Abstract
Angiogenesis leads to the formation of blood vessels from pre-existing ones, allowing tumor growth. Vascular endothelial growth factor (VEGF) and Angiopoietins (Ang-1, Ang-2) have a pivotal role in tumor angiogenesis but few data regarding their role in hereditary breast cancer are available. The aim of the present study was to analyze Ang-1, Ang-2, tyrosine-protein kinase receptor Tie2 and VEGF expression and their correlation in a cohort of familial and sporadic breast cancers in order to verify whether the presence of germline mutations in BRCA may have a role in tumor microenvironment regulation. Tumor samples from a cohort of 41 patients with a first diagnosis and a family history of breast cancer and 19 patients with sporadic breast cancers were enrolled. The expression of Tie2, Ang-1, Ang-2 and VEGF were analyzed by quantitative real-time PCR. Patients harboring BRCA mutations had higher levels of Ang-1 (P=0.05), Ang-2 (P=0.02) and VEGF (P=0.04) mRNA compared with those without BRCA mutations (BRCAX). The same was observed in triple-negative breast cancer (TNBC). Moreover, a positive correlation between Ang-2 and VEGF was found in both the familial breast cancer group (BRCA carriers: r=0.83; P<0.0001 and BRCAX: r=0.58; P=0.008) and in TNBC (r=0.62; P=0.007). The higher levels of Ang-1, Ang-2 and VEGF mRNA found in BRCA carriers and TNBCs suggest that they could be attractive angiogenic therapeutic targets in these breast cancers.
Keywords: Ang-1, Ang-2, VEGF, BRCA1, angiogenesis
Introduction
Angiogenesis, the formation of blood vessels from pre-existing ones, has a rate-limiting role in tumor development and progression. Vascular endothelial growth factor-A (VEGF) and angiopoietins are the pivotal drivers of tumor angiogenesis.1 VEGF is the best characterized member of a family that includes placental growth factor, VEGF-B, VEGF-C and VEGF-D. Binding mainly to VEGF receptor 2, VEGF activates angiogenic signals that lead to the proliferation and survival of endothelial cells.2 Angiopoietin 1 (Ang-1) is a vascular stabilizing factor that is expressed by vascular mural cells and non-vascular normal and tumor cells, whereas angiopoietin 2 (Ang-2) is primarily produced by endothelial cells during vascular remodeling. Both angiopoietins have the capability to bind to Tie2 receptor with similar affinities, but they act as antagonizing molecules: Ang-1-mediated Tie2 activation results in survival signals for endothelial cells and allows vessel maturation and maintenance of vascular integrity. Conversely, Ang-2 competes for Tie2 binding, causing vessel regression in the absence of angiogenic factors, whereas it potentiates angiogenesis in the presence of VEGF.3, 4 VEGF and Angiopoietins have both been widely studied in breast cancer, in which angiogenesis has a crucial role in tumor progression.5, 6 Some authors have reported that BRCA1, besides its role in the maintenance of genomic stability, is also involved in neovascularization through transcription regulation of some angiogenic factors.7 Specifically, VEGF and Ang-1 are negatively regulated by BRCA1 through interaction with the estrogen receptor alpha (ER-α)8 and the complex CtIP-interacting protein (CtIP) and BRCA1-interacting protein with a KRAB domain (ZBRK1),9 respectively. Inherited germline mutations in BRCA1 or BRCA2 genes, the best-known high susceptibility genes of familial breast cancer, increase the life-time risk of the development of breast and ovarian cancer10 and are responsible for 5–10% of all breast cancers.11, 12 BRCA-related cancers show morphological and immunohistochemical differences, as well as copy number aberrations compared with sporadic breast cancers. These differences may explain the activation of distinct pathways as promoters of carcinogenesis.
The aim of this study was to analyze the main angiogenic factors such as Ang-1, Ang-2, their cognate Tie2 receptor and VEGF in a cohort of familial and sporadic breast cancers to verify whether the presence of germline mutations in BRCA1/2 genes may be associated to peculiar expression of these angiogenic factors.
Materials and methods
Patients
Human breast cancer samples were obtained from 60 patients who underwent surgery at the National Cancer Research Centre ‘Giovanni Paolo II' of Bari. The cohort included 41 cases with a first diagnosis of breast cancer and with a family history (22 BRCA1/2 carriers and 19 BRCAX cases) enrolled from patients followed in our Genetic Counseling Program and 19 sporadic cancers. The BRCAX breast cancers were defined as familial breast tumors without BRCA1 and BRCA2 pathogenic mutation. DNA from peripheral blood was screened for whole BRCA1 and BRCA2 gene mutations using DHPLC as a pre-screening analysis and automatic DNA sequencing for the identification of specific alterations, as previously reported.13 All patients signed an informed consent form authorizing the research. Information about clinical parameters such as age, tumor size, nodal status, grading, status of HER2, ER and PgR (progesterone receptor) were obtained from clinical records as shown in Table 1.
Table 1. Patient clinico-pathological characteristics in familial and sporadic cancer (n=60).
| BRCA1/2 MUTATION CARRIERS (n=22) | BRCAX (n=19) | SPORADIC PATIENTS (n=19) | |
|---|---|---|---|
| Age | |||
| <45 | 14 | 7 | 2 |
| ≥45 | 8 | 12 | 17 |
| T | |||
| I | 9 | 13 | 8 |
| II–III | 12 | 6 | 11 |
| Unknown | 1 | ||
| Lymph nodes | |||
| Neg | 12 | 9 | 7 |
| Pos | 10 | 10 | 11 |
| Unknown | 0 | 0 | 1 |
| Grade | |||
| 1–2 | 8 | 10 | 1 |
| 3 | 14 | 9 | 16 |
| Unknown | 0 | 0 | 2 |
| ER | |||
| Neg | 12 | 2 | 9 |
| Pos | 10 | 17 | 10 |
| PgR | |||
| Neg | 13 | 2 | 10 |
| Pos | 9 | 17 | 9 |
| HER2/neu | |||
| Neg | 13 | 10 | 11 |
| Pos | 5 | 5 | 6 |
| Unknown | 4 | 4 | 2 |
| Immunophenotype | |||
| NTNBC | 11 | 13 | 9 |
| TNBC | 7 | 2 | 8 |
| Unknown | 4 | 4 | 2 |
Abbreviations: BRCA, breast cancer susceptibility genes; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; Neg, negative; NTNBC, non triple-negative breast cancer; PgR, progesterone receptor; Pos, positive; T, tumor size; TNBC, triple-negative breast cancer.
Familial breast cancer patients have been divided into BRCA mutation carriers and non-carriers.
RNA extraction and RT-PCR
Total RNA was extracted from formalin-fixed, paraffin-embedded breast cancer and matched adjacent non-cancerous tissues by the RNeasy FFPE Kit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol. The concentration and the quality of the isolated RNA was measured by a NanoDrop 8000 Spectrophotometer v2 1.0 (Thermo Scientific, Essex, UK). Total RNA was reverse transcribed to single-stranded cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystem, Foster City, CA, USA). Reaction was performed using 400 ng of total RNA in a total volume of 20 μl according to the manufacturer's protocol (Applied Biosystem). cDNA synthesis was performed at 25 °C for 10 min, then the reaction was incubated at 37 °C for 120 min followed by 85 °C for 5 min.
Quantitative real-time PCR
Quantitative real-time PCR was performed using 80 ng of cDNA in a final volume of 20 μl according to the manufacturer's protocol (Applied Biosystems), on the ABI Prism 7000 Sequence Detection System (Applied Biosystems). qPCR assay IDs used were the following: human Ang-1 (Hs00375822_m1), human Ang-2 (Hs01048042_m1), human Tie2 (Hs00176096_m1), human VEGF (Hs00900055_m1) and endogenous control RN18S1 (Hs03928985_g1). The samples underwent PCR analysis using the following cycling parameters: at 50 °C for 2 min, at 95 °C for 10 min, then at 95 °C for 15 s and at 60 °C for 1 min, for 45 cycles. The comparative Ct method was used by subtracting the Ct value of endogenous control from the Ct value of the target gene (ΔCt=Ct (target gene)−Ct (endogenous control)). The same calculation was done for the RNA reference given by a pool of adjacent non-cancerous tissues consisting of five samples per tumor subgroup. ΔΔCt was then calculated as the difference of these values (ΔΔCt=ΔCt sample−ΔCt reference). Finally, the amount of target was given by 2−(ΔΔCt). Each sample was tested in duplicate.
Statistical analysis
Data analysis was performed using the GraphPad Prism statistics software package (GraphPad Prism 5.01, San Diego, CA, USA). Normality of the data distribution was checked by the Kolmogorov–Smirnov test. As the values were not normally distributed, non-parametric tests were used to compare angiogenic factor levels between groups, that is, the Mann–Whitney U-test.
The χ2-test was used to compare Tie2, Ang-1, Ang-2 and VEGF frequency of overexpression between the breast cancer groups. The Spearman correlation coefficient was used to analyze the correlations between angiogenic gene expression. The disease-free survival (DFS) data were analyzed by the Log-rank (Mantel-Cox) test. The cases were divided into two groups with the expression above and below the median expression of Tie2, Ang-1, Ang-2 and VEGF. A P value≤0.05 was considered statistically significant.
Results
Ang-1, Ang-2, Tie2 and VEGF expression in familial breast cancers
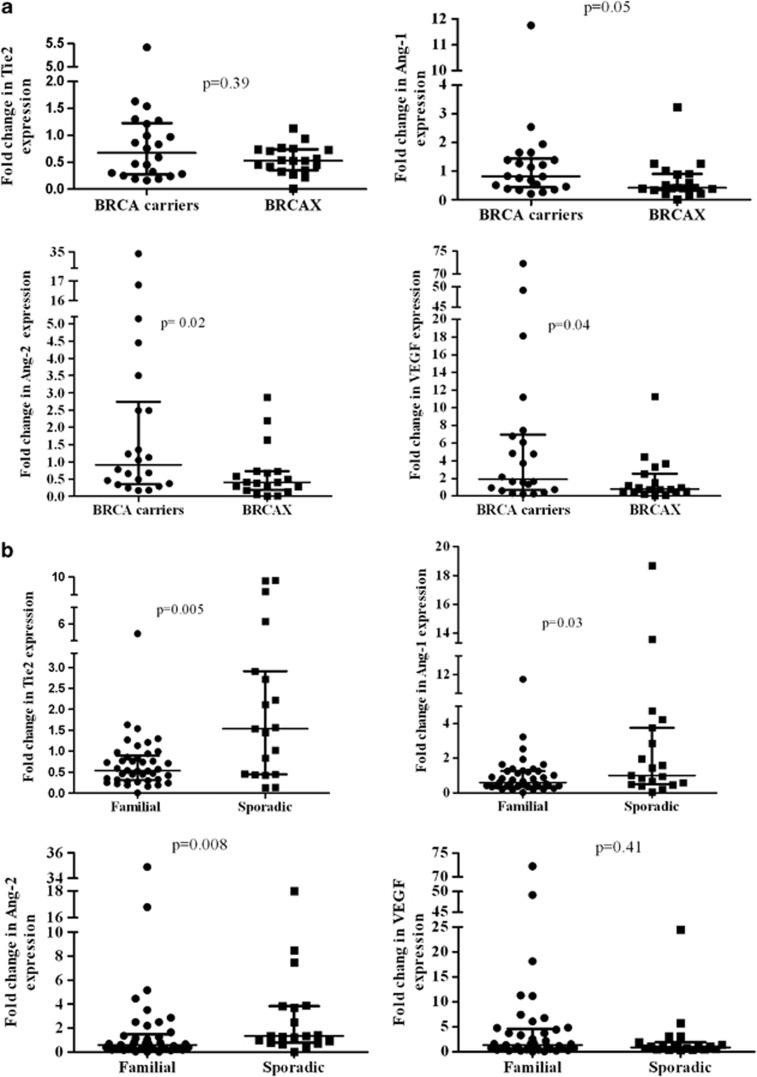
We used real-time PCR analysis to examine the expression of Tie2, Ang-1, Ang-2 and VEGF mRNA within the familial subgroups stratified by BRCA status. Figure 1 shows the distribution of the normalized expression levels of the three angiogenic factors and Tie2 receptor in BRCA1/2 carriers and BRCAX-associated tumors. The median levels of Ang-1, Ang-2 and VEGF mRNA were significantly higher in patients harboring BRCA1/2 germline mutations compared with the BRCAX group (Ang-1: 0.81 vs 0.41; P=0.05; Ang-2: 0.91 vs 0.41; P=0.02 and VEGF: 1.9 vs 0.79; P=0.04). The median levels of Tie2 mRNA showed no difference between BRCA1/2 mutation carriers and BRCAX group. The frequency of Tie2, Ang-1, Ang-2 and VEGF overexpression in BRCA1/2 mutation carriers and BRCAX-associated tumors was explored. The cases were divided into two groups based on Tie2, Ang-1, Ang-2 and VEGF expression under and over the levels in the matched adjacent non-cancerous tissues. Ang-2 and VEGF were also more frequently overexpressed in BRCA1/2 mutation carriers compared with BRCAX-associated tumors (VEGF: 68.2 vs 36.8% P=0.04 and Ang-2: 50 vs 15.8%, P=0.02). There was still a higher frequency of Ang-1 overexpression in BRCA1/2 mutation carriers compared with non-carrier breast tumors, although this was not statistically significant (45.5 vs 21.1%, P=0.1). Again, the frequency of Tie2 gene overexpression was not different between the two familial breast cancer groups.
Figure 1.
Levels of Tie2, Ang-1, Ang-2 and VEGF mRNA in BRCA1/2 mutation carriers vs BRCAX-associated tumors (a) and in familial vs sporadic breast cancer groups (b). Bars indicate median values with interquartile range.
Angiogenic factor expression in sporadic breast cancers
The median level of Tie2, Ang-1, Ang-2 and VEGF mRNA were explored in sporadic breast cancers (19 cases). These showed a significant increase of Tie2, Ang-1 and Ang-2 levels compared with the familial breast cancer group (Tie2: 1.54 vs 0.54; P=0.005; Ang-1:1.01 vs 0.6; P=0.03 and Ang-2:1.32 vs 0.59; P=0.008), whereas the expression of VEGF mRNA was higher in the familial than in the sporadic breast cancer group (P=n.s.). Sporadic breast cancer patients also presented a higher frequency of Ang-2 and Tie2 gene overexpression compared with familial breast cancers (Ang-2: 63.2 vs 34.1%, P=0.03; Tie2: 63.2 vs 17.1%, P<0.001). On the contrary, there was no evidence of differences in the frequency of Ang-1 and VEGF gene overexpression between the two breast cancer groups.
Correlations between Tie2, Ang-1, Ang-2 and VEGF mRNA expression and clinico-pathological assessment
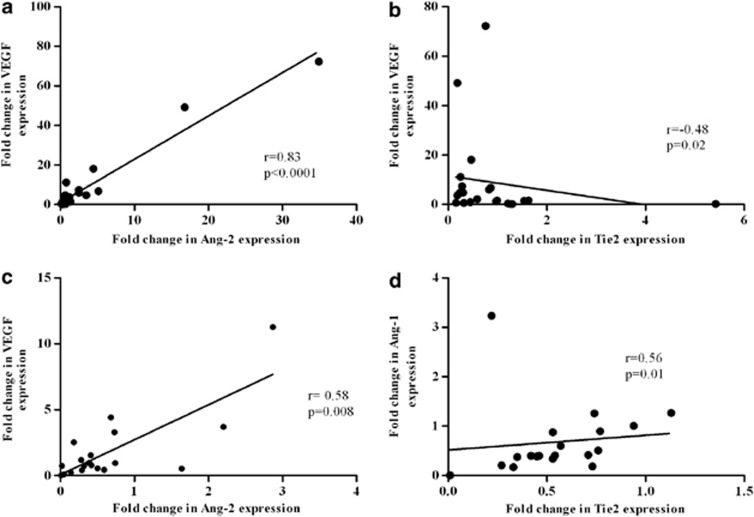
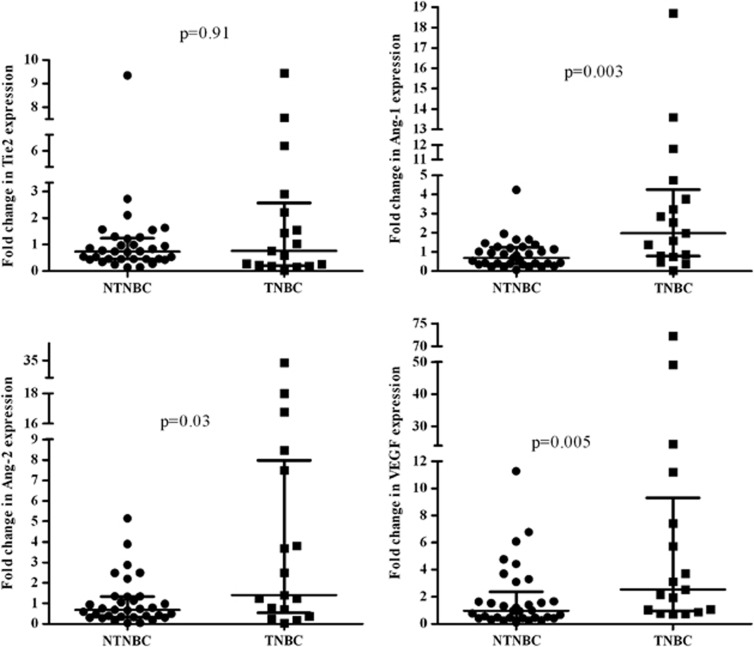
The correlation between Tie2, Ang-1, Ang-2, VEGF and clinico-pathological assessment was explored in the familial breast cancer group (Table 2). Higher median levels of VEGF correlated to negative ER (4.23 vs 0.96; P=0.01) and negative PgR (3.71 vs 0.95; P=0.009). Moreover, there was evidence of an association between a higher Tie2 median level and positive ER (0.73 vs 0.26; P=0.003) and PgR (0.72 vs 0.28; P=0.01). Ang-1 median level was higher in low differentiated grade tumors (G3 0.9 vs G1-2 0.4; P=0.01); however, there was no evidence of any correlation between Ang-2 and any clinico-pathological assessment. A correlation analysis within familial subgroups showed that patients with BRCA1/2 mutations presented a positive and significant correlation between Ang-2 and VEGF (r=0.839, P<0.0001) and an inverse and significant association between Tie2 and VEGF (r=−0.485, P=0.02). On the contrary, evidence of positive and significant association between Ang-2 and VEGF (r=0. 582, P=0.008) and between Ang-1 and Tie2 (r=0.563, P=0.012) was observed in BRCAX patients (Figure 2). The levels of Tie2, Ang-1, Ang-2 and VEGF mRNA have been also explored in immunophenotype triple-negative breast cancer (TNBC, n=17) and non TNBC (NTNBC, n=33) (Figure 3). Patients with the TNBC immunophenotype had a higher median level of Ang-1, Ang-2 and VEGF compared with the NTNBC group (Ang-1: 1.97 vs 0.68; P=0.003; Ang-2: 1.4 vs 0.68; P=0.03 and VEGF: 2.53 vs 0.96; P=0.005), whereas Tie2 receptor showed no difference between the two groups. Moreover, both Ang-2 and VEGF were more frequently overexpressed in TNBC compared with NTNBC (Ang-2: 64.7 vs 33.3%, P=0.03; VEGF: 76.5 vs 45.5%, P=0.03).
Table 2. Median levels of Tie2, Ang-1, Ang-2 and VEGF mRNA and clinico-pathological parameters in familial breast cancers.
| Tie2 median (range) | P-value | Ang-1 Median (range) | P-value | Ang-2 median (range) | P-value | VEGF median (range) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| <45 (n=21) | 0.45 (0.01–1.63) | 0.40 | 0.81 (0.01–11.75) | 0.17 | 0.5 (0.01–34.89) | 0.92 | 1.65 (0.03–72.25) | 0.34 |
| ≥45 (n=20) | 0.55 (0.16–5.42) | 0.43 (0.17–1.64) | 0.63 (0.05–4.45) | 0.87 (0.1–18.12) | ||||
| T | ||||||||
| I (n=22) | 0.53 (0.16–1.63) | 0.5 | 0.47 (0.17–11.75) | 0.2 | 0.49 (0.05–34.89) | 0.47 | 0.96 (0.1–72.25) | 0.51 |
| II-III (n=18) | 0.75 (0.01–5.42) | 1.01 (0.01–3.23) | 0.71 (0.01–16.79) | 1.58 (0.03–49.18) | ||||
| Unknown (n=1) | ||||||||
| Lymph nodes | ||||||||
| Neg (n=21) | 0.53 (0.01–1.3) | 0.47 | 0.81 (0.01–3.23) | 0.54 | 0.68 (0.02–34.89) | 0.47 | 2.16 (0.23–72.25) | 0.12 |
| Pos (n=20) | 0.55 (0.19–5.42) | 0.52 (0.19–11.75) | 0.49 (0.01–4.45) | 0.95 (0.03–18.12) | ||||
| Grade | ||||||||
| 1–2 (n=18) | 0.5 (0.27–1.63) | 0.75 | 0.40 (0.17–1.27) | 0.01 | 0.73 (0.17–5.15) | 0.1 | 1.26 (0.23–18.12) | 0.65 |
| 3 (n=23) | 0.59 (0.01–5.42) | 0.9 (0.01–11.75) | 0.46 (0.01–34.89) | 1.31 (0.03–72.25) | ||||
| ER | ||||||||
| Neg (n=14) | 0.26 (0.01–5.42) | 0.003 | 0.78 (0.01–11.75) | 0.28 | 0.73 (0.02–34.89) | 0.38 | 4.23 (0.31–72.25) | 0.01 |
| Pos (n=27) | 0.73 (0.27–1.63) | 0.51 (0.17–1.94) | 0.49 (0.01–5.15) | 0.96 (0.03–11.27) | ||||
| PgR | ||||||||
| Neg (n=15) | 0.28 (0.01–5.42) | 0.01 | 0.75 (0.01–11.75) | 0.52 | 0.78 (0.02–34.89) | 0.29 | 3.7 (0.31–72.25) | 0.009 |
| Pos (n=26) | 0.72 (0.27–1.63) | 0.52 (0.17–1.94) | 0.47 (0.01–5.15) | 0.95 (0.03–11.27) | ||||
| HER2/neu | ||||||||
| Neg (n=23) | 0.46 (0.01–1.3) | 0.1 | 0.75 (0.01–11.75) | 0.48 | 0.49 (0.02–34.89) | 0.62 | 1.63 (0.23–72.25) | 0.23 |
| Pos (n=10) | 0.77 (0.24–1.63) | 0.94 (0.34–1.94) | 0.62 (0.05–2.49) | 1.35 (0.1–6.08) | ||||
| Unknown (n=8) | ||||||||
Abbreviations: Ang-1, angiopoietin 1; Ang-2, angiopoietin 2; ER, estrogen receptor; HER2/neu, human epidermal growth factor receptor 2; PgR, progesterone receptor; T, tumor size; Tie2, tyrosine kinase with Ig and EGF (epidermal growth factor) homology domains 2; VEGF, vascular endothelial growth factor. Bold characters are significant statistic data.
Figure 2.
Relationship between expression of Ang-2 and VEGF (a) and between expression of Tie2 and VEGF (b) in BRCA1/2 mutation carriers. Relationship between expression of Ang-2 and VEGF (c) and between the expression of Tie2 and Ang-1 (d) in BRCAX.
Figure 3.
Levels of Tie2, Ang-1, Ang-2 and VEGF mRNA in triple-negative and non triple-negative breast cancer. Bars indicate median values with interquartile range.
The analysis of the different immunophenotypes revealed a strong correlation between Ang-2 and VEGF (r=0.627, P=0.007), Ang-2 and Tie2 (r=0.6, P=0.01) and between Ang-1 and Tie2 (r=0.55, P=0.02) in TNBC.
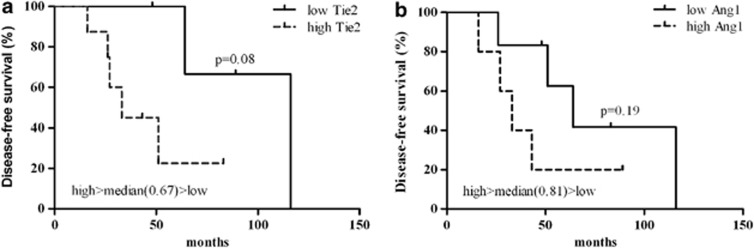
When considering the role of Angiopoietin axis expression with respect to DFS, a trend for a worse patient DFS with high expression of Ang-1 and Tie2 has been shown in particular in BRCA1/2 mutation carriers (Figure 4).
Figure 4.
Kaplan–Meier curves depicting DFS (%) of BRCA1/2 mutation carriers subset regarding the expression of Tie2 (a) and Ang-1 (b). Survival differences were assessed using Log-rank (Mantel-Cox).
Discussion
Angiogenesis leads to the formation of blood vessels from pre-existing vasculature, allowing tumor growth and progression. Central to angiogenic sprouting is the imbalance between pro-angiogenic and antiangiogenic factors.14 Our aim was to explore the hypothetical role of angiogenetic axis Angiopoietins/Tie2 and VEGF in familial breast tumors, as it has been well established that BRCA1 is involved in neovascularization. Besides its role in genomic stability, BRCA1 can modulate the tumor microenvironment through the transcriptional regulation of angiogenic factors and the stability of HIF-1 alpha.7, 15 Previous studies have suggested that BRCA1 exerts a transcriptional repression on Ang-1 and VEGF promoter through CtIP/ZBRK1 complex and via ER-α,8, 9 respectively. Upregulation of VEGF in patients who carried BRCA1 mutations has already been investigated,16 but this is the first investigation reporting Ang-1, Ang-2 and their cognate Tie2 receptor expression in a series of familial breast cancers stratified by BRCA status. In agreement with previous findings in breast cancer cell lines,8, 9 the present study showed higher levels of Ang-1 and VEGF mRNA in patients harboring BRCA1/2 germline mutations. Moreover, our analysis also showed higher levels of Ang-2 in BRCA1/2-related breast cancer. Ang-2 overexpression has also been previously reported in breast tumors6 but has never been investigated in familial breast cancer. In line with other studies,17, 18, 19 we observed a positive correlation between Ang-2 and VEGF in the familial cohort, which was more significant in BRCA1/2-related cancer. The role of Ang-2 in vascular remodeling was context-dependent. Ang-2 promoted angiogenic sprouting in the presence of VEGF, but vessel regression occurred when VEGF was absent.20 Moreover, previous evidence reported that Ang-2 and VEGF expression occurred at the front of invading sprouts during angiogenesis.21 The current data indicated that although Ang-2 transcriptional regulation by BRCA1 was unknown, mutations in BRCA1/2 genes could result in a major angiogenic sprouting through the increase of Ang 1, Ang 2 and VEGF expression. We therefore supposed that VEGF and Ang 1/2 acted in conjunction to stimulate the vascular remodeling in BRCA1/2-related cancer. More recently, it has been observed that Ang-1, besides its role in the maintenance of vascular stability, can lead to angiogenic signaling in mobile endothelial cells through ECM-anchored Tie2 and in the presence of a higher amount of VEGF.4, 22
When Tie2, Ang 1/2 and VEGF expression in familial breast tumors and sporadic breast cancers were compared, the former showed higher VEGF levels compared with the latter, which is in line with other studies.16 On the contrary, breast cancer patients without a family history showed higher levels of Tie2 and Ang 1/2 mRNA, an expression never investigated before in the two groups of sporadic and familial breast cancer. Higher levels of Ang 1/2 and Tie2 mRNA without a concurrent increment in VEGF may presuppose the presence of a more quiescent vasculature in these cancer groups. We also investigated Tie2, Angiopoietins and VEGF expression in breast cancer showing the triple-negative immunophenoype. Triple-negative are more aggressive breast cancers that lack hormone receptor expression and HER2 overexpression.23 In agreement with Linderholm's study,24 our data showed higher levels of VEGF in this group. Moreover, TNBCs, such as BRCA1/2 mutation carriers, also showed higher levels of Ang-1 and Ang-2 mRNA. Interestingly, within sporadic breast cancer, TNBCs showed higher levels of Angiopoietins and VEGF mRNA compared with non-TNBCs (data not shown). The prognostic significance of Ang-1 and Ang-2 in breast cancer is still unclear. Overexpression of Ang-2 correlated with poor prognosis in several tumors25, 26, 27 including breast cancer,28 whereas other studies were discordant.6, 29 Similarly, the role of Ang-1 as a prognostic marker in tumors is uncertain.3 The present study showed a higher expression of Ang-1 in low differentiated tumors, but no correlation between Ang-2 expression and clinico-pathological assessment was found. However, a higher VEGF expression has been found in ER and PgR-negative patients. An inverse relationship between VEGF and ER has previously been described.30 Furthermore, the preliminary data on DFS confirmed the role of Ang-1/Tie2 expression in BRCA1/2 mutation carriers in terms of a trend of correlation between higher expression of the two factors and worse patient DFS.
In conclusion, this is the first study to evaluate angiogenic axis Ang-1 and -2/Tie2 in familial breast cancer and relate them to BRCA status. Our findings showed higher levels of Ang-1, Ang-2 and VEGF expression in patients with BRCA1/2 mutations. The data indicated that interrelationship between VEGF and the Ang/Tie2 axis can stimulate the sprouting angiogenesis in BRCA-related cancer. Moreover, higher levels of these angiogenic factors in TNBC were observed and it might be presumed that, besides BRCA mutations, other mechanisms related to immunophenotype could allow a more pronounced angiogenic activity. Our data need further investigation in a larger cohort, but our evidence might suggest Ang-1, Ang-2 and VEGF as attractive angiogenic targets for BRCA1/2-related cancer and triple-negative therapy.
Acknowledgments
We thank Caroline Oakley for manuscript revision. The study was partially funded by Progetto Strategico Regionale Puglia – APQ ‘The biotechnologies for the targeted therapy in oncology (Biotecnoter)' and by Progetto regionale Puglia (DIEF 2007).
The authors declare no conflict of interest.
References
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Abdullah SE, Perez-Soler R. Mechanisms of resistance to vascular endothelial growth factor blockade. Cancer. 2011;118:3455–3467. doi: 10.1002/cncr.26540. [DOI] [PubMed] [Google Scholar]
- Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol. 2010;25:387–396. doi: 10.14670/HH-25.387. [DOI] [PubMed] [Google Scholar]
- Staton CA, Hoh L, Baldwin A, et al. Angiopoietins 1 and 2 and Tie-2 receptor expression in human ductal breast disease. Histopathology. 2011;59:256–263. doi: 10.1111/j.1365-2559.2011.03920.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Inoue H, Yasuda K, et al. Angiopoietin 2 expression in invasive ductal carcinoma of the breast: its relationship to the VEGF expression and microvessel density. Breast Cancer Res Treat. 2006;98:261–266. doi: 10.1007/s10549-005-9157-9. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos A, Deng CX, Chavakis T. Crosstalk between the DNA damage response, histone modifications and neovascularisation. Int J Biochem Cell Biol. 2010;42:193–197. doi: 10.1016/j.biocel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Li H, Chun P, Avraham S, Avraham HK. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene. 2002;21:7730–7739. doi: 10.1038/sj.onc.1205971. [DOI] [PubMed] [Google Scholar]
- Furuta S, Wang JM, Wei S, et al. Removal of BRCA1/CtIP/ZBRK1 repressor complex on ANG1 promoter leads to accelerated mammary tumor growth contributed by prominent vasculature. Cancer Cell. 2006;10:13–24. doi: 10.1016/j.ccr.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Eerola H, Aittomaki K, Asko-Seljavaara S, Nevanlinna H, von Smitten K. Hereditary breast cancer and handling of patients at risk. Scand J Surg. 2002;91:280–287. doi: 10.1177/145749690209100312. [DOI] [PubMed] [Google Scholar]
- Heerma van Voss MR, van der Groep P, Bart J, van der Wall E, van Diest PJ. Lympho-vascular invasion in BRCA related breast cancer compared to sporadic controls. BMC Cancer. 2010;10:145. doi: 10.1186/1471-2407-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Lin SY. DNA damage and breast cancer. World J Clin Oncol. 2011;2:329–338. doi: 10.5306/wjco.v2.i9.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi S, Crapolicchio A, Lacalamita R, et al. BRCA1 mutations and polymorphisms in a hospital-based consecutive series of breast cancer patients from Apulia, Italy. Mutat Res. 2005;578:395–405. doi: 10.1016/j.mrfmmm.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Munoz-Chapuli R. Evolution of angiogenesis. Int J Dev Biol. 2011;55:345–351. doi: 10.1387/ijdb.103212rm. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HJ, Rih JK, et al. BRCA1 plays a role in the hypoxic response by regulating HIF-1alpha stability and by modulating vascular endothelial growth factor expression. J Biol Chem. 2006;281:13047–13056. doi: 10.1074/jbc.M513033200. [DOI] [PubMed] [Google Scholar]
- Yan M, Rayoo M, Takano EA, Fox SB. BRCA1 tumours correlate with a HIF-1alpha phenotype and have a poor prognosis through modulation of hydroxylase enzyme profile expression. Br J Cancer. 2009;101:1168–1174. doi: 10.1038/sj.bjc.6605287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang N, Park JW, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003;63:3403–3412. [PubMed] [Google Scholar]
- Moon WS, Rhyu KH, Kang MJ, et al. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma. Mod Pathol. 2003;16:552–557. doi: 10.1097/01.MP.0000071841.17900.69. [DOI] [PubMed] [Google Scholar]
- Sun XD, Liu XE, Wu JM, Cai XJ, Mou YP, Li JD. Expression and significance of angiopoietin-2 in gastric cancer. World J Gastroenterol. 2004;10:1382–1385. doi: 10.3748/wjg.v10.i9.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metheny-Barlow LJ, Li LY. The enigmatic role of angiopoietin-1 in tumor angiogenesis. Cell Res. 2003;13:309–317. doi: 10.1038/sj.cr.7290176. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2011;16 (Suppl 1:61–70. doi: 10.1634/theoncologist.2011-S1-61. [DOI] [PubMed] [Google Scholar]
- Linderholm BK, Hellborg H, Johansson U, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20:1639–1646. doi: 10.1093/annonc/mdp062. [DOI] [PubMed] [Google Scholar]
- Anargyrou K, Terpos E, Vassilakopoulos TP, et al. Normalization of the serum angiopoietin-1 to angiopoietin-2 ratio reflects response in refractory/resistant multiple myeloma patients treated with bortezomib. Haematologica. 2008;93:451–454. doi: 10.3324/haematol.11852. [DOI] [PubMed] [Google Scholar]
- Lind AJ, Wikstrom P, Granfors T, Egevad L, Stattin P, Bergh A. Angiopoietin 2 expression is related to histological grade, vascular density, metastases, and outcome in prostate cancer. Prostate. 2005;62:394–399. doi: 10.1002/pros.20163. [DOI] [PubMed] [Google Scholar]
- Maffei R, Martinelli S, Santachiara R, et al. Angiopoietin-2 plasma dosage predicts time to first treatment and overall survival in chronic lymphocytic leukemia. Blood. 2010;116:584–592. doi: 10.1182/blood-2009-11-252494. [DOI] [PubMed] [Google Scholar]
- Sfiligoi C, de Luca A, Cascone I, et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003;103:466–474. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- Rmali KA, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Angiopoietins lack of prognostic significance in ductal mammary carcinoma. Int Semin Surg Oncol. 2007;4:6. doi: 10.1186/1477-7800-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SH, O'Donnell AL, Balu D, et al. Estrogen receptor-alpha in the inhibition of cancer growth and angiogenesis. Cancer Res. 2000;60:7094–7098. [PubMed] [Google Scholar]