Abstract
Aims:
The purpose of the study is to determine whether the nickel released from the stainless steel brackets have any cytotoxic effects on gingival fibroblast.
Materials and Methods:
Brackets are an important component of fixed orthodontics. Orthodontists are mainly concerned about the brands and various systems incorporated into the brackets. The manufactures claim bracket superiority without valid research. Since we are dealing with a biologic system factors like biocompatibility, cytotoxic potential should be taken into consideration before selecting an appliance to the patient. The cytotoxic activity of the media was investigated with MTT and comet assay.
Results:
The results of the study show that the amount of nickel leached is capable of bringing damage to the fibroblast.
Conclusion:
Our study concludes that nickel solution at minimal concentration of 1.18 μg could damage human gingival fibroblast and the nickel released from the different brands of the brackets are not uniform.
Keywords: Comet assay, cytotoxicity, inductively coupled plasma atomic emission spectroscopy, nickel
A standard orthodontic appliance consist of bands, brackets and both stainless steel and nickel titanium arch wires, which can remain in mouth for 2 years or more. The most common alloy constituents are cobalt chromium and nickel followed by other metals in different amounts. Corrosion events are very frequent in the oral cavity. The alloys used in dentistry are exposed to several aggressive physical and chemical events such as higher concentration of oxygen and chloride mixture in saliva, tartar and plaque and acid deposit from microbiologic metabolism.
Several in vitro and in vivo methods have been used to study the release of metals and their contents in biologic fluids, including saliva and blood. The main conclusion of these studies were that measurable amount of metals released from the orthodontic appliances in saliva or blood samples were significantly below the average dietary intake and did not reach the toxic concentrations.[1,2]
Although the orthodontic appliancae had no effect on the general levels of the metals, it cannot be excluded that even nontoxic concentration might be sufficient to induce important biologic effects in cells of oral mucosa. Experimental and epidemiological studies suggest that exposure to nickel components is associated with lung and nasal cancer. Even if a genotoxic potential has been demonstrated in certain systems the mechanism underlying this feature is largely unknown. But several possible pathways seems to be involved such as the interaction of metal with deoxyribonucleic acid (DNA), the generation of oxidative DNA damage or interference with DNA repair and replication process.
There have been many studies conducted to assess the nickel release and no standardization has been given yet regarding the amount of nickel that can be leached since the carcinogenic effect of the nickel[3] has been proved. The cause and effect of this in humans have never been demonstrated except that few studies show DNA damage in mucosa cells.[3,4]
Materials and Methods
Two full sets of each manufacturer′s treatment brackets, consisting of 20 brackets in each set manufactured by 3M Gemini Metal Brackets, American Orthodontics, Morelli Orthodonti Brazil, with a 0.022 inch (0.56 mm) with standard edgewise slot, were selected for the study.
The six materials group (each 20 brackets) were immersed in sterile conical flask containing 50 ml of 0.9% w/v normal saline solution and maintained at 37°C for 1 month. The saline solution samples were processed for inductively coupled plasma atomic emission spectroscopy (ICP-AES) with an ICP-AES unit (OPTIMA 4300V, perk Elmer, Norwalk, Conn). The study was conducted at trace analysis lab, cas/asd, Vikram Sarabhai Space Center; Thiruvananthapuram. The amount of nickel released from each group is calculated.
Human gingival fibroblasts (HGF) were obtained from the biopsy of the attached gingiva of sound premolars-molar and permanent molar teeth of healthy persons. Consent based on an appropriate protocol was obtained from the donors. The biopsies were stored at 4°C, for almost 24 h in Hanks salt solution supplemented with penicillin (100 u/ml), amphotericin (2.5 mg/ml), and 10% heat inactivated fetal calf serum (FCS) (PAN systems, Aidenbach, Germany). The gingival tissues were cut into 1-2 mm3 pieces, and then washed twice with Hanks salt solution. Thereafter, the cut biopsies were placed into 25 cm2 tissue culture flasks The explants were incubated with culture medium consisting of Dulbecco′s modified eagles medium, 10 mm HEPES, Glucose (4.5 g/l), NaHCO3 (3.7 g/l), Penicillin (100 u/ml), streptomycin (100 mg/ml), and amphotericin (2.5 mg/ml) all (Biochrom KG), supplemented with 10% heat inactivated FCS (PAN system, Aidenbach, Germany). Cells were detached from the monolayer by a brief treatment with trypsin - Ethylenediaminetetraacetic acid (EDTA) (0.25% trypsin, 0.02% EDTA) (Sigma, Deisenhofen, Germany) and when recultured in 75 cm2 tissue flasks unit, confluent monolayer were re-obtained. Cell counts before plating revealed 95% to 98% cell viability by the trypan blue exclusion test. Cells between the 3rd and 9th passage were used for the experiments described below.
The cytotoxicity was estimated by a modification of MTT assay. For the cytotoxicity test, the test specimens were placed in the centre of 24 well tissue culture trays. After the 24 h and 72 h incubation period, verification of the cell proliferation by trypan blue dye exclusion assay (Edmondson et al. 1988[5]) was done and then the test specimens were removed from the culture wells and the cytotoxicity of the materials was assessed using MTT (3-4, 5 dimethylthiazol) 2-5-diphenyl-S H-Tetrazolium bromide) method, after MTT (5 mg/ml in Hank’s balanced salt solution) was added to each well and the micro plates were further incubated at 37°C for 4 h. After the incubation period, 100 ml of acidified isopropanol (0.04N Hcl in isopropanol) was added to the cultures and mixed thoroughly to dissolve dark blue crystals of formazan. The solubilized reaction products were transferred to a 96 well plate and the absorbance values of each well were determined with a microplate ELISA READER-Biotech USA equipped with a 570 nm filter. Survival rates of the controls were set to represent 100% viability. Untreated cultures were used as control groups.
Comet assay is a method to measure DNA single strand breaks that caused relaxation of DNA super coils. Treating the cells with hydrogen peroxide produces a 1000 fold or more excess of single strand verses double strand breaks, even at millimolar concentration under neutral condition. The comet assay provides the information, as the number of DNA breaks increase, supercoiled loops relax, more free ends are able to migrate, and therefore a larger fraction of the DNA moves away from the comet head. The information can be obtained by measuring the distribution of DNA damage within the comet tail and by accurate fragment size measurement done by pulsed field gel electrophoresis. Comparing comet assay result with other measures of DNA damage is necessary to interpret the biological relevance of the damage.
10 μl of cell suspension (about 104 cells) cells were mixed with 75 μl of low melting point agarose (1%) and added to a slide precoated with100 μl agarose. Lysis was done overnight at 4° in the dark at ph 10. After overnight lysis the slides were submerged into a rinse solution for 20 min. After this it was taken into the electrophoresis chamber for 25 min at 0.6 v/cm.The slide were removed from the chamber neutralized, dried with 100% ethanol and stained with ethidium bromide. Using image analysis, software analyzes individual comet images for several features including total density, tail length, %DNA in tail.
Among the samples the 3M group showed more amount of nickel release and the American Orthodontics Brackets showed the least amount of nickel leached out.
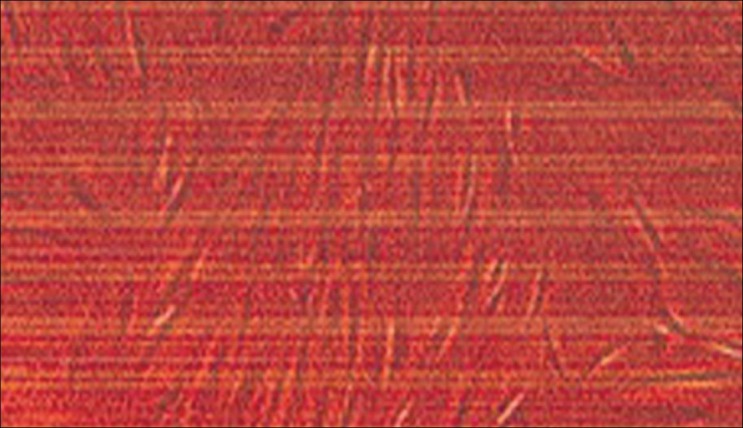
The results of the cell proliferation for the test materials are given in Table 1.
Table 1.
Results of cell proliferation
Cell growth of the fibroblast cell cultures was monitored by MTT assay after 24 h and 72 h exposure period [Figure 1]. As shown in Table 1 and Chart 1 there was a statistically significant difference between the respective control and test samples after 72 h exposure period. Group comparison revealed that significantly low number of cells was reported at 1.18 μg concentration in comparison with other specimens. None of the test samples influenced cell proliferation after the 24 h exposure period. DNA damage was evaluated by comet assay using Epifluorescent microscope fitted with CCD camera and pixel card fitted in personal computer. As anticipated no DNA damage has occurred in the control specimen [Figure 2]. No significant DNA damage was observed in all the test samples observed after 24 h exposure period. Mild DNA damage was observed only in the 1.18 μg exposed human gingival fibroblast samples, observed after 72 h [Figure 3 and Figure 4]. Findings of this study indicate that nickel release from different company brackets are not uniform, it varies. 3M brackets show more amount of nickel release. American Orthodontics brackets show least amount of nickel release. Cytotoxicity was shown when the cells were exposed to 1.18 μg of nickel alloy solution for 72 h. Mild DNA damage occurs on exposure to 1.18 μg nickel alloy solution for 72 h.
Figure 1.
Exposure on human gingival fibroblasts 24 hours
Chart 1.
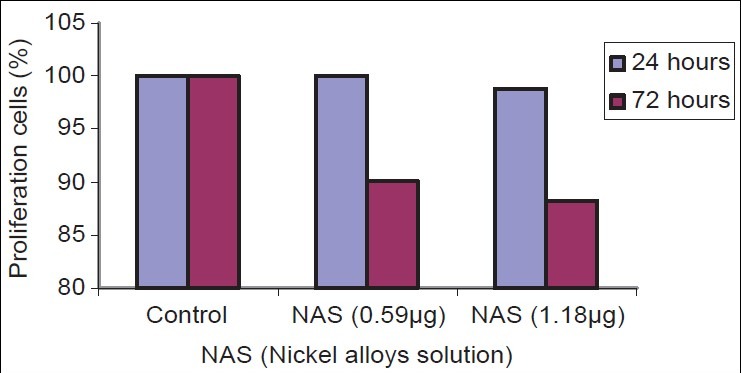
Results of cell proliferation
Figure 2.
Control showing no deoxyribonucleic acid damage
Figure 3.

Exposure on human gingival fibroblasts 72 h
Figure 4.

Mild deoxyribonucleic acid damage after 72 h
Discussion
Although the orthodontic appliance had no effect on the general levels of the metals, it cannot be excluded that even nontoxic concentration might be sufficient to induce important biologic effects in cells of oral mucosa. Experimental and epidemiological studies suggest that exposure to nickel components is associated with lung and nasal cancer. Even if a genotoxic potential has been demonstrated in certain systems the mechanism underlying this feature is largely unknown. But several possible pathways seems to be involved such as the interaction of metal with DNA, the generation of oxidative DNA damage or interference with DNA repair and replication process. Since we are dealing with a biologic system, factors like biocompatibility, cytotoxic potential etc., should be taken into consideration. This study shows that there is significant amount of nickel released from all the groups of samples. There have been many studies conducted to assess the nickel release and no standardization has been given yet regarding the amount of nickel that can be leached since the carcinogenic effect of the nickel[2] has been proved.
The amount of nickel and chromium released from an orthodontic appliance are below the average dietary intake of nickel and chromium consumed. But this amount is sufficient to elicit an allergic reaction in those who are sensitive to nickel. Although the level of nickel is minimum in concentration, it is sufficient to induce important biologic changes in cells of oral mucosa.
Regarding the cytotoxic effect of the solution on the gingival fibroblast there was a statistically significant difference between the respective control and test samples after 72 h exposure period. Group comparison revealed that a significantly low number of cells were reported at 1.18 μg concentration in comparison with other specimens. This was contradicting the study by Theodore Eliades[6].
As far as the DNA damage is considered Mild DNA damage was observed only in the 1.18 μg exposed human gingival fibroblast samples after 72 h of treatment suggesting that this much concentration is capable of bringing out the damage, because the appliance is going to be in the mouth for at least 18 months. This study correlates with results of the study conducted by Fiorenzo Faccioni.[3] These data suggests that nickel ions released from the orthodontic appliances can induce evident cytotoxic effects.
Conclusion
Since we are dealing with a biologic system factors like biocompatibility, cytotoxic potential should be taken into consideration before selecting the appliance for the patient. It is not always good to go behind the brands because manufacturer′s claim bracket superiority without valid research. There is no standardization regarding the amount of nickel that can be leached since the carcinogenic effect of the nickel[2] has been proved. The results of this study show the difference in the amount of nickel leached out from different company brackets.
This study shows the potential toxicological effects of the nickel and shows that the released ions can produce DNA breakages in gingival fibroblast. In general normal cells can repair these lesions, but loss of repair capacity due to reduction in damage detection or to an enzymatic deficiency in repair process might be an initiating event for adverse biologic effect. Because of the possible adverse biologic effect, scientific research should be directed towards dental materials that not only require mechanical resistance to wear and tear but safe for patients also.
To conclude, the research study has corroborated that nickel solution at minimal concentration of 1.18 μg could damage human gingival fibroblast and the nickel released from the different brands of the brackets are not uniform. Since the carcinogenic effect of nickel is established it is high time to think about other alloys[7] without involving the heavy metal nickel which can be used or to find standardization for the nickel leach out from the appliance.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Hwang CJ, Shin JS, Cha JY. Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2001;120:383–91. doi: 10.1067/mod.2001.117911. [DOI] [PubMed] [Google Scholar]
- 2.Lee YW, Klein CB, Kargacin B, Salnikow K, Kitahara J, Dowjat K, et al. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: A new model for epigenetic carcinogens. Mol Cell Biol. 1995;15:2547–57. doi: 10.1128/mcb.15.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop. 2003;124:687–93. doi: 10.1016/j.ajodo.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Bezzon OL. Allergic sensitivity to several base metals: A clinical report. J Prosthet Dent. 1993;69:243–4. doi: 10.1016/0022-3913(93)90098-9. [DOI] [PubMed] [Google Scholar]
- 5.Edmondson JM, Armstrong LS, Martinez AO. A rapid and simple MTT based Spectrometric assay for determining drug sensitivity in monolayer cultures. J Tissue Cult Methods; 1988;11:15–7. [Google Scholar]
- 6.Eliades T, Eliades G, Athanasiou AE, Bradley TG. Surface characterization of retrieved NiTi orthodontic archwires. Eur J Orthod. 2000;22:317–26. doi: 10.1093/ejo/22.3.317. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Johnson JW. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999;69:39–44. doi: 10.1043/0003-3219(1999)069<0039:COSSNT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]


