Abstract
Background
Extracranial atherosclerotic lesions of the carotid bifurcation cause 10% to 20% of all cases of cerebral ischemia. Until now, there have been no comprehensive evidence- and consensus-based recommendations for the management of patients with extracranial carotid stenosis in Germany and Austria.
Methods
The literature was systematically searched for pertinent publications (1990–2011). On the basis of 182 randomized clinical trials (RCTs) and 308 systematic reviews, 30 key questions were answered and evidence-based recommendations were issued.
Results
The prevalence of extracranial carotid stenosis is more than 5% from age 65 onward. Men are affected twice as frequently as women. The most important diagnostic technique is Doppler- and color-coded duplex ultrasonography. RCTs have shown that the treatment of high-grade asymptomatic carotid stenosis with carotid endarterectomy (CEA) can lower the 5-year risk of stroke from 11% to 5%. Intensive conservative treatment may lower the stroke risk still further. Moreover, RCTs have shown that CEA for symptomatic 50% to 99% carotid stenosis lowers the 5-year stroke risk by 5% to 16%. Meta-analyses of the 13 available RCTs comparing carotid artery stenting (CAS) with CEA have shown that CAS is associated with a 2% to 2.5% higher risk of periprocedural stroke or death and with a 0.5% to 1% lower risk of periprocedural myocardial infarction. If no particular surgical risk factors are present, CEA is the standard treatment for high-grade carotid stenosis. CAS may be considered as an alternative to CEA if the rate of procedure-related stroke or death can be kept below 3% or 6% for asymptomatic and symptomatic stenosis, respectively.
Conclusion
Further studies are needed so that better selection criteria can be developed for individually tailored treatment.
Atherosclerotic lesions of the extracranial brain-supplying arteries cause up to 20% of all cases of cerebral ischemia (1, e1). The prevention of cerebral ischemia due to carotid disease is thus an important matter. Because there is a great deal of uncertainty and disagreement among physicians about the diagnostic evaluation and treatment of carotid stenosis, a methodologically sound interdisciplinary guideline was created. The purpose of the guideline is to optimize the evidence-based, comprehensive care of patients with extracranial carotid stenosis in Germany and Austria.
Methods
Participating groups and the concept of the guideline
20 different medical societies and organizations participated in the creation of this S3 guideline (eBox). All members of the guideline group declared their conflicts of interest in writing, employing a procedure that is documented in the guideline report. This multidisciplinary guideline is based on evidence and consensus. Each medical society/organization had one vote on all matters that were voted on. A 75% majority of votes was considered a consensus, and a 95% majority was considered a strong consensus. The main subject areas were decided upon at the initial consensus meeting in 2005 (epidemiology, diagnostic evaluation, treatment, follow-up care), and a consensus was then obtained on the 30 key questions that were to be answered. A consensus was obtained on all recommendations in two further consensus conferences and a structured Delphi process, which took place in 2010 and 2011. The long version of the guideline was published online in August 2012 on the homepage of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der wissenschaftlichen medizinischen Fachgesellschaften e. V., AWMF) (2, 3).
eBox. Acknowledgement.
Collaborators:
Gregor Antoniadis
German Society of Neurosurgery
(Deutsche Gesellschaft für Neurochirurgie [DGNC])
Christian Arning
German Society for Ultrasound in Medicine
(Deutsche Gesellschaft für Ultraschall in der Medizin [DEGUM])
Joachim Berkefeld
German Society of Neuroradiology
(Deutsche Gesellschaft für Neuroradiologie [DGNR])
Hartmut Brückmann
German Society of Neuroradiology
(Deutsche Gesellschaft für Neuroradiologie [DGNR])
Curt Diehm
German League for Vascular Diseases
(Deutsche Gefäßliga e. V.)
Rolf R. Diel
Institute of Epidemiology, University Medical Center Schleswig-Holstein, Kiel Campus
(Institut für Epidemiologie, Universitätsklinikum Schleswig-Holstein, Campus Kiel)
Ingo Flessenkämper
German Society of Surgery
(Deutsche Gesellschaft für Chirurgie [DGCH])
Gustav Fraedrich
Austrian Union of Vascular Medicine
(Österreichischer Verband für Gefäßmedizin [ÖVG])
Andreas Fründ
German Physiotherapy Association
(Deutscher Verband für Physiotherapie [ZVK] e.V.)
Sabine George
German Ergotherapy Association
(Deutscher Verband der Ergotherapeuten)
Michael W. Görtler
German Society for Ultrasound in Medicine
(Deutsche Gesellschaft für Ultraschall in der Medizin [DEGUM])
Hartmut Görtz
German Society for Geriatrics
(Deutsche Gesellschaft für Geriatrie [DGG])
eBOX - CONTINUATION
Walter Gross-Fengels
German Society of Radiology
(Deutsche Röntgen-Gesellschaft [DRG])
Michael Hennerici
German Society of Neurology
(Deutsche Gesellschaft für Neurologie [DGN])
Ulrich Hoffmann
German Society of Angiology, German Society of Vascular Medicine
(Deutsche Gesellschaft für Angiologie /Gesellschaft für Gefäßmedizin [DGA])
Andreas Hörstgen
German Ergotherapy Association
(Deutscher Verband der Ergotherapeuten)
Peter Huppert
German Society of Interventional Radiology
(Deutsche Gesellschaft für Interventionelle Radiologie [DEGIR])
Olav Jansen
German Society of Neuroradiology
(Deutsche Gesellschaft für Neuroradiologie [DGNR])
Ralf Langhoff
German Society of Angiology, German Society of Vascular Medicine
(Deutsche Gesellschaft für Angiologie /Gesellschaft für Gefäßmedizin [DGA])
Rainer Litz
German Society of Anesthesiology and Intensive Care Medicine
(Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin [DGAI])
Harald Mudra
German Cardiac Society
(Deutsche Gesellschaft für Kardiologie [DGK])
Darius Günther Nabavi
German Stroke Society (including the German Stroke Foundation)
(Deutsche Schlaganfallgesellschaft [inkl. Deutsche Schlaganfallhilfe])
Edmund Neugebauer
German Society of Surgery
(Deutsche Gesellschaft für Chirurgie [DGCH])
Hans Niedermeier
German Society for Vascular Surgery and Vascular Medicine
(Deutsche Gesellschaft für Gefäßchirurgie und Gefäßmedizin [DGG])
Christoph Ploenes
German Society for Geriatrics
(Deutsche Gesellschaft für Geriatrie [DGG])
Robert Stingele
German Stroke Society (including the German Stroke Foundation)
(Deutsche Schlaganfallgesellschaft [inkl. Deutsche Schlaganfallhilfe])
Barbara Rantner
Austrian Union of Vascular Medicine
(Österreichischer Verband für Gefäßmedizin [ÖVG])
Josef Tacke
German Society of Interventional Radiology
(Deutsche Gesellschaft für Interventionelle Radiologie [DEGIR])
Dirk Sander
German Society of Neurology
(Deutsche Gesellschaft für Neurologie [DGN])
eBOX - CONTINUATION
Oliver Schnell
German Diabetes Society
(Deutsche Diabetes Gesellschaft [DDG])
Karl-Ludwig Schulte
German Society of Angiology, German Society of Vascular Medicine
(Deutsche Gesellschaft für Angiologie /Gesellschaft für Gefäßmedizin [DGA])
Karsten Schwerdtfeger
German Society of Neurosurgery
(Deutsche Gesellschaft für Neurochirurgie [DGNC])
Martin Storck
German Vascular Society
(Deutsche Gesellschaft für Gefäßchirurgie und Gefäßmedizin [DGG])
Dierk Vorwerk
German Radiological Society
(Deutsche Röntgen-Gesellschaft [DRG])
Knut P. Walluschek
German Society for Thoracic and Cardiovascular Surgery
(Deutsche Gesellschaft für Thorax-, Herz- und Gefäßchirurgie [DGTHG])
Gerhard Walterbusch
German Society for Thoracic and Cardiovascular Surgery
(Deutsche Gesellschaft für Thorax-, Herz- und Gefäßchirurgie [DGTHG])
Literature search, evaluation of evidence and recommendations
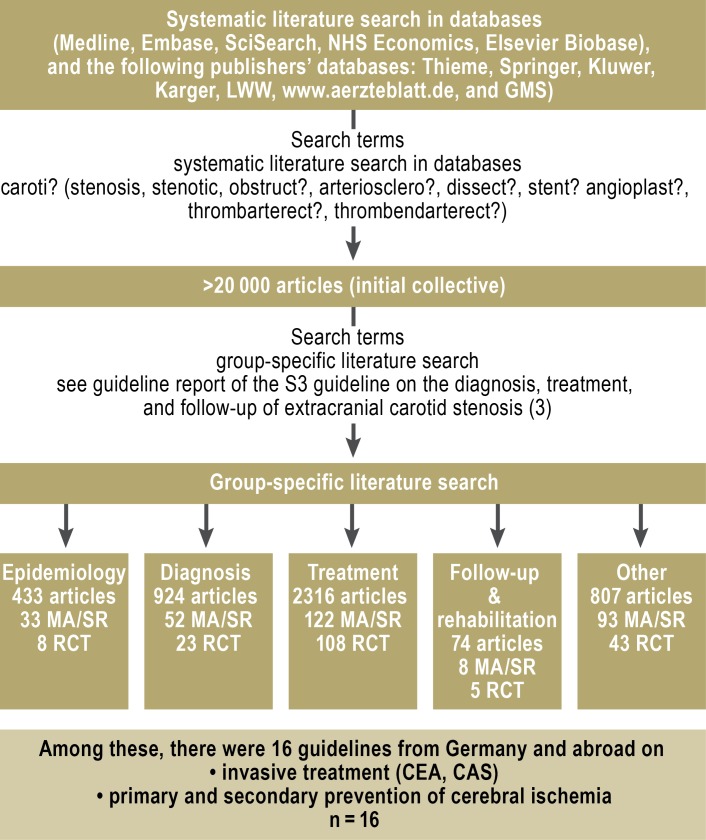
In accordance with the AWMF regulatory scheme, a systematic review was performed in the Medline, Embase, SciSearch, NHS Economic, and Elsevier Biobase databases for pertinent articles published from 1 January 1990 to 6 December 2011. More than 20 000 references were found, including 182 randomized, controlled trials and 308 systematic reviews, of which 12 were Cochrane reviews. In addition, a systematic search in the Guidelines International Network database retrieved 16 current, high-quality guidelines on extracranial carotid stenosis and on the prevention of cerebral ischemia (4– 9, e2– e5) (Figure 1).
Figure 1.
Literature-searching procedure
GMS, German Medical Science database; LWW, Lippincott Williams & Wilkins publisher’s database; MA, systematic review with metaanalysis; NHS, National Institute for Health Research; RCT, randomized controlled trial; SR, systematic review; CEA, carotid endarterectomy; CAS, carotid stenting
Three grades of recommendation (GoR) were distinguished: strong recommendation, recommendation, and open recommendation. The hereby intended strength of a recommendation is indicated by arrows (Table 1). The quality of the evidence was the basis in grading recommendations, but not necessarily the only factor. In cases where there was a clinical consensus despite a lack of adequate scientific evidence, the recommendation was issued with the label “good clinical practice” (GCP).
Table 1. Grading of evidence and recommendations.
| Level of evidence (LoE) | Grade of recommendation (GoR) | Symbol |
|---|---|---|
| 1 (high) | strong recommendation |
↑ ↑ ↓ ↓ |
| 2–3 (moderate) | recommendation | ↑ ↓ |
| 4–5 (low) | open recommendation | ↔ |
| None | good clinical practice clinical consensus |
GCP |
The grading of recommendations also took account of the harm and benefits of each intervention in question, the relevance of outcomes (trial endpoints) and effect sizes, the external validity and consistency of the findings, and ethical considerations.
Results
Epidemiology
More than 5% of persons over age 65 have an extracranial carotid stenosis measuring 50% or more. Men are affected about twice as commonly as women (10, 11). According to most studies, the risk of ipsilateral stroke rises with the degree of stenosis and is
less than 1% per year with less than 50% stenosis,
Plaques that are hypoechoic on duplex ultrasonography are presumed to be associated with a higher stroke risk than echogenic lesions (12). Moreover, histological analyses of carotid plaques and magnetic resonance imaging (MRI) studies have shown that the finding of a lipid-rich, centrally necrotic plaque, a thin or ruptured fibrotic cap, or plaque hemorrhages is associated with an elevated cerebrovascular risk (13, 14). Overall, however, the carotid-associated stroke risk seems to have declined over the last few years, possibly because of improved medical primary and secondary prevention of arteriosclerosis (15, 16).
Clinical manifestations and diagnostic evaluation
The typical manifestations of extracranial carotid stenosis include retinal ischemia, unilateral paresis, unilateral sensory disturbances, aphasia, and dysarthria. On the other hand, dizziness, diplopia, amnesia, and headache are atypical (GCP) (Table 2). Stenosis can also be classified as symptomatic if the imaging studies—in particular, diffusion-weighted MRI—reveal acute or subacute clinically silent ischemia (17).
Table 2. Important recommendations concerning the manifestations and diagnostic evaluation of carotid stenosis.
| The definition of asymptomatic and symptomatic carotid stenosis | GoR | LoE |
| The first step in distinguishing symptomatic from asymptomatic carotd stenosis consists of thorough history-taking and clinical neurological examination. This determination should be made by a neurologist with experience in the diagnosis of stroke. | GCP | – |
| Carotid stenosis is classified as asymptomatic if no symptoms or signs associated with carotid stenosis have appeared in the past six months. | GCP | – |
| The diagnostic evaluation and further clinical follow-up of extracranial carotid stenosis | GoR | LoE |
| Auscultation of the carotid artery is not a suitable method of detecting carotid stenosis. | ↓ | 2 |
| If carotid stenosis is suspected, Dopper ultrasonography or color-coded duplex ultrasonography should be performed by an experienced examiner (DEGUM criteria). | ↑ ↑ | 1 |
| If the degree of stenosis is in doubt, or if ultrasonography is rendered more difficult by concomitant intrathoracic or intracranial carotid disease or by hemodynamically relevant vascular lesions on the opposite side, contrast-enhanced MR angiography is recommended, or, alternatively, CT angiography. | GCP | – |
| A diagnostic DSA with selective catheterization of the carotid artery should only be performed if the non-invasive studies yield no definitive conclusion and the DSA findings would have therapeutic consequences. | GCP | – |
| Statements about the degree of carotid stenosis should be accompanied by information about the type of diagnostic study and the definition of stenosis that was used for quantification (preferably NASCET). | GCP | – |
| Should persons in high-risk groups undergo screening tests? | GoR | LoE |
| Routine screening for carotid stenosis should not be performed. | ↓ ↓ | 1 |
| Ultrasonographic screening is reasonable for persons with vascular risk factors. Screening should only be performed if a positive finding would have clinical consequences. | GCP | – |
| What diagnostic studies are needed before surgery or endovascular intervention? | GoR | LoE |
| All patients with carotid stenosis should have a clinical neurological examination. | GCP | – |
| Symptomatic patients should undergo brain imaging of a suitable type before any planned carotid revascularization procedure. In asymptomatic patients as well, brain imaging can provide important additional information. | GCP | – |
CT, computed tomography; DSA, digital subtraction angiography; DEGUM, German Society for Ultrasound in Medicine (Deutsche Gesellschaft für Ultraschall in der Medizin); NASCET, North American Symptomatic Carotid Endarterectomy Trial; GoR, grade of recommendation; LoE, level of evidence; GCP, good clinical practice
The main ancillary diagnostic techniques are Doppler and color-coded duplex ultrasonography, with application of the current DEGUM criteria (↑ ↑). Auscultation is not suitable (↓). By international agreement, only the distal degree of stenosis (as per the NASCET trial criteria) should be used to quantify the degree of stenosis (GCP, [Figure 2]) (18). Supplementary tests include contrast-enhanced MR angiography and CT angiography (CTA) (GCP). Digital subtraction angiography (DSA) is no longer indicated, except in special cases (GCP).
Figure 2.
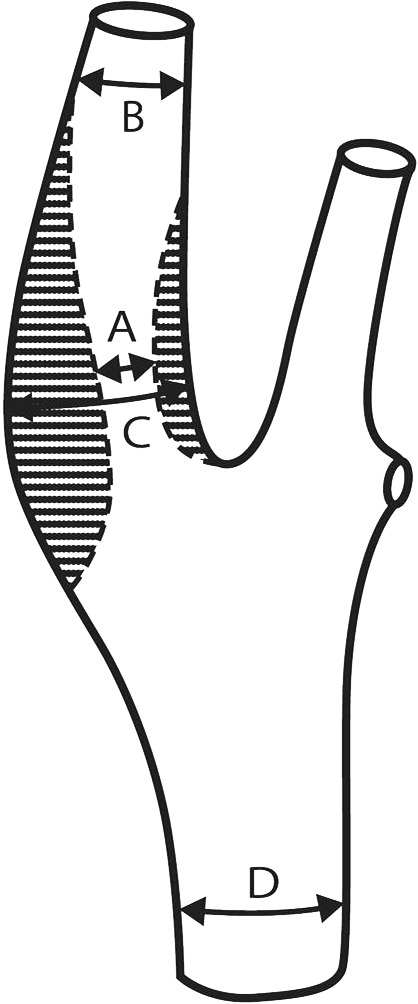
Angiographic quantifying methods for carotid stenosis: NASCET, (B–A)/B × 100%; ECST, (C–A)/C × 100%.
The former is the distal degree of stenosis, the latter is the proximal degree of stenosis.
Before any planned revascularization, symptomatic patients should have appropriate brain imaging with either computerized tomography (CT) or MRI. Such imaging studies can provide important additional information for asymptomatic patients as well, e.g., the demonstration of a clinically silent cerebral infarct (GCP). For all patients with arteriosclerotic carotid stenosis, the physician should obtain a full history of vascular risk factors and of other diseases secondary to arteriosclerosis (coronary heart disease [CHD], peripheral arterial occlusive disease [PAOD]) (GCP).
Universal screening for the presence of carotid stenosis is not recommended (↓ ↓), but screening is reasonable for persons with vascular risk factors, as long as the diagnosis of extracranial carotid stenosis would have clinical consequences (GCP): for example, in an otherwise healthy patient with normal life expectancy who is found to have a greater than 80% carotid stenosis. Patients with known carotid stenosis should be re-evaluated every 6 to 12 months (GCP).
Treatment
The conservative treatment of asymptomatic and symptomatic carotid stenosis
Only patients with a stenosis measuring at least 60% were included in the clinical trials of carotid endarterectomy (CEA) for asymptomatic carotid stenosis. Because a significant clinical advantage from CEA only became evident about five years after surgery, it is presumed that patients with an asymptomatic stenosis measuring less than 60% would benefit more from conservative treatment (GCP), and that asymptomatic patients who are at very high cardiovascular risk would not benefit at all from revascularization (GCP) (e9).
No RCTs are available regarding primary preventive treatment for patients with arteriosclerotic carotid stenosis. Nonetheless, these patients are at increased risk for vascular events, and it would be desirable for them to undergo comprehensive risk factor modification. This includes lifestyle modifications such as smoking cessation, normalization of body weight, and adequate exercise, as well as medication for the treatment of arterial hypertension, lipid metabolic disorders, and diabetes mellitus, if present. Prophylactic treatment with platelet inhibitors is of unclear benefit for patients with asymptomatic stenosis; in men, it lowers the rate of heart attack, but not that of stroke. Oral anticoagulation for patients with arteriosclerotic carotid stenosis is of no greater benefit than treatment with acetylsalicylic acid. Intermediate- and long-term secondary prevention corresponds to primary and secondary stroke prophylaxis, especially with respect to risk factor modification (5, 8, e5).
Operative and endovascular treatment
The indication for the invasive treatment of either asymptomatic or symptomatic carotid stenosis should be determined by an interdisciplinary group that includes a neurologist with experience in the diagnosis and treatment of carotid stenosis (GCP). A neurologist should monitor the complication rates of any carotid revascularization procedure (GCP).
Asymptomatic carotid stenosis—Large-scale RCTs have shown that CEA for asymptomatic carotid stenosis measuring more than 60% has a preventive effect on stroke, as long as the perioperative complication rate is less than 3% (Table 3) (19, e10, e11). The 5-year risk of stroke was 5%–6% in the operative arms of these trials, compared to ca. 11% in their conservative arms. This corresponds to an absolute risk reduction of 5% to 6% in five years, and thus to a number needed to treat (NNT) of 17 to 20 CEAs to prevent one stroke in five years. It is concluded that CEA should be considered for patients with a stenosis in the 60% to 99% range (↑ ↑), and that the complication rate must be held below 3% (↑ ↑). Men benefit more from the procedure than women, and CEA should only be performed if the patient has a life expectancy of at least 5 years (↑).
Table 3. Important recommendations about asymptomatic and symptomatic stenosis.
| Asymptomatic carotid stenosis | GoR | LoE |
| CEA should be considered for patients with 60% to 99% asymptomatic carotid stenosis, because it lowers such patients’ risk of stroke by a small but statistically significant amount. | ↑ ↑ | 1 |
| CEA is of benefit to patients with asymptomatic carotid stenosis only if it can be performed with a complication rate lower than 3%.. | ↑ ↑ | 1 |
| The benefit of CEA in patients with asymptomatic carotid stenosis is mainly in men and persons with a life expectancy of at least five years. | ↑ | 1 |
| The value of different treatments for asymptomatic carotid stenosis (CEA, CAS, BMT) should be evaluated in controlled trials. | GCP | - |
| When invasive treatment is indicated in a patient with asymptomatic carotid stenosis, CAS may be considered as an alternative if the treating center performs this procedure with quality criteria analogous to those for CEA and with a demonstrated complication rate under 3%. | ↔ | 2b |
| In cases where treatment is indicated but CEA would present special difficulties, CAS may be considered as an alternative in centers with a demonstrated complication rate under 3%. | ↔ | 2b |
| Symptomatic carotid stenosis | GoR | LoE |
| CEA is recommended for patients with 70% to 99% stenosis after TIA or a non-disabling stroke. | ↑ ↑ | 1a |
| CEA should also be considered for patients with 50% to 69% symptomatic stenosis. Men who have recently had hemispheric symptoms (AF, TIA, stroke mRS <3) benefit most. | ↑ ↑ | 1a |
| CEA is not recommended for less than 50% stenosis. | ↓ ↓ | 1a |
| It is recommended that CEA should be performed as soon as possible after the index event. | ↑ ↑ | 2 |
| CEA is the method of choice for the treatment of symptomatic carotid stenosis as long as it can be performed with normal operative risk. | ↑ ↑ | 1a |
| CAS should be considered as an alternative to CEA for symptomatic patients with high surgical risk. | ↑ | 2 |
| CAS may be considered as an alternative to CEA for symptomatic patients in centers with a documented rate of periprocedural stroke or death that is lower than 6%.. | ↔ | 2 |
AF, amaurosis fugax; BMT, best medical therapy; CAS, carotid stenting; CEA, carotid endarterectomy; GCP, good clinical practice; LoE, level of evidence; mRS, modified Rankin Scale; TIA, transient ischemic attack
In the years since these trials of CEA were performed, the pharmacological treatment of arteriosclerosis has markedly improved; we may thus ask whether their findings still apply today (20). In a recent Canadian trial, for example, the carotid-associated risk of stroke was lowered from about 3% per year to 1% per year with intensive pharmacotherapy, smoking cessation, a Mediterranean diet, and exercise (21). The nonsurgical treatment accompanying CEA includes the administration of acetylsalicylic acid (ASA) and the optimization of vascular risk factors with drugs and other means (↑ ↑).
Carotid artery stenting (CAS) may be considered as an alternative to CEA for patients in whom surgery would be technically difficult or of greater than usual risk, as long as the complication rate of CAS can be shown to be less than 3% (↔). The number of randomized, controlled trials that have been performed to compare CAS with CEA in asymptomatic aortic stenosis is still too low to permit any definitive conclusion (Table 4). In view of this uncertainty and the improvements in conservative treatment, it is recommended that patients with high-grade asymptomatic carotid stenosis should be enrolled in one of the randomized controlled trials currently in progress (e.g., SPACE-2 [22], ACST-2 [23]) (GCP).
Table 4. Endpoints in 13 randomized trials comparing CAS and CEA*1.
| Endpoints | Number of patients*2 | Number of trials*2 | CAS | CEA | Absolute risk difference (CAS versus CEA) | Pooled odds ratio(95% confidence interval) |
|---|---|---|---|---|---|---|
| Any periprocedural stroke or death | 7390 | 12 | 6.7% | 4.4% | + 2.3% | 1.54 (1.25–1.89) |
| Any periprocedural stroke | 7171 | 11 | 6.0% | 3.9% | + 2.1% | 1.53 (1.23–1.91) |
| Any stroke (mean follow-up 2.7 years) | 5359 | 9 | 10.8% | 8.2% | + 2.6% | 1.37 (1.13–1.65) |
| Periprocedural myocardial infarction | 5952 | 9 | 0.8% | 1.7% | – 0.9% | 0.48 (0.29–0.78) |
| Periprocedural cranial nerve dysfunction | 6062 | 11 | 0.3% | 5.4% | – 5.1% | 0.09 (0.05–0.16) |
*1Modified from (30)
*2Number of patients and trials in which the endpoint in question was used CAS, carotid stenting; CEA, carotid endarterectomy
Symptomatic carotid stenosis—CEA is strongly recommended for patients with symptomatic 50% to 99% carotid stenosis; the level of evidence for this is high (↑ ↑) (24– 27) (Table 3). The randomized controlled trials that compared CEA and conservative treatment for this group of patients in the 1990s showed that CEA for 70% to 99% symptomatic carotid stenosis (clinical manifestations: amaurosis fugax, transient ischemic attack [TIA], and non-disabling stroke) lowered the absolute risk of stroke within five years by 16%, which corresponds to an NNT of 6. The operation is beneficial as well for patients with 50% to 69% stenoses, with an absolute risk reduction of 4.6% (NNT 22). CEA is of no benefit for stenoses measuring less than 50%. The prophylactic effect of CEA is long-lasting; the risk of ipsilateral stroke after the operation is less than 1% per year (26, 27). The perioperative complication rate must not be above 6%.
To prevent early recurrent stroke, CEA should be performed as soon as possible after the neurological index event (↑ ↑). This can lower the five-year risk of stroke by more than 20%. Persons who are particularly likely to benefit from CEA include (↑ ↑):
Men
Patients over age 70
Patients with ulcerated stenoses
Patients with inadequate collateral circulation and recurrent symptoms.
All patients should be given ASA perioperatively (↑ ↑) (28).
CAS may be considered as an alternative to CEA for symptomatic patients in centers where the CAS-related rate of stroke or death has been documented to be under 6% (↔). This is particularly so for patients who are at high surgical risk (↑). CAS is also recommended for particular subgroups of patients (GCP): those with stenoses at surgically inaccessible sites, those with restenosis after CEA, those with radiogenic stenosis, and those with tandem stenoses (i.e., severe stenosis of the ipsilateral carotid artery, either intracranial or intrathoracic), and those with contralateral recurrent laryngeal nerve palsy. The decision regarding the mode of treatment should take account of patient-specific factors such as age and individual anatomy, as well as the patient’s preference (GCP). Further important recommendations on the technical aspects of CEA and CAS are listed in Table 5 (29, e12– e20).
Table 5. Important recommendations about treatment techniques (CEA, CAS).
| CEA | GoR | LoE |
| The surgical method (eversion endarterectomy or conventional endarterectomy) should be chosen on the basis of the surgeon’s personal experience. | ↑ | 1 |
| In conventional endarterectomy, the use of a patch significantly lowers the rate of perioperative stroke and death as well as the perioperative carotid occlusion rate and the rate of restenosis later on in the postoperative course. | ↑ ↑ | 1 |
| In elderly patients with an indication for carotid revascularization, CEA should be considered, especially if the arterial morphological anatomy is not suitable for endovascular intervention. | ↑ | 1 |
| There is inadequate evidence to support the routine (obligatory) insertion of a shunt during surgical carotid reconstruction. | ↑ ↑ | 2 |
| Intraoperative neuromonitoring during CEA is not evidence-based, but a pathological finding during monitoring can imply an indication for the selective use of a shunt while the carotid artery is clamped. | ↔ | 5 |
| Patients and surgeons can choose a procedure under local or general anesthesia; the use of one or the other yields no significant difference in outcome at 30 days. The decision regarding anesthesia should take account of the patient’s wishes and the individual experience and skills of the anesthesiologist and vascular surgeon. | ↑ ↑ | 1 |
| CAS | GoR | LoE |
| In the endovascular treatment of carotid stenosis, primary stent implantation is the method of choice. | ↑ ↑ | 2b |
| In all cases of carotid stenting, self-expanding stents that have been approved for this indication should be used. | ↑ ↑ | 3 |
| For the assessment of in-stent re-stenosis, standardized Doppler criteria must be used so that structural changes can be detected in long-term follow-up. | ↑ ↑ | 2 |
| PTA alone carries a higher rate of restenosis than CAS. | ↑ | 3 |
| Patients should be given dual platelet inhibition with acetylsalicylic acid and clopidogrel in the peri-interventional period and for at least one month after CAS. | ↑ ↑ | 1 |
CAS, carotid stenting; CEA, carotid endarterectomy; LoE, level of evidence; PTA, percutaneous transluminal angioplasty
Care coordination and structural quality indicators
Revascularization (CEA or CAS) as an outpatient procedure is rejected for reasons of patient safety (GCP) (e21). It is recommended that, in general, CEA should be performed by a vascular surgeon (↑ ↑); moreover, in any institution in which CEA is performed, there should be at least one vascular surgeon who performs at least 10 CEA procedures per year. Brain imaging tools (CT, MRT), vascular diagnostics (ultrasound, CT angiography, MR angiography, DSA), and an endovascular interventional service must be available around the clock, as well as appropriate postoperative patient monitoring (GCP) (31, e22). Registry studies on CEA—and on CAS as well, albeit with fewer patients—have shown that periprocedural complication rates are lower in high-volume hospitals (↑).
CAS should be performed by a physician with the proper clinical and endovascular qualifications who has performed at least 100 cases of selective diagnostic catheter angiography of the brain-supplying arteries and has independently carried out at least 25 supra-aortic interventions. Furthermore, the hospital should have at least one physician doing endovascular procedures who performs at least 10 cases of CAS per year. 24-hour availability of cranial CT and MRI, a neuro-interventional service, a neurovascular service, and appropriate monitoring of high-risk patients, are obligatory (GCP).
Discussion
All of the recommendations found in this S3 guideline are based on a consensus of at least 75% of the participating scientific societies and organizations; most are based on a strong consensus (≥ 95%). This document thus represents a broadly accepted basis for the treatment of extracranial carotid stenosis.
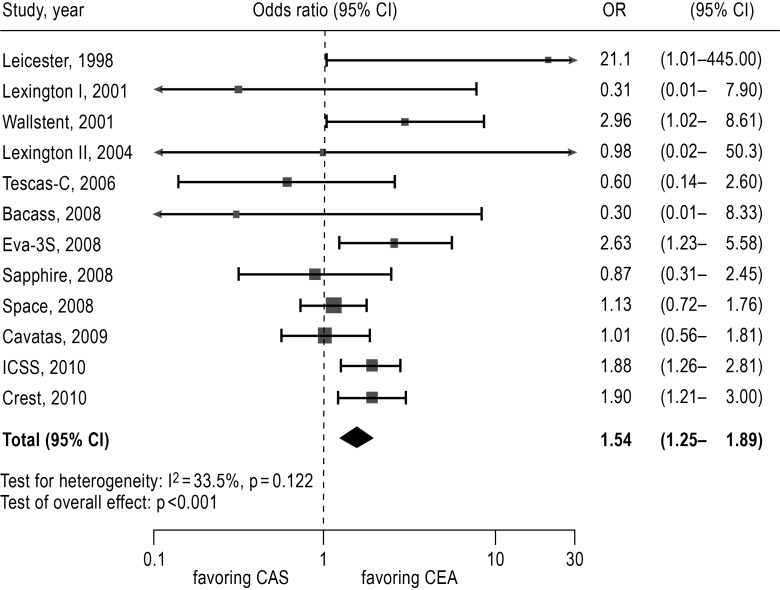
Regarding the ongoing debate on CAS versus CEA, 13 RCTs including a total of 7480 patients have been summarized in several meta-analyses (30, 32, 39). A recent meta-analysis found CAS to be inferior with respect to the endpoint “periprocedural stroke or death,” with a risk of 6.7% compared to 4.4% for CEA (odds ratio 1.54, 95% confidence interval [CI] 1.25–1.89) (Table 4, Figure 3). The difference came about mainly because of a higher risk of stroke with stenting (30). As most of the RCTs to date have included a majority of patients with symptomatic stenoses, adequate data are not yet available regarding asymptomatic stenoses. The meta-analysis also shows that CEA carries a higher rate of myocardial infarction (1.7% versus 0.8%), as well as a markedly higher risk of an at least transient cranial nerve dysfunction (Table 4). As procedure-related stroke is rare, the overall risk of stroke is lower after CEA than after CAS, even in the intermediate term (2.7 years) (Table 4). Nonetheless, the available trials show no difference in ipsilateral stroke rates after a successful procedure (CEA or CAS) (3, 33– 37).
Figure 3.
Forest plot for estimation of the procedural risk for the combined endpoint “any procedure-related stroke or death”
OR, odds ratio; CI, confidence interval;
CEA, carotid endarterectomy;
CAS, carotid stenting.
From: Economopoulos KP, Sergentanis TN, Tsivgoulis G, Mariolis AD, Stefanadis C: Carotid artery stenting versus carotid endarterectomy: a comprehensive meta-analysis of short-term and long-term outcomes. Stroke; a Journal of Cerebral Circulation. 2011; 42: 687–692. Reprinted with the permission of Wolters Kluwer Health (30).
The age of the patient was an important prognostic factor in all RCTs, with a trend toward fewer CAS-associated complications in patients under age 68 and a significantly lower risk with CEA in patients over age 68. The North American CREST trial also included an investigation of patients’ quality of life after CEA and CAS. It was found that, four weeks after the procedure, patients who had undergone CAS had less pain and dysphagia, while those who had undergone CEA were less likely to have difficulty with walking. These differences were no longer detectable twelve months after the procedure. Procedure-related stroke causes a persistent, statistically significant impairment of quality of life, which procedure-related myocardial infarction and cranial nerve dysfunction does not (38) .
The recommendations in this S3 guideline generally accord with those of the current guideline of the European Society for Cardiology (ESC), in which CAS is considered an alternative to CEA only for patients at high operative risk (simple recommendation). CAS can also be considered as an alternative to CEA for symptomatic patients in high-volume hospitals where the complication rate of CAS is under 6% (weak recommendation) (10). In contrast, the American Heart Association (AHA), in its current guideline, states that CAS is indicated as an alternative to CEA for symptomatic patients with >70% stenoses at average or low risk of complications associated with endovascular intervention (strong recommendation). This AHA recommendation is largely based on the findings of the North American CREST trial; it barely considers the three large-scale randomized controlled trials that have been performed in Europe to address his issue (34, 35, e24).
The guideline group consented that CAS is undergoing rapid technical development but has not yet achieved the high standard set by surgical treatment. The RCTs show a higher rate of periprocedural stroke after CAS, but higher rates of perioperative myocardial ischemia and (mainly transient) cranial nerve dysfunction after CEA. The age dependency of the CAS complication rate suggests that more pronounced atherosclerosis (e.g., in the aortic arch and the proximal portion of the common carotid artery) is a risk factor for endovascular treatment. After successful interventions, however, the RCTs show no significant difference in the secondary preventive effect of CEA and CAS (30, 32, 34– 36). All of the RCTs performed to date have been fraught with weaknesses of content and method, so that no absolutely reliable comparison of CAS and CEA under randomized conditions can yet be made. As for factors that might predict a better or worse outcome for CAS, the trial data do not permit any reliable statement about any of the tested criteria (study design, patient selection, endpoints, subgroups, data monitoring, experience of the treating physician, standardization of periprocedural treatment, etc.). This need not imply, however, that these factors are irrelevant to the quality of the outcome (e23).
Perspectives and overview
In the future, further high-quality trials will be needed, so that better selection criteria can be developed for individually optimized conservative, operative, or endovascular treatment, particularly for patients with asymptomatic carotid stenoses. It will presumably be important to use modern imaging techniques (MRI, PET-CT, duplex ultrasonography) for the reliable assessment of plaque morphologies that might be predictive of cerebral ischemia.
In this S3 guideline, CEA is recommended as the standard treatment for high-grade asymptomatic and mid- and high-grade symptomatic carotid stenosis. CAS may be considered as an alternative to CEA if the treating center meets quality criteria that are analogous to those for CEA, with a complication rate under 3% for asymptomatic stenoses or 6% for symptomatic stenoses. The guideline group welcomes the introduction of obligatory quality assurance for CAS as of 1 January 2012, after having been in place for CEA since 2003. An update of this guideline is projected for 2015.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Eckstein is a member of the steering committee of the SPACE-1 trial and co-director of the SPACE-2 trial.
Prof. Dörfler, Dr. Kühnl and Dr. Lawall state that they have no conflict of interest.
Prof. Kopp is an employee of the AWMF.
Prof. Ringleb has received reimbursement of meeting participation fees and of travel and accommodation expenses from GlaxoSmithKline and Boehringer Ingelheim. He has also received lecture honoraria from GlaxoSmithKline. He is a member of the steering committee of the SPACE-2 trial.
References
- 1.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke; a Journal of Cerebral Circulaton. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 2.Leitlinienreport der S3-Leitlinie zur Diagnostik. Therapie und Nachsorge der extracraniellen Carotisstenose, AWMF-Registernummer 004-028. www.awmf.org. Last accessed on 25 May 2013.
- 3.S3-Leitlinie zur Diagnostik. Therapie und Nachsorge der extracraniellen Carotisstenose, AWMF-Registernummer 004-028. www.awmf.org. Last accessed on 25 May 2013.
- 4.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the management of patients with extracranial carotid and vertebral artery disease. Journal of the American College of Cardiology. 2011;57:1002–1044. doi: 10.1016/j.jacc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a Journal of Cerebral Circulation. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 6.Tendera M, Aboyans V, Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) European Heart Journal. 2011;32:2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 7.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. Journal of Vascular Surgery. 2011;54:e1–e31. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 9.Liapis CD, Bell PR, Mikhailidis D, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2009;37:1–19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 10.de Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis Stroke. a Journal of Cerebral Circulation. 2009;40:1105–1113. doi: 10.1161/STROKEAHA.108.532218. [DOI] [PubMed] [Google Scholar]
- 11.Sander D DC, Eckstein HH, et al. S3 Guideline Extracranial Carotid Stenosis, chapter 6: Epidemiology. Gefässchirurgie. 2012;17:497–501. [Google Scholar]
- 12.Topakian R, King A, Kwon SU, Schaafsma A, Shipley M, Markus HS. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology. 2011;77:751–758. doi: 10.1212/WNL.0b013e31822b00a6. [DOI] [PubMed] [Google Scholar]
- 13.Salem MK, Sayers RD, Bown MJ, et al. Features of unstable carotid plaque during and after the hyperacute period following TIA/stroke. European Journal of Vascular and Endovascular Surgery: the Official Journal of the European Society for Vascular Surgery. 2013;45:114–120. doi: 10.1016/j.ejvs.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Bogiatzi C, Cocker MS, Beanlands R, Spence JD. Identifying high-risk asymptomatic carotid stenosis. Expert opinion on medical diagnostics. 2012;6:139–151. doi: 10.1517/17530059.2012.662954. [DOI] [PubMed] [Google Scholar]
- 15.Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke; a Journal of Cerebral Circulation. 2004;35:2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 16.Naylor AR. What is the current status of invasive treatment of extracranial carotid artery disease? Stroke; a Journal of Cerebral Circulation. 2011;42:2080–2085. doi: 10.1161/STROKEAHA.110.597708. [DOI] [PubMed] [Google Scholar]
- 17.Ringleb P GM, Nabavi DG, et al. S3 Guideline Extracranial Carotid Stenosis, chapter 7: Symptoms and diagnostics of carotid stenosis. Gefässchirurgie. 2012;17:502–519. [Google Scholar]
- 18.Arning C, Widder B, von Reutern GM, Stiegler H, Gortler M. Revision of DEGUM ultrasound criteria for grading internal carotid artery stenoses and transfer to NASCET measurement. Ultraschall Med. 2010;31:251–257. doi: 10.1055/s-0029-1245336. [DOI] [PubMed] [Google Scholar]
- 19.Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376:1074–1084. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke; a Journal of Cerebral Circulation. 2009;40:e573–e583. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]
- 21.Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Archives of Neurology. 2010;671:80–86. doi: 10.1001/archneurol.2009.289. [DOI] [PubMed] [Google Scholar]
- 22.Reiff T, Stingele R, Eckstein HH, et al. Stent-protected angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy: SPACE2—a three-arm randomised-controlled clinical trial. International journal of stroke. Official Journal of the International Stroke Society. 2009;4:294–299. doi: 10.1111/j.1747-4949.2009.00290.x. [DOI] [PubMed] [Google Scholar]
- 23.Rudarakanchana N, Dialynas M, Halliday A. Asymptomatic Carotid Surgery Trial-2 (ACST-2): rationale for a randomised clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. European Journal of Vascular and Endovascular Surgery: the Official Journal of the European Society for Vascular Surgery. 2009;38:239–242. doi: 10.1016/j.ejvs.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 24.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 25.Clinical alert benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy Trial (NASCET) investigators Stroke. a journal of cerebral circulation. 1991;22:816–817. doi: 10.1161/01.str.22.6.816. [DOI] [PubMed] [Google Scholar]
- 26.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. doi: 10.1016/s0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]
- 27.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 28.Ringleb P BJ, Eckstein HH. S3 Guideline Extracranial Carotid Stenosis, chapter 8.2: When is surgery or endovascular therapy indicated in patients with an asymptomatic or a symptomatic carotid stenosis (including subgroups and emergency procedures)? what is the best interval between the neurological index event and the surgical/endovascular procedure? Gefässchirurgie. 2012;17:522–542. [Google Scholar]
- 29.Rerkasem K, Rothwell PM. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting) Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD000190.pub2. CD000190. [DOI] [PubMed] [Google Scholar]
- 30.Economopoulos KP, Sergentanis TN, Tsivgoulis G, Mariolis AD, Stefanadis C. Carotid artery stenting versus carotid endarterectomy: a comprehensive meta-analysis of short-term and long-term outcomes. Stroke; a Journal of Cerebral Circulation. 2011;42:687–692. doi: 10.1161/STROKEAHA.110.606079. [DOI] [PubMed] [Google Scholar]
- 31.Storck M BJ, Doerfler A. S3 Guideline Extracranial Carotid Stenosis, chapter 8.19: What are the requirements for training and the organisational structure on facilities, in which angiography or surgical/endovascular revascularization procedures of the extracranial carotid artery are performed? Gefässchirurgie. 2012;17:599–602. [Google Scholar]
- 32.Bangalore S, Kumar S, Wetterslev J, et al. Carotid artery stenting vs carotid endarterectomy: meta-analysis and diversity-adjusted trial sequential analysis of randomized trials. Archives of Neurology. 2011;68:172–184. doi: 10.1001/archneurol.2010.262. [DOI] [PubMed] [Google Scholar]
- 33.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. The New England Journal of Medicine. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurology. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 35.Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurology. 2008;7:885–892. doi: 10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 36.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. The New England Journal of Medicine. 2008;358:1572–1579. doi: 10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 37.Ederle J, Bonati LH, Dobson J, et al. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurology. 2009;8:898–907. doi: 10.1016/S1474-4422(09)70228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen DJ, Stolker JM, Wang K, et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: results from CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial) Journal of the American College of Cardiology. 2011;58:1557–1565. doi: 10.1016/j.jacc.2011.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naggara O, Touze E, Beyssen B, et al. Anatomical and technical factors associated with stroke or death during carotid angioplasty and stenting: results from the endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial and systematic review. Stroke; a Journal of Cerebral Circulation. 2011;42:380–388. doi: 10.1161/STROKEAHA.110.588772. [DOI] [PubMed] [Google Scholar]
- e1.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke; a Journal of Cerebral Circulation. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- e2.Scottish Intercollegiate Guidelines Network. 2008. Management of patients with stroke or TIA: assessment, investigation, immediate management and secondary prevention. [Google Scholar]
- e3.Intercollegiate Stroke Working Party National clinical guideline for stroke. national clinical guideline for diagnosis and initial management of acute stroke and transient ischaemic attack (TIA) by the National Institute for Health and Clinical Excellence. 2008. Third edition Incorporating the recommendations from Stroke. [Google Scholar]
- e4.European Stroke Orgaisation (ESO) Executive Committee and ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovascular Diseases. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- e5.Deutsche Gesellschaft für Neurologie Primär- und Sekundärprävention der zerebralen Ischämie. Gemeinsame Leitlinie der DGN und der Deutschen Schlaganfallgesellschaft. www.dgnorg/images/stories/dgn/leitlinien/LL2008/ll08kap_024pdf. 2008.
- e6.Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study European Journal of Vascular and Endovascular Surgery. the Official Journal of the European Society for Vascular Surgery. 2005;30:275–284. doi: 10.1016/j.ejvs.2005.04.031. [DOI] [PubMed] [Google Scholar]
- e7.Halliday A MA, Marro J, Menken M. Immediate carotid endarterectomy reduced non-perioperative stroke in severe asymptomatic carotid artery stenosis. Evidence Based Medicine. 2004;9 [PubMed] [Google Scholar]
- e8.Nadareishvili ZG, Rothwell PM, Beletsky V, Pagniello A, Norris JW. Long-term risk of stroke and other vascular events in patients with asymptomatic carotid artery stenosis. Archives of Neurology. 2002;59:1162–1166. doi: 10.1001/archneur.59.7.1162. [DOI] [PubMed] [Google Scholar]
- e9.Ringleb P. S3 Guideline Extracranial Carotid Stenosis, chapter 81.7: What patients should be treated conservatively with which medication? Gefässchirurgie. 2012;17:593–595. [Google Scholar]
- e10.Toole JF BW, Castaldo JE, Chambless LE, Moore WS, Robertson JT, Young B, Howard VJ. Endarterectomy for asymptomatic carotid artery stenosis. Journal of the American Medical Association. 1995;273:1421–1428. [Google Scholar]
- e11.Hobson RW, 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. The New England Journal of Medicine. 1993;328:221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- e12.Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD000160.pub3. CD000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Rerkasem K, Rothwell PM. Local versus general anaesthesia for carotid endarterectomy. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD000126.pub3. CD000126. [DOI] [PubMed] [Google Scholar]
- e14.Naylor AR, Bown MJ. Stroke after cardiac surgery and its association with asymptomatic carotid disease: an updated systematic review and meta-analysis. European Journal of Vascular and Endovascular Surgery. The Official Journal of the European Society for Vascular Surgery. 2011;41:607–624. doi: 10.1016/j.ejvs.2011.02.016. [DOI] [PubMed] [Google Scholar]
- e15.Eckstein HH. S3 Guideline Extracranial Carotid Stenosis, chapter 84: Is eversion endarterectomy associated with different rates of clinical success, complications or recurrent stenoses by comparison to conventional endarterectomy with or without patch? Gefässchirurgie. 2012;17:545–547. [Google Scholar]
- e16.Berkefeld J, Doerfler A. S3 Guideline Extracranial Carotid Stenosis, chapter 8.5: Is carotid artery balloon dilatation alone associated with different rates of clinical success, complications or recurrent carotid stenoses by comparison to primary carotid artery stenting? Gefässchirurgie. 2012;17:548–549. [Google Scholar]
- e17.Storck M, Eckstein HH. S3 Guideline Extracranial Carotid Stenosis, chapter 8.7. Which patients with a high-grade extracranial carotid stenosis benefit from an intraluminal shunt during carotid endarterectomy? Gefässchirurgie. 2012;17:563–564. [Google Scholar]
- e18.Storck M. S3 Guideline Extracranial Carotid Stenosis, chapter 8.8: Do patients in whom carotid endarterectomy is performed under general anesthesia benefit from an intraoperative monitoring? :Gefässchirurgie. 2012;17:565–566. [Google Scholar]
- e19.Eckstein HH. S3 Guideline Extracranial Carotid Stenosis, chapter 8.10: Which kind of anesthesia is preferable during carotid endarterectomy? Gefässchirurgie. 2012;17:575–576. [Google Scholar]
- e20.Berkefeld J, Doerfler A. S3 Guideline Extracranial Carotid Stenosis, chapter 8.13: Which kind of materials (catheters, stents, protection devices) are preferable in CAS? Gefässchirurgie. 2012;17:582–584. [Google Scholar]
- e21.Storck M. S3 Guideline Extracranial Carotid Stenosis, chapter 8.18: Ambulatory treatment of carotid stenosis by CEA or CAS - is it possible or reasonable? Gefässchirurgie. 2012;17:597–598. [Google Scholar]
- e22.Eckstein HH, Berkefeld J, Doerfler A. S3 guideline Extracranial Carotid chapter 8.2: Is there a relationship between qualification, volume and outcome for CEA and CAS? Gefässchirurgie. 2012;17:603–605. [Google Scholar]
- e23.Nothacker M, Rütters D, Weinbrenner S. Therapie der Karotisstenose mittels Endarteriektomie oder Angioplastie mit Stenting - Analyse der vorliegenden Evidenz. ÄZQ-Ärztliches Zentrum für Qualität in der Medizin. Version 1.1. www.aezq.de/mdb/edocs/pdf/literatur/evidenzbericht-karotisstenose.pdf. 2010 [Google Scholar]
- e24.Ederle J, Dobson J, Featherstone RL, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analy- sis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]