7,48,49. Furthermore, LTP and LTD in the developing barrel cortex require NMDAR activation50.
The first convincing evidence that NMDARs are a key player in the development of patterned connections along the rodent somatosensory pathway came with the description of mice with complete deletion of the crucial subunit, NR1 of the NMDAR complex51. These mice had normal topographic axonal projections between the whiskerpad and the brainstem, but completely lacked barrelettes, that is, patterns within the whiskerfield map in the brainstem trigeminal complex (BSTC). Similar results were later reported for the NR2B subunit KO mice52. Because both mice are postnatally lethal, pattern formation at higher trigeminal centers could not be studied. Iwasato et al.53 addressed this problem by ‘rescuing’the NR1 KO mice by ectopic expression of a transgene of NR1 splice variants. When high levels of NR1 transgene were expressed along the somatosensory pathways in the NR1 KO background, whisker-specific patterns were restored. By contrast, low levels of transgene expression in NR1 KO background failed to restore patterns in the principal sensory nucleus of the trigeminal nerve, VPM, and the face region of the somatosensory cortex. In these mice, absence of cortical and thalamic patterns could be attributed to the lack of patterning in the principal sensory nucleus, which conveys whisker patterns to the upstream somatosensory centers. Therefore, such genetic manipulations could not directly assess the role of NMDARs at the cortical level.
Spatial precision of NMDAR deletion at the cortical level has been a major technical challenge. This technical hurdle was recently overcome54 by generating region specific NR1 KO mice using crelox gene excision under the control of the cortex-specific Emx1 promoter. In these mice (CxNR1 KO), the NR1 gene was deleted in all Emx1-expressing excitatory cortical neurons (and in hippocampus and olfactory bulbs), but remained intact elsewhere in the brain. In CxNR1 KO mice, whisker-specific patterns developed normally in the BSTC and thalamus. In the somatosensory cortex, TCAs formed rudimentary patches representing large whiskers. By contrast, layer IV granule cells failed to develop barrels, even in regions in which TCAs showed patterns. Thus, in the absence of NMDAR function, granule cells fail to detect any patterning of their presynaptic partners, and fail to organize into barrel modules.
These data further suggest that NMDAR activation is necessary for the normal maturation of a full complement of thalamocortical patterning in the somatosensory cortex. The presynaptic effect in CxNR1 KO animals might be as a result of misregulation of an NMDAR-stimulated release of a retrograde signal because the loss of NMDAR expression is restricted to cortex. Furthermore, whereas presynaptic NMDARs have been identified in the hippocampus, NMDARs in the adult somatosensory cortex appear to be largely postsynaptic55. The rudimentary patterning of TCAs in CxNR1 KO mice indicates that cortical NMDARs are not necessary for the gross patterning of TCAs. However, detailed analyses in CxNR1 KO mice showed that GABAergic interneurons escaped the NR1 deletion, because of their origin from the ganglionic eminence in which Emx1 is not expressed56,57. Hence, patterning of TCAs could be attributed to NMDAR activation in these cells, which also participate in cortical barrels58,59.
Similar to normal mice, neonatal lesions of a row of whiskers on the contralateral snout of CxNR1 KO mice resulted in fusion of the thalamocortical terminals representing the lesioned row of whiskers54. This finding appears to contradict an earlier report in which lesion-induced plasticity was prevented by APV infusion over the barrel cortex40. A notable possibility in explaining plasticity of TCAs in the CxNR1 KO mice is the residual NMDAR function in GABAergic cells; a possibility that awaits further experimental analyses.
Metabotropic glutamate receptor 5 and barrel formation
Group1 metabotropic glutamate receptors (mglu1 and mglu5) have been implicated in hippocampal and cortical plasticity60. Furthermore, they are present along the developing somatosensory pathway during the sensitive period61–63. These receptors activate intracellular messenger pathways, positively regulating phospholipase C (PLC) and adenylyl cyclase (Adcy). Mice with deletion of the Mglu5 receptor gene display segregation of large whisker TCAs into rows but not individual patches within rows, and lack barrels45. The complete loss of barrels, in spite of partial segregation of thalamic axons, indicates that mglu5 receptor signaling is also crucial for the normal transfer of patterns from TCAs to their postsynaptic partners during barrel development. Interestingly, TCAs are segregated into a primordial barrel pattern as they traverse the cortical plate, before reaching layer IV (Ref. 12). It is not known whether the mglu5 receptor is necessary for the initial segregation of TCAs or whether it is necessary for the maintenance of segregation in layer IV.
The deletion of the gene encoding the mglu5 receptor was not restricted to cortex64; thus, the defect in thalamocortical axon patterning might be caused by incomplete barreloid formation in VPM and/or improper afferent signaling. There is evidence for a presynaptic mglu receptor in cortex that couples to PLC signaling pathways65, although the precise mglu receptor subtype is not known. However, in adult animals, ultrastructural localization of mglu5 indicates postsynaptic localization63. If it is the case that the locus of the defect in Mglu5 KO mice is postsynaptic, retrograde signal(s) activated via mglu5 and NMDAR signaling might be crucial for the maintenance of thalamocortical patterning. Furthermore, the differences in the defects in the TCAs in these two animals suggest multiple retrograde molecules might be necessary.
Intracellular pathways
An understanding of the intracellular mechanisms by which receptor activity leads to altered structural and functional changes requires a dissection of the second messenger systems activated following receptor stimulation (Fig. 3). Several mutations in genes downstream from serotonergic and glutamatergic pathways in mice revealed phenotypes with disrupted thalamocortical or barrel patterning.
In 1996, a spontaneous mutation was reported, in which TCAs failed to pattern and no barrels formed66. These ‘barrelless’mice exhibited normal laminar and topographic distribution of TCAs, but the overall thalamocortical projection field was larger, similar to that seen in MAOA KO mice (see Fig. 2). The mutation in the barrelless mice has been identified as mutation of the Adcy type I gene67. The gene product is a membrane-bound enzyme that catalyses the formation of cAMP and subsequently regulates the activity of the cAMP-dependent protein kinase A (PKA) and cyclic nucleotide-gated ion channels68. Because the 5-HT1B receptor negatively couples to Adcy activity, these findings suggest that cAMP-dependent pathways regulate thalamocortical glutamatergic transmission and synaptic refinement, but additional postsynaptic defects cannot be excluded67. Several postsynaptic receptors, including mglu5 receptors, also regulate Adcy activity. Indeed, differential effects on cortical neurons might explain functional differences in the barrel cortex of Adcy1 KO and MAOA KO mice69. Indeed, deletion of the RIIβ isoform of PKA results in a near complete loss of cortical barrels, in spite of TCA segregation in the posterior medial barrel subfield (P. Neumann, pers. comm.). Therefore, the loss of TCA segregation in the Adcy1 KO mice is not solely mediated by PKA. Furthermore, the dramatic effects on cortical segregation strongly suggest a postsynaptic role for PKA in barrel formation, possibly as a downstream target of cAMP generated following mglu activation or via NMDA activation of calmodulin70.
Mice lacking the gene encoding phospholipase C-β1 (PLC-β1) also exhibit defects in barrel formation without affecting patterning of TCAs (Ref. 45) (Fig. 2). PLC-β1 is a G-protein-activated phosphodiesterase that hydrolyses phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] into two second messengers: inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3] and diacylglycerol (DAG). Ins(1,4,5)P3 regulates intracellular Ca2+ levels by causing its release from the endoplasmic reticulum, and DAG controls the level of phosphorylating agent protein kinase C (PKC). The expression of PLC-β1 in the developing cortex is largely postsynaptic71, and phosphoinositide hydrolysis following activation of the group 1 mglu receptors is dependent on PLC-β1 expression during the first postnatal45 week β1 is indicating that mglu5 receptor activation of PLC-crucial for barrel formation (Fig. 3). However, whereas the cortical deficits observed in Mglu5 KO mice appear to be mediated through receptor activation of PLC-β1, the effects on thalamic afferent segregation in Mglu5 KO mice must be mediated by another pathway, possibly via activation of Adcy1.
Ultrastructural localization of PLC-β1 gave insight into a possible cellular locus for the defects manifested in these knockout mice. PLC-β1 is concentrated in intermediate-compartment-like organelles called ‘botrysomes’, which are found within and near dendritic roots72,73. Apart from their more traditional roles in intracellular signaling (see above) PtdIns(4,5) P2, Ins(1,4,5)P3 and DAG have all been shown to play a role in vesicular trafficking. Thus, PLC-β1 activation might regulate protein trafficking into dendrites in response to synaptic activity during dendritic differentiation, perhaps by regulating the release of neurotrophins because preproBDNF can be purified from the botrysome (Fig. 3)72. The primary locus of the postsynaptic neuronal defect in CxNR1, Mglu5, PkarIIβ and PLC-β1 KO mice is unknown. Three distinct, yet potentially overlapping, possibilities exist: (1) dendrites fail to develop, remaining in a relatively immature state; (2) dendrites develop, but are unable to respond to cues from TCAs and fail to reorient into the barrel hollows and (3) cortical neurons are unable to migrate to the barrel walls. Our preliminary evidence from PLC-β1 and CxNR1 KOs suggests that dendrites do develop a relatively normal level of complexity, indicating that they might be unable to organize around incoming TCAs.
Postsynaptic pathways involved in transfer of patterns from TCAs to barrel cells
Do the defects in the CxNR1 and Mglu5 KO mice reflect distinct cellular mechanisms, both necessary, but insufficient for cortical differentiation? Or, do these receptors activate overlapping intracellular pathways and similar downstream effector molecules? Recent isolation and characterization of a postsynaptic NMDA receptor complex, or ‘Hebbosome’, suggests that NMDARs and group 1 mglu receptors might be acting via overlapping, or synergistic mechanisms74,75. This 2000 kDa multiprotein complex contains at least 77 proteins, 49 of which participate in NMDAR signaling. The component proteins fall into five main categories: (1) NMDARs and mglu receptors (but not the AMPA receptors), (2) adaptor proteins, (3) protein kinases and phosphatases, (4) cytoskeletal proteins, and (5) cell adhesion proteins. Both PKARIIβ (Ref. 74) and PLC-β1 (S.G.N. Grant and H. Husi, pers. commun.) are components of the Hebbosome, further supporting a role for this multiprotein complex in cortical differentiation. A striking feature of the composition of the Hebbosome is that there appears to be ‘modules’ or sets of signaling proteins that are known to comprise key components of signal transduction pathways. This raises the possibility of dissecting distinct pathways within the Hebbosome that underlie barrel formation. The points of potential cross-talk between NMDAR and mglu5 receptor signaling in the developing barrel cortex are not known, however, there are several candidate Hebbosome constituents including mitogen-activated protein kinase (MAPK), which can be activated by both PKA and PKC (Ref. 76), and calmodulin-dependent protein kinase (CAMK). A point mutation in the Camk2α gene (α-Cam Kinase II) that abolishes autophosphorylation prevents physiological plasticity in the barrel cortex of juvenile and adult animals77. Furthermore, CAMK, MAPK and PKA can activate transcription factors including cAMP response element-binding protein (CREB), which has been implicated in adult barrel plasticity78,79. Taken together these findings suggest that Hebbosomes integrate signals arising from different patterns of activity and then instruct the subsequent intracellular changes.
Concluding remarks
It has been a little over thirty years since the coining of the term ‘barrels’by Woolsey and Van de Loos1. Hendrik Van der Loos envisioned that the key to understanding barrel cortex development and plasticity lies in the analysis of genetic alterations. To this end, he and his colleagues bred numerous mice expecting to obtain spontaneous mutations that would help us understand how the barrel cortex organization is matched to the sensory periphery5. The ‘barrelless’ mice emerged in the colony established by them, a few years after the untimely passing away of Van der Loos. Present-day mouse molecular genetics and transgenic and knockout strategies are now expediting this vision, unveiling the molecular and cellular mechanisms of sensory map formation, patterning and plasticity in the mammalian brain.
Acknowledgments
We thank E. New, B. Genc, and A. Haeberle for production of Figs 1 and 2, and J. Gordon and Biomedical Illustration for producing Fig. 3. We also thank P. Neumann for helpful discussions and comments on the manuscript.
Contributor Information
Reha S. Erzurumlu, Email: rerzur@lsuhsc.edu, Dept of Cell Biology and Anatomy and Neuroscience Center, LSUHSC, New Orleans, LA 70112, USA
Peter C. Kind, Email: pkind@ed.ac.uk, Dept of Biomedical Sciences, Edinburgh University, Edinburgh, UK EH8 9XD
References
- 1.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of the mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 2.Woolsey TA. In: Development of Sensory Systems in Mammals. Coleman EJ, editor. John Wiley & Sons; 1990. pp. 461–516. [Google Scholar]
- 3.O’Leary DDM, et al. Development, critical period plasticity, and adult reorganizations of mammalian somatosensory systems. Curr Biol. 1994;4:535–544. doi: 10.1016/0959-4388(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 4.Killackey HP, et al. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- 5.Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W, et al. Contribution of supragranular layers to sensory processing and plasticity in adult rat barrel cortex. J Neurophysiol. 1998;80:3261–3271. doi: 10.1152/jn.1998.80.6.3261. [DOI] [PubMed] [Google Scholar]
- 7.Rema V, et al. Experience-dependent plasticity of adult rat S1 cortex requires local NMDA receptor activation. J Neurosci. 1998;18:10196–10206. doi: 10.1523/JNEUROSCI.18-23-10196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebedev MA, et al. Experience-dependent plasticity of rat barrel cortex: redistribution of activity across barrel-columns. Cereb Cortex. 2000;10:23–31. doi: 10.1093/cercor/10.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- 10.Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- 12.Agmon A, et al. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molnar Z, et al. The role of the first postmitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J Neurosci. 1998;18:5746–5765. doi: 10.1523/JNEUROSCI.18-15-05746.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molnar Z, Hannan AJ. Development of thalamocortical projections in normal and mutant mice. Results Probl Cell Differ. 2000;30:293–332. doi: 10.1007/978-3-540-48002-0_13. [DOI] [PubMed] [Google Scholar]
- 15.Miyashita-Lin EM, et al. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- 16.Tuttle R, et al. Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development. 1999;126:1903–1916. doi: 10.1242/dev.126.9.1903. [DOI] [PubMed] [Google Scholar]
- 17.Pratt T, et al. A role for Pax6 in the normal development of dorsal thalamus and its cortical connections. Development. 2000;127:5167–5178. doi: 10.1242/dev.127.23.5167. [DOI] [PubMed] [Google Scholar]
- 18.Leighton PA, et al. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- 19.Vanderhaeghen P, et al. A mapping label required for normal scale of body representation in the cortex. Nat Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- 20.Erzurumlu RS, et al. Transient patterns of GAP-43 expression during the formation of barrels in the rat somatosensory cortex. J Comp Neurol. 1990;292:443–456. doi: 10.1002/cne.902920310. [DOI] [PubMed] [Google Scholar]
- 21.Maier DL, et al. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci U S A. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimiya M, et al. Postnatal development of serotonin nerve fibers in the somatosensory cortex of mice studied by immunohistochemistry. J Comp Neurol. 1986;246:191–201. doi: 10.1002/cne.902460205. [DOI] [PubMed] [Google Scholar]
- 23.D’Amato RJ, et al. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erzurumlu RS, Jhaveri S. Emergence of connectivity in the embryonic rat parietal cortex. Cereb Cortex. 1992;2:336–352. doi: 10.1093/cercor/2.4.336. [DOI] [PubMed] [Google Scholar]
- 25.Blue ME, et al. A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cereb Cortex. 1991;1:380–389. doi: 10.1093/cercor/1.5.380. [DOI] [PubMed] [Google Scholar]
- 26.Rhoades RW, et al. Development and lesion induced reorganization of the cortical representation of the rat’s body surface as revealed by immunocytochemistry for serotonin. J Comp Neurol. 1990;293:190–207. doi: 10.1002/cne.902930204. [DOI] [PubMed] [Google Scholar]
- 27.Bennett-Clarke CA, et al. The source of the transient serotonergic input to the developing visual and somatosensory cortices in rat. Neuroscience. 1991;43:163–183. doi: 10.1016/0306-4522(91)90425-n. [DOI] [PubMed] [Google Scholar]
- 28.Lebrand C, et al. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 29.Bennett-Clarke CA, et al. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on TCAs. Proc Natl Acad Sci U S A. 1993;90:153–157. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebrand C, et al. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- 31.Rhoades RW, et al. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol. 1994;72:2438–2450. doi: 10.1152/jn.1994.72.5.2438. [DOI] [PubMed] [Google Scholar]
- 32.Cases O, et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 33.Cases O, et al. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitalis T, et al. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: Determination of a sensitive developmental period. J Comp Neurol. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Salichon N, et al. Excessive action of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase A and 5-HT transporter knockout mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blue ME, Johnston MV. The ontogeny of glutamate receptors in rat barrel field cortex. Dev Brain Res. 1995;14:11–25. doi: 10.1016/0165-3806(94)00147-r. [DOI] [PubMed] [Google Scholar]
- 37.Rema V, Ebner FF. Postnatal changes in NMDAR1 subunit expression in the rat trigeminal pathway to barrel field cortex. J Comp Neurol. 1996;2:165–184. doi: 10.1002/(SICI)1096-9861(19960429)368:2<165::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Chiaia NL, et al. Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissa-related patterns in the rat’s somatosensory cortex. Dev Brain Res. 1992;66:244–250. doi: 10.1016/0165-3806(92)90086-c. [DOI] [PubMed] [Google Scholar]
- 39.Henderson TA, et al. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Dev Brain Res. 1992;66:146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- 40.Schlaggar BL, et al. Postsynaptic control of synaptic plasticity in developing somatosensory cortex. Nature. 1993;364:623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- 41.Fox K, et al. Glutamate receptor blockade at cortical synapses disrupts development of thalamocortical and columnar organization in somatosensory cortex. Proc Natl Acad Sci U S A. 1996;93:5584–5589. doi: 10.1073/pnas.93.11.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitrovich N, et al. Effects of NMDA receptor blockade in the developing rat somatosensory cortex on the expression of the glia-derived extracellular matrix glycoprotein tenascin-C. Eur J Neurosci. 1996;8:1793–1802. doi: 10.1111/j.1460-9568.1996.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 43.Chiaia NL, et al. Evidence for prenatal competition among the central arbors of trigeminal primary afferent neurons. J Neurosci. 1992b;12:62–76. doi: 10.1523/JNEUROSCI.12-01-00062.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlaggar BL, et al. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- 45.Hannan AJ, et al. Phsopholipase C-β1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosci. 2001;4:282–288. doi: 10.1038/85132. [DOI] [PubMed] [Google Scholar]
- 46.Welker E, et al. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Majid RM, et al. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nature Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- 48.Cramer K, Sur M. Activity-dependent remodeling of connections in the mammalian visual system. Curr Opin Neurobiol. 1995;5:106–111. doi: 10.1016/0959-4388(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 49.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 50.Feldman DE, et al. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- 51.Li Y, et al. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:422–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 52.Kutsuwada T, et al. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 53.Iwasato T, et al. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 54.Iwasato T, et al. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valtschanoff JG, et al. Expression of NR2 receptor subunit in rat somatic sensory cortex: synaptic distribution and colocalization with NR1 and PSD-95. J Comp Neurol. 1999;410:599–611. [PubMed] [Google Scholar]
- 56.Lavdas AA, et al. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parnavelas JG. The origin and migration of cortical neurones: new vistas. Trends Neurosci. 2000;23:126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- 58.Lin CS, et al. Glutamic acid decarboxylase immunoreactivity in layer IV barrel cortex of rat and mouse. J Neurosci. 1985;7:1934–1939. doi: 10.1523/JNEUROSCI.05-07-01934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiser PJ, et al. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 1998;402:62–74. [PubMed] [Google Scholar]
- 60.Bortolotto ZA, et al. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol. 1999;9:299–304. doi: 10.1016/s0959-4388(99)80044-0. [DOI] [PubMed] [Google Scholar]
- 61.Blue ME, et al. Ontogeny of non-NMDA glutamate receptors in rat barrel field cortex: I. Metabotropic receptors. J Comp Neurol. 1997;386:16–28. [PubMed] [Google Scholar]
- 62.Munoz A, et al. Development of metabotropic glutamate receptors from trigeminal nuclei to barrel cortex in postnatal mouse. J Comp Neurol. 1999;409:549–566. [PubMed] [Google Scholar]
- 63.Romano C, et al. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 64.Lu Y-M, et al. Mice lacking mGluR5 show impaired learning and reduced CA1 LTP, but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez-Prieto J, et al. Presynaptic receptors and the control of glutamate exocytosis. Trends Neurosci. 1996;19:235–239. doi: 10.1016/0166-2236(96)10031-x. [DOI] [PubMed] [Google Scholar]
- 66.Welker E, et al. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- 67.Abdel-Majid RM, et al. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- 68.Roy DR, Barnstable CJ. Temporal and spatial pattern of expression of cyclic nucleotide-gated channels in developing rat visual cortex. Cereb Cortex. 1999;9:340–347. doi: 10.1093/cercor/9.4.340. [DOI] [PubMed] [Google Scholar]
- 69.Yang Z, et al. Differences in somatosensory processing in S1 barrel cortex between normal and monoamine oxidase A knockout (Tg8) adult mice. Cereb Cortex. 2001;11:26–36. doi: 10.1093/cercor/11.1.26. [DOI] [PubMed] [Google Scholar]
- 70.Kind PC, Neumann PE. Plasticity: downstream of glutamate. Trend Neurosci. 2001;24:553–555. doi: 10.1016/s0166-2236(00)01921-4. [DOI] [PubMed] [Google Scholar]
- 71.Spires T, et al. Ultrastructural analysis of PLC–β expression in developing cortex. Soc Neurosci Abstr. 2000;26:68. [Google Scholar]
- 72.Kind PC, et al. Phospholipase C-β1 is present in the botrysome, an intermediate compartment-like organelle, and its regulation by visual experience in cat visual cortex. J Neurosci. 1997;17:1471–1480. doi: 10.1523/JNEUROSCI.17-04-01471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hannan AJ, et al. Phospholipase C-β1 expression correlates with neuronal differentiation and synaptic plasticity in rat somatosensory cortex. Neuropharmacol. 1998;37:593–605. doi: 10.1016/s0028-3908(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 74.Husi H, et al. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 75.Husi H, Grant S. Proteomics of the nervous system. Trends Neurosci. 2001;24:259–266. doi: 10.1016/s0166-2236(00)01792-6. [DOI] [PubMed] [Google Scholar]
- 76.Roberson ED, et al. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glazewski S, et al. The role of α-CamKII autophosphorylation in neocortical experience-dependent plasticity. Nat Neurosci. 2000;3:911–918. doi: 10.1038/78820. [DOI] [PubMed] [Google Scholar]
- 78.Glazewski S, et al. Impaired experience-dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb Cortex. 1999;9:249–256. doi: 10.1093/cercor/9.3.249. [DOI] [PubMed] [Google Scholar]
- 79.Barth AL, et al. Upregulation of cAMP response element-mediated gene expression during experience-dependent plasticity in adult neocortex. J Neurosci. 2000;20:4206–4216. doi: 10.1523/JNEUROSCI.20-11-04206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


