Abstract
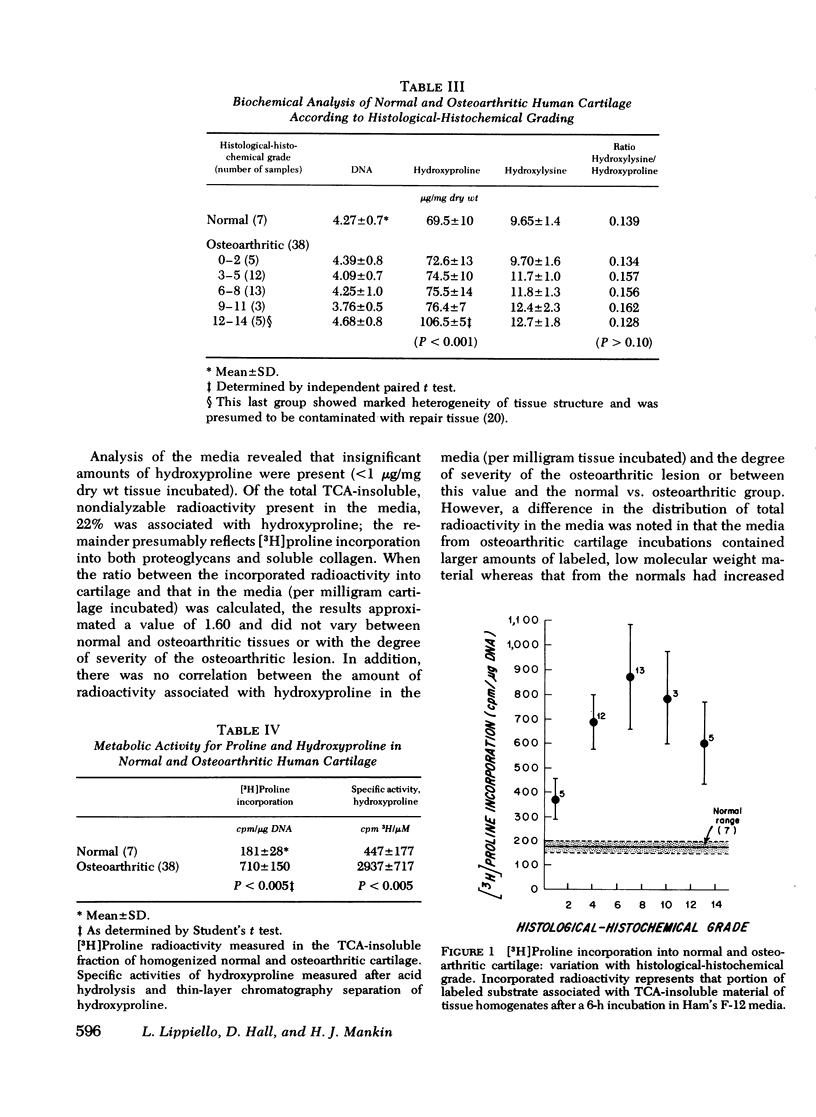
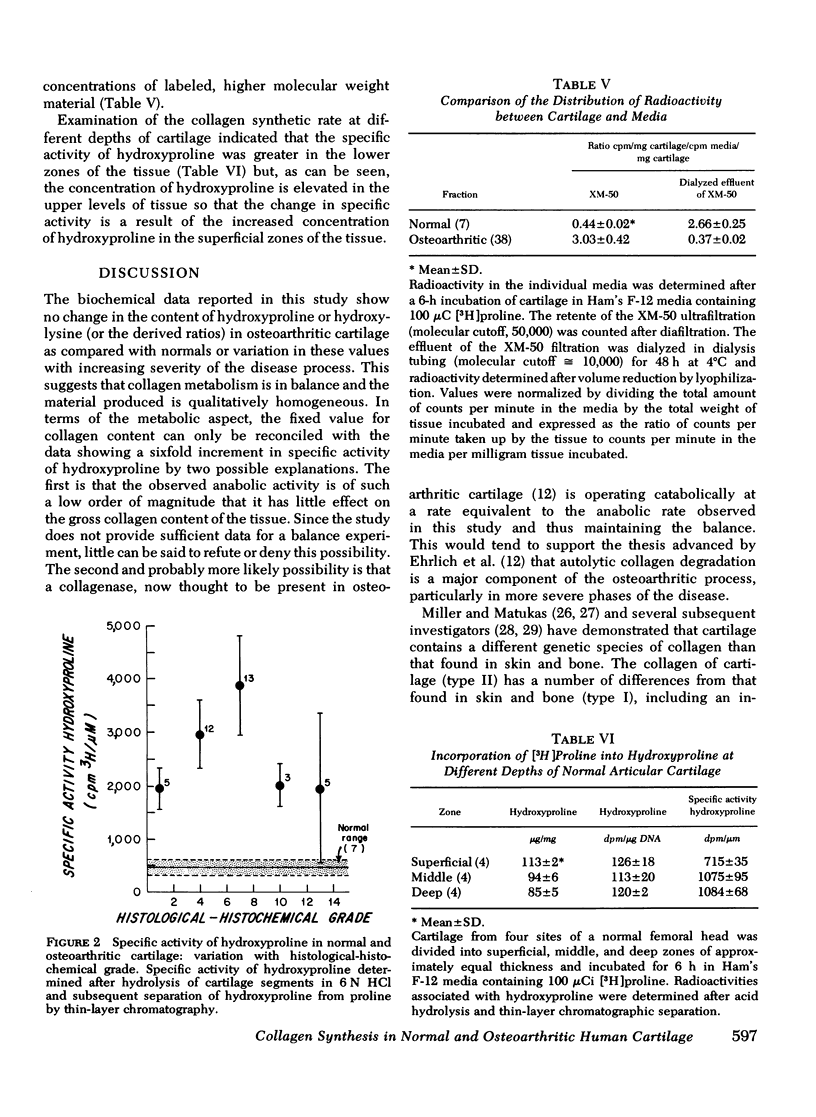
Collagen metabolism in osteoarthritic human articular cartilage was compared to that in normal cartilage and was also correlated with the degree of severity of the osteoarthritic lesion as determined by a histological-histochemical grading system. No correlation was apparent between the concentrations of DNA, hydroxyproline, and hydroxylysine and the degree of severity of the osteoarthritic lesion (except in far-advanced lesions). Similarly, there was no correlation in levels of these components in tissues from the normal vs. osteoarthritic group. The similarity of the values of the ratio hydroxylysine/hydroxyproline in osteoarthritic tissue compared with normal, and the lack of variation in these with increasing severity of the disease process argues against the possibility that osteoarthritis is associated with a major shift in the synthesis of type II collagen to type I. [3H]Proline incorporation into osteoarthritic cartilage was increased fourfold as compared to normal cartilage and varied with advancing histological-histochemical grade. Measurement of the specific activity of insolubilized hydroxyproline-containing material of the cartilage matrix, as an index of the turnover of collagen, showed a sixfold increase in osteoarthritic cartilage which also varied with grade. These data suggest that collagen synthesis in these tissues is substantially greater than in nonosteoarthritic tissues and varies directly with the severity of the disease process up to a point and then varies inversely as the lesion becomes more severe.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L. Enzymic degradation of cartilage in osteoarthritis. Fed Proc. 1973 Apr;32(4):1494–1498. [PubMed] [Google Scholar]
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLET A. J., HANDY J. R., STURGILL B. C. Chondroitin sulfate concentration and protein-polysaccharide composition of articular cartilage in osteoarthritis. J Clin Invest. 1963 Jun;42:853–859. doi: 10.1172/JCI104777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONTING S. L., JONES M. Determination of microgram quantities of deoxyribonucleic acid and protein in tissues grown in vitro. Arch Biochem Biophys. 1957 Feb;66(2):340–353. doi: 10.1016/s0003-9861(57)80009-5. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. An improved method for the assay of hydroxylysine. Anal Biochem. 1973 Nov;56(1):10–15. doi: 10.1016/0003-2697(73)90163-2. [DOI] [PubMed] [Google Scholar]
- Brandt K. D. Enhanced extractability of articular cartilage protoglycans in osteoarthrosis. Biochem J. 1974 Nov;143(2):475–478. doi: 10.1042/bj1430475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. G., Mankin H. J., Treadwell B. V. Acid hydrolase activity in osteoarthritic and normal human cartilage. J Bone Joint Surg Am. 1973 Jul;55(5):1068–1076. [PubMed] [Google Scholar]
- Fukae M., Mechanic G. L. Chromatographically different type II collagens from human normal and osteoarthritic cartilage. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1575–1580. doi: 10.1016/0006-291x(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Howell D. S. Pemberton lecture. Degradative enzymes in osteoarthritic human articular cartilage. Arthritis Rheum. 1975 Mar-Apr;18(2):167–177. doi: 10.1002/art.1780180215. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970 Apr;52(3):424–434. [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The turnover of adult rabbit articular cartilage. J Bone Joint Surg Am. 1969 Dec;51(8):1591–1600. [PubMed] [Google Scholar]
- Maroudas A., Evans H., Almeida L. Cartilage of the hip joint. Topographical variation of glycosaminoglycan content in normal and fibrillated tissue. Ann Rheum Dis. 1973 Jan;32(1):1–9. doi: 10.1136/ard.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A. Biochemistry of articular cartilage. Nature of proteoglycans and collagen of articular cartilage and their role in ageing and in osteoarthrosis. Ann Rheum Dis. 1973 Jul;32(4):364–378. doi: 10.1136/ard.32.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Van der Korst J. K., Sokoloff L. Collagen of human articular and costal cartilage. Arthritis Rheum. 1969 Feb;12(1):21–29. doi: 10.1002/art.1780120105. [DOI] [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- Repo R. U., Mitchell N. Collagen synthesis in mature articular cartilage of the rabbit. J Bone Joint Surg Br. 1971 Aug;53(3):541–548. [PubMed] [Google Scholar]
- Sapolsky A. I., Altman R. D., Woessner J. F., Howell D. S. The action of cathepsin D in human articular cartilage on proteoglycans. J Clin Invest. 1973 Mar;52(3):624–633. doi: 10.1172/JCI107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M., Brickley D. M., Glimcher M. J. The identification of two types of collagen in the articular cartilage of postnatal chickens. Calcif Tissue Res. 1974;17(1):43–55. doi: 10.1007/BF02547213. [DOI] [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Weiss C., Rosenberg L., Helfet A. J. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg Am. 1968 Jun;50(4):663–674. doi: 10.2106/00004623-196850040-00002. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Cartilage cathepsin D and its action on matrix components. Fed Proc. 1973 Apr;32(4):1485–1488. [PubMed] [Google Scholar]



