Abstract
Background
Hantavirus disease is a zoonosis of increasing clinical importance. A new incidence peak was reached in Germany in 2012, with more than 2800 reported cases. These viruses are transmitted from small mammals to human beings. The disease begins with high fever and non-pathognomonic manifestations that can end in shock and organ failure.
Methods
This article is based on a selective literature search, on the authors’ experiences at the National Referral Laboratory for Hantavirus Infections (Nationales Konsiliarlaboratorium für Hantaviren), and on published recommendations from Germany and abroad.
Results
Two hantavirus species cause clinically relevant infections in Germany. Puumala virus, which is transmitted by bank voles, causes large outbreaks of disease every 2 to 3 years in the southwestern and western regions of Germany and in the Bavarian Forest. Dobrava-Belgrad virus, transmitted by striped field mice, causes infections in the north and east of the country. Serological tests are available for primary and confirmatory diagnosis; moreover, viral nucleic acids can be amplified in the early phase of illness and compared with the viral nucleic acids from the reservoir hosts of the corresponding type of infection. Infections with American types of hantavirus have ca. 35% case fatality, and hantaviruses from southeastern Europe and Asia are also highly pathogenic; in contrast, the febrile illnesses caused by hantaviruses in Germany are usually relatively mild.
Conclusion
When persons living in high-risk areas present with fever of unknown origin or with renal dysfunction of unknown origin, physicians should consider the possibility of a hantavirus infection and should initiate the appropriate diagnostic evaluation.
The threat to human health from transferred zoonotic viruses is a highly current debate. In addition to the repeated transmission of influenzaviruses from animals to humans, the threat from the new coronaviruses is also under intense discussion. After the SARS outbreak in 2003, it was only recently that a new coronavirus, HCoV-EMC—which triggers lung infections with high case fatality—was introduced into Germany (1). This virus is assumed to be transmitted to humans by certain species of bat (2). Another zoonotic disease, which affects many more patients, is hantavirus disease (3, 4). Human pathogenic hantaviruses are transmitted to humans from infected rodents. However, these viruses have recently also been shown to be present in shrews, moles, and now even bats (5).
Recent developments
In 2012, 2824 cases of hantavirus disease in Germany were reported to the Robert Koch Institute. This is the highest number since hantavirus infection was made a notifiable disease in 2001 (Robert Koch Institute, SurvStat, www3.rki.de/SurvStat). In Germany, the most striking features of hantavirus disease are initially high fever and impaired renal function. Although this infectious disease was almost unknown in Germany until quite recently, both among doctors and among the general public, hantavirus disease (along with norovirus and rotavirus disease, flu, and hepatitis C) is among the five most common notifiable viral diseases today. This calls for renewed efforts in research into this disease, including its epidemiology, prevention, and treatment.
In addition, at the beginning of September 2012, news made the headlines that in the Yosemite National Park in California, an outbreak of hantavirus disease had occurred in which cardiopulmonary complications were the most striking feature. Of the ten patients affected, three died (6). Hantavirus disease is rarer in America than in Europe and Asia, but the clinical course is more severe (7).
Clinical manifestation
The incubation time is usually 2 to 3 weeks, although both shorter and longer times (up to 6 weeks) have been observed (8). The disease develops through various stages (3, 4, 9– 11); the symptoms are not pathognomonic, especially the early ones. The disease starts with:
Abrupt fever
Headache, stomach ache, aching flanks and/or back
In many cases, nausea/vomiting
Chills
Conjunctivitis
Often transient visual disturbances.
In this first stage, patients are often treated with analgetics and antipyretics, which are later mistakenly blamed for the later-occurring renal failure that is actually due to the infection. The different clinical stages can be identified more or less clearly depending on the severity of the disease. The 3- to 4-day febrile stage is followed by a hypotensive stage during which other hemostatic disturbances may also occur, which may manifest as microhemorrhage (including skin petechiae and conjunctival bleeding). Next comes the oliguric stage (Box 1). This critical stage is followed by polyuria and convalescence. Clinical symptoms and parameters on which a suspected diagnosis of hantavirus disease may be based in Germany are summarized in Box 2.
Box 1. Characteristic symptoms and findings in the acute stages of hantavirus disease.
Stage 1
High fever (3–4 days); non-specific “flu-like” symptoms such as headache, myalgia, chills, and conjunctivitis; severe, often colic-like flank pain, abdominal pain, nausea and vomiting
Stage 2
Hypotension; other hemostatic disorders; conjunctival bleeding and skin petechiae
Stage 3
Renal failure; marked proteinuria and microhematuria; creatinemia; thrombocytopenia, leukocytosis, uremia and oligo-/anuria; extrarenal manifestations (e.g., CNS involvement or severe pulmonary symptoms) may be seen
Further stages
Polyuria
Convalescence
Course of disease due to hantaviruses occurring in America
High fever, cough, hypoxia, shock, pulmonary edema with tachypnea, dyspnea, and non-productive cough, interstitial pneumonia with mononuclear infiltrates, adult respiratory distress syndrome (ARDS)
Box 2. Criteria for a suspected diagnosis of hantavirus disease.
Acute onset of illness with fever >38.5 °C
Back pain/headache/abdominal pain
Proteinuria and/or hematuria
Raised serum creatinine
Thrombocytopenia
Oliguria and subsequent polyuria
If at least four of the above criteria are present, a suspected diagnosis of hantavirus disease (hemorrhagic fever with renal syndrome) should be made (From 33)
Life-threatening developments can occur in the form of shock during the hypotensive stage or the occurrence of renal and/or cardiopulmonary failure. The frequency with which these life-threatening processes develop correlates with the degree of virulence of the various hantaviruses and the case fatality they trigger. In severe cases, the patient requires dialysis or extracorporeal oxygenation. Externally visible bleeding is typical of infection by the hantaviruses found in Asia, and for this reason the World Health Organization (WHO) has suggested the name “hemorrhagic fever with renal syndrome” (HFRS). In hantavirus-induced cardiopulmonary syndrome (HCPS), which is caused by hantaviruses circulating in America, the striking features are hypoxia and bilateral interstitial pulmonary infiltrates that start 2 days after disease onset (3, 7). Since, however, organ damage is not always restricted to the main target organ, and HFRS in Europe is associated with much less external hemorrhage than in Asia, the general term “hantavirus disease” is coming into increasing use (12). The Robert Koch Institute, by the way, has been using this term for years.
Renal retention values start to rise during the febrile stage, reaching their highest during the oliguric stage. The rise in serum creatinine concentration, which is often dramatic (>620 µmol/L) (13), is combined with proteinuria and microhematuria in the reduced amount of urine. Paraclinically, thrombocytopenia and, often, leukocytosis are found. In addition to the renal and/or pulmonary manifestations, in some cases other organ involvement is seen, for example concomitant hepatitis, myocarditis, thyroiditis, panhypopituitarism, or cerebral manifestations. Patients who survive hantavirus disease usually recover without sequelae, but renal hypertension is under discussion as a possible late consequence (14). The severity of hantavirus disease depends very much on which type of the virus has infected the patient (Table).
Table. Important human pathogenic hantaviruses.
| Disease | Virus species ►Genotype | Reservoir host | Distribution of the virus and of the disease | Mortality (approx.) (4, 7, 30– 32) |
|---|---|---|---|---|
| HFRS | Puumala virus (PUUV)*1 | Bank vole (Myodes glareolus) | Europe | < 1% |
| Dobrava-Belgrade virus (DOBV)*2 ► Kurkino (DOBV-Aa)*1 ► Dobrava (DOBV-Af) ► Sochi (DOBV-Ap) |
Striped field mouse (Apodemus agrarius) Yellow-necked mouse (Apodemus flavicollis) Black Sea field mouse (Apodemus ponticus) |
Central and Eastern Europe Balkans Russia (Crimea) |
0.3–0.9% 10–12% >6% |
|
| Tula virus (TULV)*1 | Common vole (Microtus arvalis) | Europe*3 | ? | |
| Hantaan virus (HTNV) | Striped field mouse (Apodemus agrarius) | Asia | 10–12% | |
| Seoul virus (SEOV) | Rats (Rattus rattus, Rattus norvegicus) | Asia and possibly worldwide | 1–2% | |
| HCPS | Sin Nombre virus (SNV) | Deer mouse (Peromyscus maniculatus) | North America | 35% |
| Andes virus (ANDV) | Long-tailed pygmy rice rat (Oligoryzomys longicaudatus) | South America | 35% |
*1Hantaviruses in circulation in Germany. The large majority of clinical cases are due to PUUV infection; in the north-eastern parts of the country, DOBV infections occur.
*2The fourth genotype of the Dobrava–Belgrade virus demonstrated in Europe is the Saaremaa genotype, which is sometimes regarded as a separate species of the virus. Since no case of infection of a human by the Saaremaa genotype has yet been verified, it is not regarded as a human pathogen (19).
*3There is only one clinical report of a case of TULV infection.
HFRS, hemorrhagic fever with renal syndrome; HCPS, hantavirus-induced cardiopulmonary syndrome
The pathogenesis of hantavirus disease is marked by vasodilation and disturbances of the capillary endothelial barrier, resulting in extravasation of blood and inflammatory processes in the internal organs, such as the kidneys. At the same time, changes occur in the coagulability of the blood. The strength of the specific CD8-T-cell response and the synthesis of inflammatory cytokines appears to be correlated to the severity of disease (4). In addition, there have been first observations of an interaction between pathogenic hantaviruses and platelet precursor cells and with the platelets themselves (15). So far, attempts to turn these first results about the immune pathogenesis into a therapeutic strategy have failed.
Pathogens and their natural hosts
Hantaviruses are negative-sense RNA viruses with three genome segments and a capsid. They form a genus to themselves within the Bunyavirus family. Unlike representatives of other Bunyavirus genera (for example, the pathogens that cause Crimean–Congo hemorrhagic4) fever and sandfly fever in the region around the Mediterranean Sea), they are transmitted, not by arthropod bites, but usually by aerosols. According to their genetic and serological differences as well as the animal reservoirs they colonize, the hantaviruses can be subdivided into various species, including the Puumala virus and Dobrava–Belgrade virus species found in Germany (Table).
Human beings are “accidental hosts” for hantaviruses and are infected via virus-containing secretions (feces, urine, saliva) of the host animals. Maintenance of viral infectivity outside the host organism depends on a variety of factors such as temperature, humidity, and presence of protective protein, and under optimal conditions is estimated at several weeks (16). Horizontal transmission of the pathogen from person to person has not been described in Europe.
A number of different hantavirus species are in circulation in Germany (Table). The one that most commonly causes disease in humans is the Puumala virus. So far as is known at present, the large disease outbreaks that occurred in 2007, 2010, and 2012 were caused by the Puumala virus (17, 18). The virus’s natural host is the bank vole (Myodes glareolus), a cricetine rodent that is ubiquitous in Germany. A second human pathogenic hantavirus in Germany is a variant (Kurkino genotype) of the Dobrava–Belgrade virus, transmitted by the striped field mouse (Apodemus agrarius). The Dobrava–Belgrade virus exists in various genotypes that are hosted by different Apodemus species and cause disease of very varying degrees of severity (19). In Germany, infection of humans by this virus have so far only been described in the north and north-east of the country. This is because the distribution of the striped field mouse is restricted to those parts of Germany. In addition, a third hantavirus, the Tula virus, is present in the common vole (Microtus arvalis) and related cricetine rodents. It is not yet known how important the Tula virus is as a pathogen in humans; so far only one case of disease has been associated with infection by this virus (20). Finally, two hantaviruses (Seewis virus and Asikkala virus) have been demonstrated in shrews in Germany, but their pathogenicity for humans is unclear at present (21, 22).
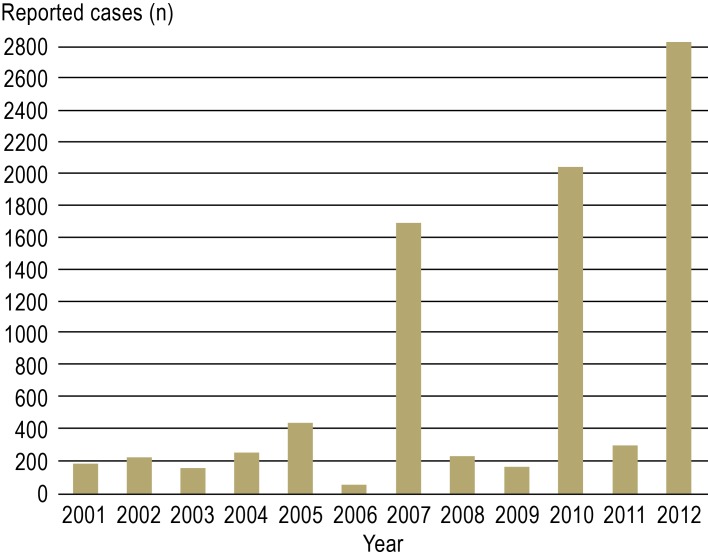
Looking at the number of hantavirus infections reported annually since 2001, marked variations can be seen (Figure 1). The average number of reported cases each year is around 230, but much higher numbers were reported in 2007, 2010, and 2012 (www3.rki.de/SurvStat). These hantavirus outbreaks affected in particular known endemic areas in Baden–Württemberg, Bavaria, North Rhine–Westphalia, and Lower Saxony. During the 2010 outbreak, for the first time accumulations of cases were seen in Hesse and Thuringia (23). Cases of human infection have also been seen in cities, in Cologne, for example, where they were presumed to be due to pathogen exposure in a city park (24).
Figure 1.
Number of reported cases of hantavirus disease in Germany since 2001. (Source: Robert Koch Institute, SurvStat, www3de.rki./SurvStat)
Possible causes of the variation in annual case numbers
It is believed that mass reproduction of the bank vole is the cause of the increased occurrence (outbreaks) of disease due to Puumala virus infection. This increase in the population density of rodents is taken to be due to climatic factors and beech mast in the preceding year. Mild and snowy winters probably favor survival of the animals, which then achieve a high population density in the year of the outbreak. The denser the population and the more prevalent the virus in the animal hosts, the more likely it is that transmission to humans as accidental hosts will take place. This therefore suggests a connection between mass reproduction in the bank vole and hantavirus outbreaks in humans. Interestingly, in both winter 2009/spring 2010 and in winter 2011/spring 2012, there was a rise in the number of reported cases very early in the season. Whether such observations can be used as a future early warning system of hantavirus outbreaks remains to be elucidated by further studies.
Virological diagnosis
Diagnosis of infection in patients is usually by serological procedures such as enzyme immunoassay, immunoblot, or immunofluorescence test (4, 27). Evidence of a de novo hantavirus infection is given by parallel demonstration of IgM and IgG antibodies that react with hantavirus antigen. In routine diagnostics, it is often difficult to distinguish between Puumala and Dobrava–Belgrade virus, either because the antibody demonstration is carried out with antigen of only one of those two viruses, or because serological cross-reactions occur. The hitherto inadequate state of knowledge about the occurrence of Tula virus infections could also be due to the antigenic relationship between Tula virus and Puumala virus. For this reason, the reported virus typing given with case reports passed to the Robert Koch Institute is not very reliable.
A more exact hantavirus typing than the serological methods mentioned above is possible by us of the focus reduction neutralization test (FRNT), in which neutralizing antibodies (directed against the virus envelope) are determined. This method is time-consuming and requires biosafety level 3 laboratory conditions. FRNT is not helpful in finding hitherto unknown types of hantavirus, or for differentiating between infections by the various genotypes of Dobrava–Belgrade virus.
For fine differentiation of viral strains and, for example, mapping them to various different outbreak regions in Germany, viral nucleic acid amplification and sequence analysis is required. This molecular diagnostic procedure is performed on whole blood or serum; however, it is only possible during the first days of symptoms since after that the virus is eliminated from the blood.
Molecular epidemiological fine differentiation of Puumala virus strains
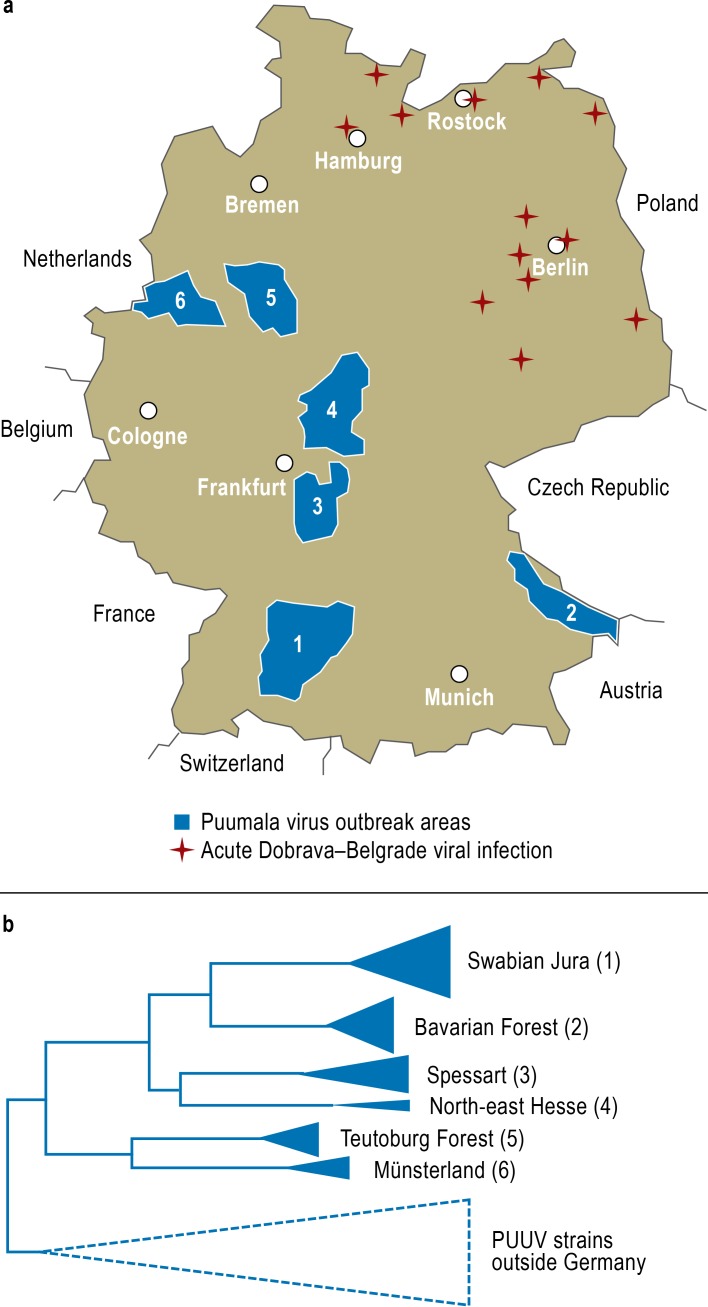
During the Puumala virus outbreaks in Germany in 2007 and 2010, for the first time viral nucleic acid from a large number of patients was successfully amplified and analyzed (17, 18). In parallel with this, viral genetic material was demonstrated in bank voles in the same areas that the patients with proven hantavirus nucleic acid came from. Various outbreak areas were characterized, and Puumala virus nucleotide sequences from patients and from bank voles in these areas were investigated: the Swabian Jura, the Bavarian Forest, Spessart, north-east Hesse, the Teutoburg Forest, and Münsterland (Figure 2a). The viral strains from these areas each form their own sequence cluster in the molecular phylogenetic analysis, and in each cluster the nucleotide sequences from patients and host animals are very closely related (Figure 2b). It is also entirely possible to specify the origin of the individual virus strain more and more closely, i.e., to carry out further molecular subtyping within one outbreak area.
Figure 2.
Molecular epidemiological cadastre of hantavirus strains in Germany
a) Areas of Puumala virus outbreaks in 2010 (blue). In these areas, viral nucleic acids from both bank voles and patients were analyzed and compared. Places with evidence of patients with Dobrava–Belgrade viral infection in the north and east of Germany are also shown (from: [18]: Ettinger J, Hofmann J, Enders M, et al.: Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg Infect Dis 2012; 18: 1461–4; with the addition of new, unpublished data in the possession of the present authors).
b) Schematic representation of the phylogenetic relationships between the viral strains from six Puumala virus outbreak areas, from which viral sequences from patients and local bank voles were analyzed. The viral strains of each outbreak area form their own “molecular cluster,” which is different from that of the neighboring outbreak areas (from [34]: Epidemiologisches Bulletin des Robert Koch-Institutes: Molekulare Unterscheidbarkeit der zirkulierenden Hantavirus-Stämme in den verschiedenen Ausbruchsregionen Deutschlands. Epidem Bull 2012, Nr. 25, 228–31; reproduced by kind permission of the Robert Koch Institute)
Vertical transmission
As the number of hantavirus infections rises, the risk increases that pregnant women will become infected with this virus. Despite this, to date there are very few reports of cases where sensitive methods were used to monitor for infection of the child. The largest European study so far reported two pregnant women with acute Puumala virus infection and two others with acute Dobrava–Belgrade virus infection (28). Despite the fact that all four women became ill, in no case did the maternal infection lead to intrauterine damage or infection of the child (observation period up to 12 months after birth).
Treatment and prevention
For hantavirus infection, according to § 7 of the German Law on the Prevention and Control of Infectious Diseases, all laboratories have a statutory duty to notify the Ministry of Health when they identify a case of infection associated with acute illness.
Treatment of the disease is symptomatic, with the focus on maintaining cardiovascular stability and compensating temporary organ failure (dialysis, oxygenation). No causative antiviral treatment option exists; results of studies using the virostatic ribavirin are unclear. The same is true of the first, limited attempts to use antiviral cytokines (interferon type I) or immunomodulatory substances (4, 9).
There is no approved vaccine for active immunization against the human pathogenic hantaviruses that are circulating in Europe (4). Genetically engineered vaccines that exist at the laboratory stage have so far not been adopted into development programs by the pharmaceutical industry. For this reason, the main focus of attempts to protect humans from infection is prevention of exposure, i.e., preventing transmission of the pathogen from animal to human. Hantavirus infections could be largely prevented if contact with the voles and their secretions could be prevented (29). This involves controlling the voles in and around human dwellings. When working in places where voles may have been (e.g., when cleaning out stables, sheds, or summer houses at the end of the winter), especially in endemic areas, single-use gloves and masks that fit closely over nose and mouth (e.g., the FFP3 respiratory masks available from DIY stores) should be worn. When out of doors, contact with vole nests and secretions should likewise be avoided. Other measures are to keep food safely both indoors and out of doors, and to disinfect and appropriately dispose of any trapped or dead voles. Since the infection can be transmitted by rodents kept in the laboratory, such as brown rats, common voles and bank voles, these animals too should be regularly examined for any persistent hantavirus infection—even though at present this is only prescribed for brown rats by the Federation of European Laboratory Animal Science Associations (FELASA).
Future perspectives
In the future, renewed efforts must be made to develop virostatics and a vaccine for use in Europe. In the short term, however, preventive measures aimed at reducing exposure of people living in the endemic areas to the pathogen will be the most important. This requires a strengthening of public awareness of this infectious disease. In addition, targeted training courses are needed for hospital physicians and those in private practice—especially in endemic areas—to familiarize them with the symptoms of the disease. The improved understanding of the (immune) pathogenesis underlying hantavirus disease could lead to novel therapeutic pathways. One task to be performed is molecular epidemiological mapping of the outbreak areas at the finest resolution possible and—connected with this—to determine precisely the place where patients were infected, by molecular analyses of viral samples. Further research into the ecological causes of viral outbreaks might in the future be used in the development of an “early warning system.”
Key Messages.
Hantavirus diseases are zoonoses; the pathogens are transmitted from small mammals (rodents) to humans.
The disease starts with a high fever; depending on severity, shock and renal and/or pulmonary failure may occur.
The special viral diagnostic procedure relies on serological and molecular biological tests, the last of which enable exact mapping of the hantavirus outbreak areas.
Given the lack of an approved vaccine, infection prophylaxis consists of avoiding contact with rodents and their secretions.
In 2012, the number of cases of hantavirus disease in Germany reached a new high at more than 2800 recorded cases.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Krüger has received lecture fees from Synlab.
Dr. Hofmann has received lecture fees from Abbott and Siemens.
Dr. Ulrich declares that no conflict of interest exists.
References
- 1.Gesellschaft für Virologie e.V. Stellungnahme zu Infektionen mit dem neuen Coronavirus, Stand. www.g-f-v.org. 2013. Mar 27,
- 2.Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Inf Dis. 2013;19:456–459. doi: 10.3201/eid1903.121503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters CJ, Simpson GL, Levy H. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med. 1999;50:531–545. doi: 10.1146/annurev.med.50.1.531. [DOI] [PubMed] [Google Scholar]
- 4.Krüger DH, Schönrich G, Klempa B. Human pathogenic hantaviruses and prevention of infection. Hum Vacc. 2011;7:685–693. doi: 10.4161/hv.7.6.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss S, Witkowski PT, Auste B, et al. Hantavirus in bat, Sierra Leone. Emerg Infect Dis. 2012;18:159–161. doi: 10.3201/eid1801.111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MMWR. Notes from the field: Hantavirus Pulmonary Syndrome in visitors to a National Park - Yosemite Valley, California, 2012. Morbid Mortal Weekly Rep. 2012;61 [PubMed] [Google Scholar]
- 7.Mertz GJ, Hjelle B, Crowley M, Iwamoto G, Tomicic V, Vial PA. Diagnosis and treatment of new world hantavirus infections. Curr Opin Infect Dis. 2006;19:437–442. doi: 10.1097/01.qco.0000244048.38758.1f. [DOI] [PubMed] [Google Scholar]
- 8.Kramski M, Achazi K, Klempa B, Krüger DH. Nephropathia epidemica with a 6-week incubation period after occupational exposure to Puumala hantavirus. J Clin Virol. 2009;44:99–101. doi: 10.1016/j.jcv.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Krüger DH, Ulrich R, Lundkvist A. Hantavirus infections and their prevention. Microbes Infect. 2001;3:1129–1144. doi: 10.1016/s1286-4579(01)01474-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee HW, van der Groen G. Hemorrhagic fever with renal syndrome. Prog Med Virol. 1989;36:62–102. [PubMed] [Google Scholar]
- 11.Robert Koch-Institut. www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Hantaviren.html. Stand. 2012. Oktober Infektionskrankheiten A-Z: Hantavirus-Infektionen. RKI-Ratgeber für Ärzte. [Google Scholar]
- 12.Clement J, Maes P, Lagrou K, van Ranst M, Lameire N. A unifying hypothesis and a single name for a complex globally emerging infection: hantavirus disease. Eur J Clin Microbiol Infect Dis. 2012;31:1–5. doi: 10.1007/s10096-011-1456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasche FM, Uhel B, Krüger DH, et al. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg Infect Dis. 2004;10:1420–1425. doi: 10.3201/eid1008.031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen MH, Mäkelä SM, Ala-Houhala IO, et al. Ten-year prognosis of Puumala hantavirus-induced acute interstitial nephritis. Kidney Int. 2006;69:2043–2048. doi: 10.1038/sj.ki.5000334. [DOI] [PubMed] [Google Scholar]
- 15.Lütteke N, Raftery MJ, Lalwani P, et al. Switch to high-level virus replication and HLA class I upregulation in differentiating megakaryocytic cells after infection with pathogenic hantavirus. Virology. 2010;405:70–80. doi: 10.1016/j.virol.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Hardestam J, Simon M, Hedlund KO, Vaheri A, Klingström J, Lundkvist A. Ex vivo stability of the rodent-borne Hantaan virus in comparison to that of arthropod-borne members of the Bunyaviridae family. Appl Environ Microbiol. 2007;73:2547–2551. doi: 10.1128/AEM.02869-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann J, Meisel H, Klempa B, et al. Hantavirus outbreak, Germany, 2007. Emerg Infect Dis. 2008;14:850–852. doi: 10.3201/eid1405.071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger J, Hofmann J, Enders M, et al. Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg Infect Dis. 2012;18:1461–1464. doi: 10.3201/eid1809.111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klempa B, Avsic-Zupanc T, Clement J, et al. Complex evolution and epidemiology of Dobrava-Belgrade hantavirus: definition of genotypes and their characteristics. Arch Virol. 2013;158:521–529. doi: 10.1007/s00705-012-1514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klempa B, Meisel H, Räth S, Bartel J, Ulrich R, Krüger DH. Occurrence of renal and pulmonary syndrome in a region of northeast Germany where Tula hantavirus circulates. J Clin Microbiol. 2003;41:4894–4897. doi: 10.1128/JCM.41.10.4894-4897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlegel M, Radosa L, Rosenfeld UM, et al. Broad geographical distribution and high genetic diversity of shrew-borne Seewis hantavirus in Central Europe. Virus Genes. 2012;45:48–55. doi: 10.1007/s11262-012-0736-7. [DOI] [PubMed] [Google Scholar]
- 22.Radosa L, Schlegel M, Gebauer P, et al. Detection of shrew-borne hantavirus in Eurasian pygmy shrew (Sorex minutus) in Central Europe. Infect Genet Evol. 2013 Apr 16; doi: 10.1016/j.meegid.2013.04.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Faber M, Wollny T, Schlegel M, et al. Puumala virus outbreak in Western Thuringia, Germany 2010: epidemiology and strain identification. Zoon Publ Health. 2013 Feb 8; doi: 10.1111/zph.12037. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Essbauer SS, Schmidt-Chanasit J, Madeja EL, et al. Nephropathia epidemica outbreak in a metropolitan area, Germany. Emerg Infect Dis. 2007;13:1271–1273. doi: 10.3201/eid1308.061425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faber MS, Ulrich RG, Frank C, et al. Steep rise in notified hantavirus infections in Germany, April 2010. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- 26.Boone I, Wagner-Wiening C, Reil D, et al. Early rise of notified human hantavirus infections since October 2011 in Baden-Wuerttemberg, Southern Germany. Euro Surveill. 2012;17 [PubMed] [Google Scholar]
- 27.Krüger DH. Hantaviren. In: Doerr HW, Gerlich WH, editors. Medizinische Virologie. 2nd edition. Stuttgart: Thieme; 2010. pp. 580–588. [Google Scholar]
- 28.Hofmann J, Führer A, Bolz M, et al. Hantavirus infections by Puumala or Dobrava-Belgrade virus in pregnant women. J Clin Virol. 2012;55:266–269. doi: 10.1016/j.jcv.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Konsiliarlaboratorium für Hantaviren (Institut für Virologie der Charité)/Robert Koch-Institut/Friedrich-Loeffler-Institut/Julius Kühn-Institut. Informationen zur Vermeidung von Hantavirusinfektionen. www.virologie-ccm.charite.de/hantapraev.pdf. Stand. 2010. Juni
- 30.Klempa B, Tkachenko EA, Dzagurova TK, et al. Hemorrhagic fever with renal syndrome caused by 2 lineages of Dobrava hantavirus, Russia. Emerg Infect Dis. 2008;14:617–625. doi: 10.3201/eid1404.071310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzagurova TK, Klempa B, Tkachenko EA, et al. Molecular diagnostics of hemorrhagic fever with renal syndrome during a Dobrava virus infection outbreak in the European part of Russia. J Clin Microbiol. 2009;47:4029–4036. doi: 10.1128/JCM.01225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avsic-Zupanc T, Petrovec M, Furlan P, Kaps R, Elgh F, Lundkvist A. Hemorrhagic fever with renal syndrome in the Dolenjska region of Slovenia-a 10-year survey. Clin Infect Dis. 1999;28:860–865. doi: 10.1086/515185. [DOI] [PubMed] [Google Scholar]
- 33.Krüger DH, Ulrich R, Schütt M, Meisel H. Hantavirusinfektionen als Ursache des akuten Nierenversagens. Dtsch Arztebl. 2002;99:A 645–A 651. [Google Scholar]
- 34.Epidemiologisches Bulletin des Robert Koch-Institutes. Molekulare Unterscheidbarkeit der zirkulierenden Hantavirus-Stämme in den verschiedenen Ausbruchsregionen Deutschlands. Epidem Bull. 2012;25:228–231. www.rki.de. [Google Scholar]