Abstract
Background
0.5% to 0.8% of all adults suffer from rheumatoid arthritis (RA). The main considerations for persons with new-onset RA are early diagnosis, disease-modifying anti-rheumatic drugs (DMARDs), remission, and interdisciplinary treatment.
Method
As part of the process of creating a new S3 guideline on the management of early RA and a new S1 guideline on stage-adapted pharmacotherapy for RA, the authors conducted a selective search and review of the literature and specifically updated it to 20 March 2013.
Results
In patients presenting with joint inflammation, the diagnosis of RA can be directly confirmed (positive predictive value, 85% to 97%), and its prognosis assessed, on the basis of the following findings: joint examination, acute phase reaction, serology (rheumatoid factor [RF], antibody against citrullinated peptides/proteins [ACPA], and duration of symptoms (ACR/Eular classification criteria, 2010). Early, remission-oriented and adapted treatment with DMARDs (“treating to target”) leads to several years of normal bodily function without disability in 40% to 60% of patients. Treatment by an interdisciplinary team promotes the achievement of this goal. The risks associated with this form of treatment are low, with a dropout rate of less than 1 per 100 patient-years. Life-threatening complications are rare.
Conclusion
Early diagnosis, intervention with DMARDs in the first three months of disease, and the achievement of a remission minimize the adverse sequelae of RA. The sequential introduction of DMARDs, including biological agents in non-responders, as part of a treat-to-target concept optimizes the long-term outcome, as has been demonstrated in clinical trials for periods of up to eight years.
In Germany, around every third person suffers chronic joint pain from the age of 20 years onward, and every second person from the age of 40 (1). One of the principal causes of this pain, with serious consequences, is rheumatoid arthritis (RA). RA is the most frequent inflammatory rheumatic disease, with a prevalence of 0.5% to 0.8% in the adult population (e1). The incidence increases with age; overall, it is 25 to 30 per 100 000 for adult men and 50 to 60 per 100 000 for adult women (e2, e3).
Twenty-five years ago, rheumatoid arthritis was diagnosed 2 years on average after disease onset and the treatment was gradually intensified depending on the clinical symptoms (e4). This resulted in disability, chronic pain, early retirement, and mortality of over 20% within 10 years (2, 3).
The current guidelines demand:
Disease-modifying treatment in the first 3 months
Continuous monitoring of disease activity
Remission as treatment goal
Management by a multidisciplinary team (4; ↑ ↑ [↑ ↑, these recommendations are based on studies with a high level of certainty in which the benefits are shown to clearly outweigh the risks]).
Comparison with the approach to accepted medical emergencies such as myocardial infarction, with indications for early detection, patient information, and intervention as needed, is by no means far-fetched: The onset of rheumatoid arthritis is also an emergency. An optimal long-term prognosis requires coordination of the various levels of care.
Material and methods
A systematic survey of the literature up to 31 August 2011 was carried out in the framework of the S1 guideline on the stage-adapted drug treatment of rheumatoid arthritis (5) and the S3 guideline on management of early rheumatoid arthritis (4).
We identified and added publications up to 20 March 2013 on the topics of “diagnosis,” “remission,” and “multidisciplinary team”. Most of these studies were randomized controlled trials.
Diagnosis
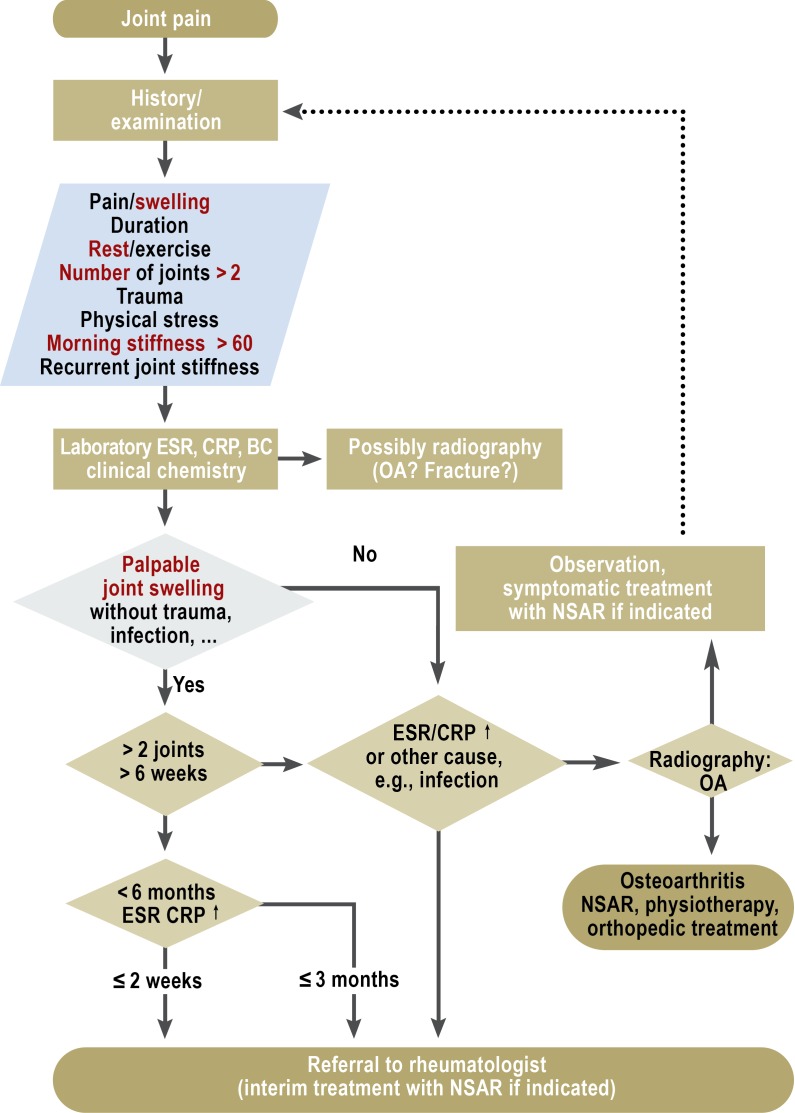
The first important step towards optimizing the long-term prognosis is early confirmation of the diagnosis of RA. The cardinal symptom of RA is joint swelling, and other possible causes of this swelling must be excluded (Figure).
Figure.
Diagnostic algorithm for investigation of patients with joint swelling. From (4) Schneider M, Lelgemann M, Abholz HH, et al.: Management der frühen rheumatoiden Arthritis. Heidelberg: Springer-Verlag 2011; reproduced by kind permission of Springer, Heidelberg.
ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; BC, blood count; OA osteoarthritis; NSAR, nonsteroidal antirheumatics; ↑, elevated
Although the new ACR/EULAR 2010 classification criteria for RA (Table 1) (6) are not primarily diagnostic criteria, they can be used as such. These classification criteria were based on the findings in nine early cohorts of patients with joint swellings (n = 3115) (6). The endpoint “diagnosis of RA” was a precondition for disease-modifying treatment with methotrexate after a year (7).
Table 1. ACR/EULAR 2010 classification criteria for rheumatoid arthritis (RA) (6)*.
| Points | 0 | 1 | 2 | 3 | 5 |
|---|---|---|---|---|---|
| Swollen/painful joints | ≤ 1 (medium to) large joint |
2–10 (medium to) large joints |
1–3 small joints |
4–10 small joints |
≥ 11 including small joints |
|
Serology RF and ACPA |
Negative | One or both weakly positive |
One or both strongly positive |
||
|
Acute phase CRP und ESR |
Normal | One or both elevated |
|||
| Duration of symptoms | <6 weeks | ≥ 6 weeks |
*Preconditions for the use of the criteria are confirmation of a swollen joint and exclusion of other possible causes of the joint complaints. With a total score of 6 points or more the diagnosis of RA is considered confirmed. The demonstration of typical erosions confirms RA without reference to the other criteria. Positive predictive value 85–97%. ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; RF, rheumatoid factor; ACPA, antibodies to citrullinated peptides/proteins; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate
Apart from the criterion of joint swelling, the classification includes items such as:
Number and type (small/large) of joints with painful swelling
Laboratory data (rheumatoid factor, ACPA [anti-citrullinated protein antibody], and CRP [C-reactive protein])
These items are weighted according to their value. The weighting is based essentially on the odds ratios for differentiation between early arthritis patients who did and those who did not receive treatment with disease-modifying antirheumatic drugs (DMARDs) after 12 months. A total score of ≥ 6 signifies RA, provided other possible causes of the symptoms have been excluded (6). The AUC (receiver operating characteristic: sensitivity [rate of true positives] as ordinate, 1-specificity [rate of false positives] as abscissa) for this limit of 6 ranges from 0.66 to 0.82 in various cohorts (6).
Since the aim is to diagnose RA before any damage has been caused, the disease must be classifiable without reference to erosions. Under the new criteria, however, typical radiologically detectable erosions in several joints suffice to classify a disease as RA regardless of all other findings.
After the joint findings, antibody results carry the greatest weight for classification and diagnosis. In daily clinical practice, determination of one antibody, rheumatoid factor, or antibodies against cyclic citrullinated peptide (anti-CCP or ACPA) is sufficient to pinpoint the cause of the joint pain.
The classification criteria help to confirm the diagnosis of RA at an early stage. Overall, early analyses show a high positive predictive value (85% to 97%) for the necessity of DMARD treatment (e5) and that many presentations previously classified as undifferentiated arthritis are now identified as RA (e6). The negative predictive value, however, is relatively low, at 27% to 59%, so patients with early arthritis must still be monitored closely even if they do not initially meet the criteria (e5). This is true particularly for seronegative patients (those without rheumatoid factors and ACPA) (e7). Up to 20% of the patients who fulfill the new criteria have diseases other than RA (e5).
If RA is suspected, the erythrocyte sedimentation rate (ESR), CRP, ACPA, and rheumatoid factor should be determined (4; ↑ ↑).
Treatment
All those involved in treatment should coordinate their efforts to ensure that the patient receives consistent information. Standardized patient information documents and courses such as those developed by the German Society for Rheumatology (Deutsche Gesellschaft für Rheumatologie) in cooperation with the German Rheumatism League (Deutsche Rheuma-Liga) form an essential part of the treatment of patients with RA, even though the studies on their effects are small, heterogeneous, and yield no significant results due to the many other factors involved (8).
Especially at the commencement of treatment, when they are confronted with much that is new and threatening and have to make a lot of decisions in a short time, patients are often overburdened. For this reason, all patients—particularly those in whom signs of poor ability to cope contribute to persistence of symptoms—should be offered psychological intervention.
Physical function and active exercise are central to living with RA. German guidelines on management of early RA recommend therapists to encourage their patients to perform regular dynamic exercises and follow an individually adjusted program of strength and endurance training, despite the absence of valid studies (4; ↑ [↑, these recommendations are based on studies in which the certainty of the findings is limited and/or the benefits only slightly outweigh the risks]).
Qualified ergotherapeutic advice is recommended for patients with limited hand function or limited ability to perform daily activities (4; ↑). A systematic review published in 2002 showed that patients benefit significantly from training in joint protection (9).
DMARD treatment
Disease-modifying drugs (DMARDs) are a central element in the treatment of RA patients (4; ↑ ↑). This group of drugs includes:
The classic synthetic DMARDs (cDMARDs) (Table 2)
The biological DMARDs (bDMARDs, biologics)
The glucocorticoids (GCs).
Table 2. DMARDs approved for the treatment of rheumatoid arthritis (cDMARD white, bDMARD olive).
| Active substance | Dosage | Strength of recommendation (S1 guideline [40]) | When? | Frequent adverse effects (>1/100; recorded using the treatment monitoring form of the DGRh [http://dgrh.de/therapieueberwachen.html] unless otherwise specified) |
|---|---|---|---|---|
| Abatacept | 10 mg/kg BW/ 4 weeks after induction phase | ↑ ↑ | As 1st or 2nd bDMARD following inadequate response to 2 cDMARDs | Hyperlipidemia, headache, dizziness, drowsiness, bronchitis, coughing, upper airway infections (including tracheitis, nasopharyngitis), rhinitis, abdominal pain, nausea, diarrhea, dyspepsia, skin rash, herpes simplex, urinary tract infection, tiredness, weight loss, hypertension, flushing |
| Adalimumab | 40 mg/2 weeks | ↑ ↑ | As 1st or 2nd bDMARD following inadequate response to 2 cDMARDs | Decreased hemoglobin concentration, hypertension, headache, dizziness, drowsiness, upper airway infections, rhinitis, sinusitis, bronchitis, increased coughing, pneumonia, nausea, diarrhea, sore throat, elevated transaminases, reactions at injection site, skin rash, pruritus, herpes simplex, urinary tract infection, weight loss, influenza syndrome |
| Anakinra | 100 mg/day | ↑ | As alternative to 1st or 2nd bDMARD | Reactions at injection site, headache |
| Antimalarial drugs | 4 mg chloroquine/kg or >6.5 mg hydroxychloroquine/kg BW/day | ↑ | As alternative to 2nd cDMARD or in combination with cDMARDs | Nausea, lack of appetite, diarrhea |
| Azathioprine | 2–3 mg/kg BW/day | – | As alternative to 2nd cDMARD | Nausea, vomiting, diarrhea, leukopenia, anemia, infection, drug fever |
| Certolizumab | 200 mg/ 2 weeks after induction phase | ↑ ↑ | As 1st or 2nd bDMARD following inadequate response to 2 cDMARDs | Urinary tract infection, herpes simplex, upper airway infections, headache, dizziness, skin rash, pruritus, exhaustion, pyrexia, pain and reddening at site of administration (product information 04/2009) |
| Cyclosporine | 2.5–3.5 mg/kg BW/day | ↑ | As alternative to 2nd cDMARD or in combination with cDMARDs | Lack of appetite, nausea and occasional vomiting, diarrhea, muscle twitching and cramp may indicate magnesium deficiency, slight trembling of hands, slight increase in body hair, swelling and inflammation of gums, hypertension, tiredness |
| Etanercept | 50 mg/week | ↑ ↑ | As 1st or 2nd bDMARD following inadequate response to 2 cDMARDs | Irritation at injection site, infections |
| Golimumab | 50 mg/month | ↑ ↑ | As 1st or 2nd bDMARD following inadequate response to 2 cDMARDs | Upper airway infections (nasopharyngitis, pharyngitis, laryngitis, rhinitis), bacterial infections (e.g., inflammation of subcutaneous tissue), viral infections (e.g., influenza and herpes), bronchitis, sinusitis, superficial fungal infections, anemia, allergic reactions (bronchospasm, hypersensitivity, urticaria), autoantibody positive, depression, sleeplessness, dizziness, paresthesias, headache, hypertension, obstipation, dyspepsia, gastrointestinal and abdominal pain, nausea, elevated ALT/GPT levels, elevated AST/GOT levels, alopecia, dermatitis, itching, skin rash, fever, asthenia, reaction at injection site (e.g., erythema, urticaria, induration, pain, bruising, itching, irritation und paresthesias), delayed wound healing, thoracic symptoms (product information 11/2012) |
| Infliximab | 3–5 mg/kg BW every 8 weeks after induction phase | ↑ ↑ | As 1st or 2nd bDMARD following inadequate response to 2 cDMARDs | Headache, dizziness, drowsiness, infections of upper and lower respiratory tract (e.g., sinusitis, pneumonia), nausea, diarrhea, elevated transaminases, urticaria, skin rash, pruritus, reactions to infusion |
| Leflunomide | 10–20 mg/day | ↑ ↑ | As alternative to 1st or 2nd cDMARD or in combination with cDMARDs | Diarrhea, nausea, vomiting, abdominal pain, mouth ulcers, elevated liver parameters, leukocytopenia, headache, dizziness, asthenia, hypertension, eczema, hair loss, skin rash, itching, weight loss, mutagenicity, teratogenicity (both in animal experiments) |
| Methotrexate | 10–25 mg/week | ↑ ↑ | As 1st cDMARD or in combination with cDMARDs and especially bDMARDs | Stomatitis, hair loss, nausea, vomiting, elevated transaminases |
| Parenteral gold | 50 mg/ 2 weeks after initial phase | ↑ | As alternative to 2nd cDMARD | Dermatitis, stomatitis, pruritus, eosinophilia, proteinuria, deposits on cornea/lens if gold dose >1500 mg (harmless), metallic taste |
| Rituximab | Two doses of 1000 mg at a 2-week interval every 6–12 months | ↑ ↑ | As 2nd bDMARD following inadequate response to 2 cDMARDs | Airway infections, reactions to infusion, influenza-like symptoms, infections, transitory hyperuricemia (15%) |
| Sulfasalazine | 2 g/day after initial phase | ↑ | As alternative to 1st or 2nd cDMARD or in combination treatment with cDMARDs | Exanthema, pruritus, nausea, abdominal pain, lack of appetite, hyperchromasia, oligospermia, reversible loss of fertility in men, headache, feeling of weakness, tiredness |
| Tocilizumab | 8 mg/kg BW every 28 days | ↑ ↑ | As 1st or 2nd bDMARD following inadequate response to 2 cDMARDs | Skin and subcutaneous infections, pneumonia, oral herpes simplex, herpes zoster, mouth ulcers, gastritis, exanthema, pruritus, headache, dizziness, elevated transaminases, hypertension, leukopenia, neutropenia, conjunctivitis |
↑, These recommendations are based on studies in which the certainty of the findings is limited and/or the benefits only slightly outweigh the risks. ↑ ↑, These recommendations are based on studies with a high level of certainty in which the benefits are shown to clearly outweigh the risks. DMARD, disease-modifying antirheumatic drug; bDMARD, biological DMARD; cDMARD, classic synthetic DMARD; DGRh, Deutsche Gesellschaft für Rheumatologie (German Society for Rheumatology)
In contrast to the general perception, DMARDs are tolerated well. Their toxicity is no greater than that of the frequently used nonsteroidal antirheumatics (NSARs), which act only against the symptoms (e8) (Table 2).
Basic principles: begin early, have a target—We now know that the most important aim is not rapid alleviation of symptoms (achievable with NSARs and GCs), but swift blocking of disease progression. This requires proper consistent use of DMARDs.
The kind of long-term placebo-controlled trials that would compare the course of RA with and without adequate treatment are prohibited on ethical grounds. Cohort studies, however, show that 47% to 53% of patients with early RA develop moderate to severe functional impairments over a period of 5 years (10– 12). Administration of cDMARDs in the first 6 months after diagnosis can reverse the initial loss of function in over 80% of patients (13) and bring about remission of the disease in 42% to 48% of cases (13, 14). The number of structurally damaged joints is significantly associated with the time from disease onset to commencement of treatment (15).
A systematic review of 38 placebo-controlled studies showed that all cDMARDs with the exception of the antimalarials significantly inhibited radiological disease progression (16). If the DMARD treatment is initiated in the first 6 months, the rate of radiological progression is halved compared to beginning treatment later (in the 2nd year) (17– 19).
In a Dutch early arthritis study (610 patients with mean symptom duration of 17 weeks), immediate commencement of DMARD treatment (methotrexate + prednisolone) achieved complete remission in around 60% of patients after only 4 months (20). In comparison with rapid initiation of DMARD treatment, a delay of 3 to 6 months led to a statistically significant doubling of the radiologically detected progression score over a period of 4 years (2.5 points versus 1.3 points per year) (21). Another Dutch study compared patients in whom RA was diagnosed within 12 weeks of disease onset with those whose diagnosis was delayed beyond 12 weeks (22). Even this delay of just a few months led to an almost 30% higher rate of destruction and a nearly 50% reduction in DMARD-free remission over a period of 6 years. Consistent treatment with the DMARD methotrexate (MTX), for example, reduced mortality by 60% (compared with no MTX) (23). In a study from Finland, immediate initiation of DMARD treatment in the early phase of the disease completely prevented RA-related mortality (24). The use of modern treatment strategies—predominantly interventions with MTX alone or in combination with other DMARDs (in a Dutch long-term study)—at the beginning of DMARD administration brought about a reduction in mortality to the level in the normal population (25).
Treat to target
In addition to initiating treatment as early as possible, it is advisable to have a treatment goal in mind and to adjust the treatment at intervals of no more than 3 months (4; ↑ ↑).
The target of treatment is suppression of disease activity, i.e., clinical remission (4, 26; ↑ ↑). In a Finnish study, remission was achieved in 35% of patients in whom DMARD treatment began within 4 months of diagnosis, compared with only 10% in those whose treatment started later (14). Systematic reviews show that enduring clinical remission is a good surrogate marker for better long-term functionality and only slight radiological progression (27, 28). Further important parameters of long-term outcome are early radiologically detectable destruction (29) and the ACPA status (patients with or without antibodies to citrullinated peptides/proteins) (e9). Radiologically detectable structural joint damage significantly affects physical function. Annual radiological registration of the damage is therefore a control parameter for treatment efficacy (4).
The use of bDMARDs leads to minimization of radiologically detectable progression independent of clinical response (e10). This has not been proved for cDMARDs. In a randomized controlled trial comparing treatment with MTX and treatment with MTX + infliximab, the MTX patients in remission showed no radiologically detectable progression but those with high disease activity had an annual increase of 6.5 points in their radiological score. In contrast, the patients on MTX + infliximab exhibited negligible progression regardless of whether they were in remission or had high activity (30).
Disease remission is assessed by scores that take account of clinical and laboratory findings (28, 31). The scale most often used in Germany is the Disease Activity Score (DAS28), which comprises the following parameters:
Number of swollen and pressure-sensitive joints
ESR or CRP
Patient’s subjective wellbeing (e11).
A score of ≤ 2.6 shows low disease activity, while ratings >5.1 indicate high disease activity. The treatment goal is thus to reduce the score to <2.6, corresponding to low disease activity; in advanced disease the target is <3.2.
The disease activity should be monitored at regular intervals, e.g., by means of the DAS28 and the treatment should be adjusted accordingly. Long-term studies following this strategy have shown an optimal long-term prognosis (with average disease duration of 23 weeks). For example, 48% of the 508 RA patients in the Dutch BeST study showed complete remission after 5 years (and in 14% the remission was even drug-free); the mean functional status was practically normal (13). The annual rate of radiologically detectable progression was also minimal (1.5 points on a 400-point scale). Further evidence for the benefit of the “treat to target” principle is provided by the comparison between two French cohorts, one with this remission-oriented strategy and the other with standard treatment (depending on the preference of the treating physician) (32). The combined goal of remission, normal functional status, and lack of radiologically detectable progression was attained by 20 patients (32.3%) and 13 patients (10.2%) respectively, corresponding to an odds ratio of 3.65.
Treatment sequence
Numerous treatment trials over the past decade have set out to establish the optimal sequence of steps in treatment with DMARDs. Ultimately an individual decision always has to be taken after consideration of the risk profile and the patient’s preferences. The consensus view, however, is that MTX is the agent of choice for monotherapy and should also form one component of any combination of cDMARDs (4; ↑ ↑). The principle reason for this is MTX’s very good risk–benefit ratio and long-term adherence compared with other cDMARDs. Numerous randomized controlled trials (RCTs) have shown that under study conditions MTX monotherapy (in combination with a low-dose GC) can achieve remission in 25% to 30% of cases (13, 33). With optimized dosing and parenteral administration of MTX and optimal GC dosing (including intra-articular administration) the remission rate can be doubled. In the Danish CIMESTRA study the rate was 43% (34), and in the Dutch IMPROVED study it reached 65% (20).
cDMARDs, including MTX, have a delayed onset of action; therefore, until the basic treatment starts to have its full effect the cDMARD should be accompanied by administration of a GC (4; ↑ ↑). GCs have been proved to take effect immediately, slowing the development or progression of destruction (e12) and raising the remission rate (e13). There is currently no conclusive evidence regarding the optimal dose of this cortisone application. In most cases cortisone is started at around 0.5 mg/kg BW prednisolone equivalent; study dosages vary between 10 and 60 mg/day (e14). Depending on the clinical symptoms, the dose should be reduced gradually to ≤ 7.5 mg prednisolone equivalent and continued at this lower dose together with cDMARDs.
In the event that a patient does not respond adequately to the initial cDMARD treatment (usually MTX + GC), or right from the outset in high-risk patients, two types of treatment escalation can be employed (e15): MTX is usually first combined with another cDMARD. The available evidence indicates that particularly the combinations MTX + sulfasalazine + hydroxychloroquine (35, e15) and MTX + leflunomide (36) increase the response rate significantly. In the first of the two studies cited, the triple combination achieved a good response in 24/31 patients (77%), against 12/36 (33%) for MTX alone. In the second study, an ACR-70 response (roughly equivalent to remission) of 10% was attained by MTX + leflunomide compared with 2.3% for MTX alone.
Even when treatment is started early and all resources are exhausted with regard to cDMARD + GC treatment, the disease is satisfactorily controlled in a maximum of 60% to 80% of patients. The use of bDMARDs provides a solution for most of the remaining cases. In patients with high disease activity and/or an unfavorable prognosis, international guidelines such as the EULAR recommendations advise administration of bDMARDs directly after failure of the initial cDMARD treatment (37). In all other cases this step should be taken after failure of the second cDMARD strategy, at the latest (4; ↑ ↑). RCTs have shown that bDMARDs in combination with MTX achieve remission rates at least twice as high as MTX + placebo. For instance, the remission rate with etanercept + MTX was 50%, while with MTX alone it was only 28% (38); adalimumab + MTX resulted in a rate of 34%, MTX alone 17% (39). Furthermore, practically all RCTs have shown that in nearly all cases bDMARDs prevent the occurrence or progression of radiologically detectable changes and the associated functional impairments (38, e16). This effect was demonstrated in a long-term extension study of adalimumab over a period of 8 years (e17). In a meta-analysis of treatment trials in patients not previously treated with MTX or biologics, the likelihood of zero progression was around twice as high for the combination biologic + MTX as it was for MTX alone (odds ratio 2.19) (e18).
The approach we have described is currently being extended to embrace strategies of treatment de-escalation.
Key Messages.
Newly diagnosed rheumatoid arthritis is an eminently treatable disease with prospects of remission for more than half of those afflicted.
Early diagnosis is a precondition for successful treatment.
Early diagnosis is facilitated by new classification criteria.
The long-term prognosis is optimized by early commencement of treatment and management following the “treat to target” principle with sequential use of disease-modifying antirheumatic drugs (DMARDs), including biologics in the event of lack of response to classic DMARDs.
Provided the current guidelines on administration and monitoring are followed, the benefits of treatment with DMARDs, biologics, and cortisone far outweigh the risks.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Professor Schneider has received consulting fees from Abbott, Essex, Roche, UCB, and Pfizer. He has received reimbursement of conference attendance fees and travel costs from Abbott, Pfizer, and Merck, and payments for lectures from Essex, Abbott, MSD, Roche, Pfizer, and UCB. Moreover, he has received third-party funding for research from Abbott, Pfizer, Roche, and UCB.
Professor Krüger has received reimbursement of conference attendance fees and travel costs from Abbvie and Roche, and payments for lectures from Abbvie, Roche, BMS, MSD, Pfizer, and UCB. He has received consulting fees from Abbvie, BMS, Pfizer, Roche, MSD, and UCB.
References
- 1.Westhoff G, Schneider M, Raspe H, et al. Advance and unmet need of health care for patients with rheumatoid arthritis in the German population-results from the German Rheumatoid Arthritis Population Survey (GRAPS) Rheumatology. 2009;48:650–657. doi: 10.1093/rheumatology/kep045. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Coulton BL, Symmons DPM, Popert AJ. Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet. 1987:1108–1111. doi: 10.1016/s0140-6736(87)91672-2. [DOI] [PubMed] [Google Scholar]
- 3.Pincus T, Callahan LF, Sale WG, et al. Severe functional decline, work disability, and increased mortality in 75 rheumatoid arthritis patients studied over 9 years. Arthritis Rheum. 1984;27:864–872. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 4.Schneider M, Lelgemann M, Abholz HH, et al. http://dgrh.de/leitlinien.html. Heidelberg: Springer Verlag; 2011. Management der frühen rheumatoiden Arthritis. [Google Scholar]
- 5.Albrecht K, Krüger K, Müller-Ladner U, Wollenhaupt J. Systematische Literaturrecherche für die S1-Leitlinie der DGRh zur sequenziellen medikamentösen Therapie der rheumatoiden Arthritis 2011. Z Rheumatol. 2012;71:604–618. doi: 10.1007/s00393-012-1048-y. [DOI] [PubMed] [Google Scholar]
- 6.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 7.Neogi T, Aletaha D, Silman AJ, et al. American College of Rheumatology; European League Against Rheumatism The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Phase 2 methodological report. Arthritis Rheum. 2010;62:2582–2591. doi: 10.1002/art.27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedermann K, Fransen J, Knols R, Uebelhart D. Gap between short- and long-term effects of patient education in rheumatoid arthritis patients: a systematic review. Arthritis Rheum. 2004;51:388–398. doi: 10.1002/art.20399. [DOI] [PubMed] [Google Scholar]
- 9.Steultjens EM, Dekker J, Bouter LM, et al. Occupational therapy for rheumatoid arthritis. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003114.pub2. 1 CD003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Zeben D, Hazes JM, Zwinderman AH, Vandenbroucke JP, Breedveld FC. The severity of rheumatoid arthritis: a 6-year followup study of younger women with symptoms of recent onset. J Rheumatol. 1994;21:1620–1625. [PubMed] [Google Scholar]
- 11.Wiles NJ, Lunt M, Barrett EM, et al. Reduced disability at five years with early treatment of inflammatory polyarthritis: results from a large observational cohort, using propensity models to adjust for disease severity. Arthritis Rheum. 2001;44:1033–1042. doi: 10.1002/1529-0131(200105)44:5<1033::AID-ANR182>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Lindqvist E, Saxne T, Geborek P, Eberhardt K. Ten year outcome in a cohort of patients with early rheumatoid arthritis: health status, disease process, and damage. Ann Rheum Dis. 2002;61:1055–1059. doi: 10.1136/ard.61.12.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klarenbeek NB, Güler-Yüksel M, van der Kooij SM, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis. 2011;70:1039–1046. doi: 10.1136/ard.2010.141234. [DOI] [PubMed] [Google Scholar]
- 14.Mottonen T, Hannonen P, Korpela M, et al. Delay to institution of therapy and induction of remission using single-drug or combination disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002;46:894–898. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 15.Jansen LM, van der Horst-Bruinsma IE, van SD, Bezemer PD, Dijkmans BA. Predictors of radiographic joint damage in patients with early rheumatoid arthritis. Ann Rheum Dis. 2001;60:924–927. doi: 10.1136/ard.60.10.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones G, Halbert J, Crotty M, et al. The effect of treatment on radiological progression in rheumatoid arthritis: a systematic review of randomized placebo-controlled trials. Rheumatology (Oxford) 2003;42:6–13. doi: 10.1093/rheumatology/keg036. [DOI] [PubMed] [Google Scholar]
- 17.Bukhari MA, Wiles NJ, Lunt M, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: Results from a large observational inception study. Arthritis Rheum. 2003;48:46–53. doi: 10.1002/art.10727. [DOI] [PubMed] [Google Scholar]
- 18.Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year followup of a prospective double blind placebo controlled study. J Rheumatol. 1995;22:2208–2213. [PubMed] [Google Scholar]
- 19.Tsakonas E, Fitzgerald AA, Fitzcharles MA, et al. Consequences of delayed therapy with second-line agents in rheumatoid arthritis: a 3 year followup on the hydroxychloroquine in early rheumatoid arthritis (HERA) study. J Rheumatol. 2000;27:623–629. [PubMed] [Google Scholar]
- 20.Wevers-de Boer K, Visser K, Heimanns L, et al. Remission induction therapy with methotrexate and prednisone in patients with early rheumatoid and undifferentiated arthritis (the IMPROVED study) Ann Rheum Dis. 2012;71:1472–1477. doi: 10.1136/annrheumdis-2011-200736. [DOI] [PubMed] [Google Scholar]
- 21.van Aken J, Lard LR, Le Cessie S, et al. Radiological outcome after four years of early versus delayed treatment strategy in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004;63:274–279. doi: 10.1136/ard.2003.010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Linden MPM, Le Cessie S, Raza K, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62:3537–3546. doi: 10.1002/art.27692. [DOI] [PubMed] [Google Scholar]
- 23.Choi HK, Hernan MA, Seeger JD, et al. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 24.Peltomaa R, Paimela L, Kautiainen H, Leirisalo-Repo M. Mortality in patients with rheumatoid arthritis treated actively from the time of diagnosis. Ann Rheum Dis. 2002;61:889–894. doi: 10.1136/ard.61.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Nies JAB, de Jong Z, van der Helm-van Mil AHM, et al. Improved treatment strategies reduce the increased mortality risk in early arthritis patients. Rheumatology. 2010;49:2210–2216. doi: 10.1093/rheumatology/keq250. [DOI] [PubMed] [Google Scholar]
- 26.Smolen JS, Aletaha D, Bijlsma JWJ, et al. for the T2T Expert Committee: Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Tuyl LHD, Felson DT, Wells G, Smolen J, Zhang B, Boers M. ACR & EULAR committee to define remission for clinical trials. Systematic review: Evidence for predictive validity of remission on long term outcome in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:108–117. doi: 10.1002/acr.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klarenbeek NB, Koevoets R, van der Heijde DM, et al. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1815–1821. doi: 10.1136/ard.2010.149260. [DOI] [PubMed] [Google Scholar]
- 29.van den Broek M, Dirven L, de Vries-Bouwstra JK, et al. Rapid radiological progression in the first year of early rheumatoid arthritis is predictive of disability and joint damage progression during 8 years of follow-up. Ann Rheum Dis. 2012;71:1530–1533. doi: 10.1136/annrheumdis-2011-201212. [DOI] [PubMed] [Google Scholar]
- 30.Smolen JS, Han C, van der Heijde DMFM, et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Ann Rheum Dis. 2009;2011:823–827. doi: 10.1136/ard.2008.090019. [DOI] [PubMed] [Google Scholar]
- 31.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism: Provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–413. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 32.Soubrier M, Lukas C, Sibilia J, et al. Disease activity score-driven therapy versus routine care in patients with recent-onset active rheumatoid arthritis: data from the GUEPARD trial and ESPOIR cohort. Ann Rheum Dis. 2011;70:611–615. doi: 10.1136/ard.2010.137695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374:459–466. doi: 10.1016/S0140-6736(09)60944-2. [DOI] [PubMed] [Google Scholar]
- 34.Hetland ML, Stengaard-Pedersen K, Junker P, et al. Combination treatment with Methotrexate, Cyclosporine, and intraarticular Betamethasone compared with Methotrexate and intraarticular Betamethasone in early active rheumatoid arthritis. Arthritis Rheum. 2006;54:1401–1409. doi: 10.1002/art.21796. [DOI] [PubMed] [Google Scholar]
- 35.O’Dell JR, Leff R, Paulsen G. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1164–1170. doi: 10.1002/art.10228. [DOI] [PubMed] [Google Scholar]
- 36.Kremer JM, Genovese MC, Cannon GW, et al. Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate. A randomized, double-blind, placebo- controlled trial. Ann Intern Med. 2002;137:726–733. doi: 10.7326/0003-4819-137-9-200211050-00007. [DOI] [PubMed] [Google Scholar]
- 37.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 39.Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72:64–71. doi: 10.1136/annrheumdis-2011-201247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krüger K, Wollenhaupt J, Albrecht K, et al. S1-Leitlinie der DGRh zur sequenziellen medikamentösen Therapie der rheumatoiden Arthritis 2011: adaptierte EULAR Empfehlungen und aktualisierter Therapiealgorithmus. Z Rheumatol. 2012;71:592–603. doi: 10.1007/s00393-012-1038-0. [DOI] [PubMed] [Google Scholar]
- e1.Symmons DP. Epidemiology of rheumatoid arthritis: determinants of onset, persistence and outcome. Best Pract Res Clin Rheumatol. 2002;16:707–722. doi: 10.1053/berh.2002.0257. [DOI] [PubMed] [Google Scholar]
- e2.Silman AJ, Hochberg M. 2nd ed. Oxford: Oxford University Press; 2001. Epidemiology of the rheumatic diseases. [Google Scholar]
- e3.Doran MF, Pond GR, Crowson CS, O’Fallon M, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year-period. Arthritis Rheum. 2002;46:625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- e4.Brooks PM, Buckwalter JA. Principles of Management of Patients with Rheumatic Disease. In: Klippel JH, Dieppe PA, editors. Rheumatology. Philadelephia: Mosby; 1998. [Google Scholar]
- e5.van der Helm-van Mil A HM, Huizinga TWJ. The 2010 ACR/EULAR criteria for rheumatoid arthritis: do they affect the classification or diagnosis of rheumatoid arthritis? Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201426. doi:10.1136/annrheumdis-2012-201426. [DOI] [PubMed] [Google Scholar]
- e6.Krabben A, Huizinga TWJ, van der Helm-van Mil AHM. Undifferentiated arthritis characteristics and outcomes when applying the 2010 and 1987 criteria for rheumatoid arthritis. Ann Rheum Dis. 2012;7:238–241. doi: 10.1136/annrheumdis-2011-200205. [DOI] [PubMed] [Google Scholar]
- e7.Fautrel B, Combe B, Rincheval N, Dougados M. for the ESPOIR Scientific Committee: Level of agreement of the 1987 ACR and 2010 ACR/EULAR rheumatoid arthritis classification criteria: an analysis based on ESPOIR cohort data. Ann Rheum Dis. 2012;71:386–389. doi: 10.1136/annrheumdis-2011-200259. [DOI] [PubMed] [Google Scholar]
- e8.Fries JF, Williams CA, Ramey D, Bloch DA. The relative toxicity of disease-modifying antirheumatic drugs. Arthritis Rheum. 1993;36:297–306. doi: 10.1002/art.1780360303. [DOI] [PubMed] [Google Scholar]
- e9.Knevel R, van Nies JAB, le Cessie S, et al. Evaluation of the contribution of cumulative levels of inflammation to the variance in joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2013;72:307–308. doi: 10.1136/annrheumdis-2012-201931. [DOI] [PubMed] [Google Scholar]
- e10.Keystone EC. Clinical Implications of Understanding Radiographic Findings in Relation to Clinical Outcomes in Rheumatoid Arthritis. J Rheumatol. 2009;36(Suppl 82):11–16. doi: 10.3899/jrheum.090125. [DOI] [PubMed] [Google Scholar]
- e11.van der Heijde DM, van ’t Hof MA, van Riel PL, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49:916–920. doi: 10.1136/ard.49.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Kirwan JR, Bijlsma JW, Boers M, Shea BJ. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Syst Rev. 2007;24 doi: 10.1002/14651858.CD006356. CD 006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Todoerti M, Scire CA, Boffini N, et al. Early disease control by low-dose prednisone comedication may affect the quality of remission in patients with early rheumatoid arthritis. Ann N Y Acad Sci. 2010;1193:139–145. doi: 10.1111/j.1749-6632.2009.05367.x. [DOI] [PubMed] [Google Scholar]
- e14.Bharadwaj A, Alves C. The wide variation in corticosteroid use in early rheumatoid arthritis - There is need for guidelines. Arthritis Rheum. 2012;64 [Google Scholar]
- e15.de Jong PH, Hazes JM, Barendregt PJ, et al. Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: first results of the tREACH trial. Ann Rheum Dis. 2013;72:72–78. doi: 10.1136/annrheumdis-2011-201162. [DOI] [PubMed] [Google Scholar]
- e16.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER Study: A Multicenter, Randomized, Double-Blind Clinical Trial of Combination Therapy With Adalimumab Plus Methotrexate Versus Methotrexate Alone or Adalimumab Alone in Patients With Early, Aggressive Rheumatoid Arthritis Who Had Not Had Previous Methotrexate Treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- e17.Breedveld F, Keystone E, van der Heijde D, et al. Initial combination therapy with adalimumab plus methotrexate leads to better long-term outcomes in patients with early rheumatoid arthritis: 8-Year Results of the Premier Trial. Ann Rheum Dis. 2011 Suppl., THU0230. [Google Scholar]
- e18.Pierreisnard A, Issa N, Barnetche T, et al. Meta-analysis of clinical and radiological efficacy of biologics in rheumatoid arthritis patients naive or inadequately responsive to methotrexate. Joint Bone Spine. 2012 doi: 10.1016/j.jbspin.2012.09.023. online Nov 6, doi: 10.1016/j.jbspin.2012.09.023. [DOI] [PubMed] [Google Scholar]