Abstract
Based on their mode of action and preclinical data, one would expect bisphosphonates to improve the healing of fractures in cancellous bone, and bone morphogenetic proteins (BMPs) to reduce the risk of non-union in severe shaft fractures. Parathyreoid hormone (PTH) can be expected to accelerate fracture healing in general. The clinical data in support of this is meager. Stimulation of cancellous bone healing and strength by bisphosphonates has been inadvertently shown in the context of implant fixation, but not convincingly in fractures per se. The clinical BMP literature is confusing, and the chance of ever demonstrating reduced numbers of non-union are small, due to power issues. Still, acceleration of ‘normal' healing may be possible, but largely remains to show. For PTH, the two available clinical trials both show accelerated healing, but none of them is flawless, and there is a need for better studies.
Introduction
This paper intends to discuss the sparse evidence on pharmacological acceleration of human fracture repair that is available, against the background of some of the preclinical literature. I have not had the intention to make a meta-analysis, as the randomized clinical trials in this area are few and different. Moreover, their quality is not always good.
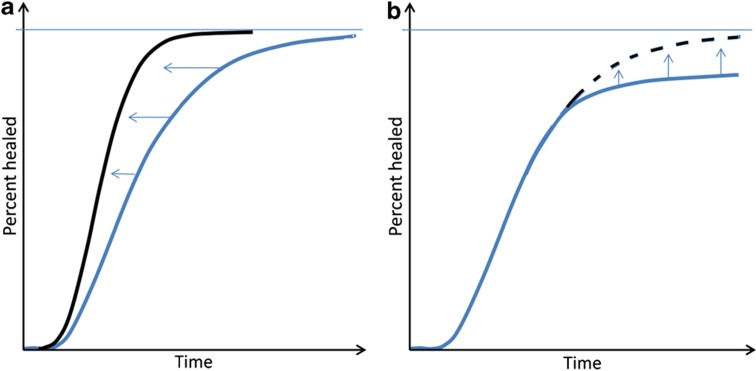
Almost all papers discussing this topic mention in the first paragraph that there is a 5–10% rate of healing complications in fractures (delayed union or worse). This precentage might not describe the biology of fracture healing as much as the impatience of orthopedic surgeons, who decide when it is time to intervene. If we imagine a certain ‘standard fracture', and presume that we have a good definition for healing of that fracture, the healing time should of course have some kind of random variation. The healing time would depend on the sum of several normally distributed factors, which together would yield a log-normal distribution of the healing time (Figure 1). Therefore, a curve describing the incidence density of different healing times would have a long right tail, which implies that ‘delayed union' in many cases lies within the normal biological variation. In other words: by mere chance, we should expect some normal patients to have a considerably longer healing time than average. The subject of this paper is whether there are methods to reliably shift this entire curve to the left, by reducing the expected healing time for all, or at least the majority, of the patients.
Figure 1. Time to healing for a large number of hypothetical ‘standard' fractures.
A log-normal distribution, or a mixture of log-normal distributions, can be expected, here shown as cumulative log-normal plots. (a) A homogenous fracture population (blue). Note that half of the patients have healed rather quickly, but the curve approaches 100% slowly. A too short time limit for definition of delayed union will, therefore, label normal cases as pathological. If bone formation could be accelerated in all patients, perhaps with PTH, the curve would shift to the left (black). (b) A mixture of two populations (blue): a number of non-healers have been added to the population in a. If the deficient initiation of healing could be prevented, perhaps with a peroperative BMP, the right part of the curve would shift upwards (black).
Prevention of atrophic non-union, on the other hand, may be a different thing than accelerating the healing process. If we disregard infections and other more or less iatrogenic complications, atrophic non-union may be the end result of early events, leading to an inability to get the healing process started.1 This would explain the higher risk of non-union after open fractures,2 and the poorer healing with increased soft tissue trauma.3 These early events may soon become better understood than they are today, as the inflammatory and immunologic components of the early healing process are now subject to increasing research activity.4,5 Although it is surely an oversimplification to regard slow healing and atrophic non-union as two entirely separate entities, this dichotomy may help to sort things out. The subject of this discussion is mainly acceleration or repair, that is, accelerating ‘normal' repair.
Based on their histological appearance, fractures in shafts and metaphyses can be expected to heal by different mechanisms and respond differently to drugs. There is a myriad of papers on the biology of shaft fracture healing, but only a very sparse literature on the biology of cancellous bone fractures.6 This might be due to the paucity of animal models for cancellous bone healing, especially for mechanical evaluation.
The healing process in a shaft fracture faces the problem of recruiting cells to the area, either from the thin periosteum, surrounding muscle, endothelium or blood. In contrast, the broken trabeculae in cancellous bone are surrounded by marrow, with readily available stromal cells that can differentiate to osteoblasts. Later in the process, shaft fractures show more or less endochondral ossification. Therefore, a scarcity of cartilage, formed early on, might be a limiting factor. In contrast, it is thought that cancellous fractures appear to heal almost entirely by metaplastic bone formation. Indeed, own unpublished clinical biopsies show fetal-type woven bone, forming primarily within the marrow space, with no contact with bone surfaces. These observations suggest that drugs that can stimulate cell recruitment and cartilage formation may be beneficial mainly in shaft fractures, whereas drugs that increase osteoblast number and activity might accelerate both, and perhaps cancellous fractures to a larger extent.
Clinical studies of drugs for acceleration of fracture healing are hampered by the lack of good definitions.7 Many fracture types have a large number of subtypes, and their classification often has a limited reproducibility. Therefore, inaccessibly large numbers of study patients may be needed to overcome variation in fracture geometry.
It is a general orthopedic experience that clinical healing occurs before a bridging callus can be convincingly shown on X-rays. Decades ago, when many shaft fractures were treated non-operatively, it could be shown in tibial shaft fractures that measurements of the mechanical stiffness were superior to radiographs in their ability to predict when the patient could bear weight without pain or complications.8,9 With internal fixation, this is now difficult. It is not certain that improved radiologic modalities will solve the problem, and measurement of patient satisfaction is a blunt instrument.
In contrast to the difficulties in measuring fracture healing in patients in mechanical terms, it is possible to quantitate aspects of implant fixation in humans.10,11 Especially in cancellous bone, the processes following the insertion of an implant are likely to be closely related, or identical, to the processes after fracture. Therefore, I believe that measurements of implant fixation can yield information of drug effects on cancellous fracture healing in general.
Bisphosphonates
Bisphosphonates reduce the remodeling of the bony callus, but according to both animal and clinical data, this has no detrimental effects on healing in biomechanical or clinical terms.12,13,14 Theoretically, bisphosphonates could be expected to impair the intracortical bridging of perfectly reduced cortical fractures. It was suggested several decades ago that very narrow fracture gaps need to be widened by osteoclastic resorption before they can be filled with new bone.15 This is also the case around perfectly fitted implants in bone.16 Fatigue fractures, such as the so called atypical fractures of the femoral shaft, form very thin fracture gaps before they dislocate.17 The atypical fractures occur almost exclusively in patients using bisphosphonates.18 Bisphosphonates adhere to bone and may stay for decades in the skeleton.19 Still, the bisphosphonate-associated risk diminishes rapidly after cessation of bisphosphonate use.18 This suggests that the bisphosphonate causing the crack is derived from ongoing treatment. A possible explanation would depend on the fact that thin cracks need to be widened before they can be replaced with new bone (targeted remodeling).20 Bisphosphonates from the circulation might accumulate on the exposed crack surfaces, blocking the resorption necessary for targeted remodeling. This interpretation is further supported by histological observations of resorption around, but not in, the crack.17 This principle could apply to perfectly reduced and fixed shaft fractures in general.
Conversely, fractures in cancellous bone might heal faster with bisphosphonates. Trauma to cancellous bone in rats appears to cause transient metaplastic bone formation in the marrow, which is then quickly and completely resorbed. If this resorption is reduced by a bisphosphonate, there will remain some bone that can act as a scaffold for further bone formation, yielding a stronger construct.21 My group has studied this phenomenon in the context of implant fixation.22,23,24 Placement of an implant in cancellous bone constitutes a considerable trauma and will elicit a repair response, likely to be similar to cancellous fracture healing. Therefore, data on the benefit of bisphosphonates for implant fixation might be relevant also for metaphyseal fractures. Two double-blinded clinical trials show improved early fixation of knee joint replacements,25,26 and one similar trial shows improved fixation of hip replacements, with also better clinical outcome (Harris hip score).27 Recently, improved fixation of dental implants was shown, with the use of a bisphosphonate coating directly on the metal implant surface.28 A randomized, placebo-controlled trial of clodronate in distal radial fractures showed a significantly increased radiodensity of the fracture region in the bisphosphonate group at 2 months.29 Taken together, these studies suggest a beneficial effect that has remained unexplored.
Bone Morphogenetic Proteins
Bone morphogenetic proteins (BMPs) are thought to mainly initiate fracture healing. BMP treatment would then primarily be useful to reduce the risk of non-union. Indeed, non-union in human patients seems to be related to genetic changes in the BMP signaling system.30 However, BMP treatment for faster ‘normal' healing has been suggested as well.
The recent scandal with misleading reports of randomized trials of BMP2 versus cancellous bone grafting in spine surgery unfortunately has cast doubt on clinical use of BMPs in general.31,32 In several papers, adverse events were seriously under-reported, and the comparator—a cancellous bone grafting procedure—was suboptimal, thus favoring the BMP2 group. The unwanted side effects include local inflammation with serious swelling, as well as resorption of cancellous bone. The latter is a well-known effect of BMP implants since more than a decade.33 Recently, three trials with BMP2 on a new, calcium-phosphate based carrier in long bone shaft fractures have been prematurely terminated after review of interim data ( http://www.clinicaltrials.gov).
There are two published large multi-center trials of BMP2 for tibial shaft fractures. The first one, published already in 2002, recruited 450 open fractures, randomly allocated to three treatment groups: 12 or 6 mg of BMP2 on a collagen sponge carrier, or untreated control.34 As the controls received no carrier, the physician in charge was not blinded. The study showed that 12 mg of BMP2 reduced the proportion of the patients needing a secondary intervention (the predetermined primary effect variable) from 46 to 26 percent. There were also fewer infections. Variables unsensitive to bias, such as radiographic healing time (blinded review) and the number of failing fixation devices also showed an advantage for the 12 mg BMP2 group. This study has been criticized because the subgroup receiving reamed marrow nailing, which is believed to be optimal, showed no effect of BMP2. Therefore, a new similar trial was started, where 277 patients who received a reamed marrow nail were randomized to 12 mg BMP2 as above, or untreated control.35 This trial had to be terminated prematurely because of an alarming number of wound infections in the BMP2 group. The infections may be associated with the local inflammation at the treatment sites, which is a known side effect. However, the infections must not necessarily be caused by the BMP2 at all: the need for a wider dissection in this group in order to make space for the BMP2 carrier may be a sufficient explanation. However, the healing time, based on clinical and radiographic criteria, showed only a slight advantage for BMP2, which was not (but almost) statistically significant.
To conclude, BMP2 had beneficial effects in tibial fractures with a high risk of healing complications and non-unions. With more favorable conditions and optimal surgery, the negative effects seem to dominate. Clearly, more studies are warranted. It must be noted that, except for the criticized spine studies, no study so far has shown that BMPs are better than cancellous bone grafts.
Parathyreoid Hormone
Parathyreoid hormone (PTH) increases bone remodeling with a net-positive effect on bone mass. Osteoblast number and activity are increased, primarily at remodeling sites.36 Animal experiments have shown a striking effect of PTH on cortical and cancellous bone healing or regeneration in rats.37,38,39 This response is more dramatic than the response in untraumatized bone.40 Therefore, it has been speculated that PTH should be able to accelerate fracture healing in humans, in spite of the fact that its effects on bone mass in the treatment of osteoporosis appear slowly. However, the large doses of PTH used in animal experiments have cast doubt on their clinical relevance.
There are only two randomized trials on PTH and fracture healing. One of them randomized patients with non-operatively treated distal radial fractures to 40 or 20 μg of teriparatide daily, or placebo.41 The time to restoration of cortical radiographic continuity (the primary variable) was reduced from 9 to 7 weeks with 20 μg compared with placebo, but there was no significant effect of the higher dose, possibly due to increased remodeling that made new-formed cortex less visible on radiographs.42,43 While the researchers were still blinded for treatment allocation, the patients from the first author's treatment facility were scored for the radiographic appearance 5 weeks after fracture (n=27). This analysis showed a clear correlation with dose of teriparatide, so that 9 of 10 patients with the highest dose showed a visible bony callus, and only 1 of the 8 placebo patients did.44 These results suggest that PTH has a strong effect on early callus formation. Future radiographic studies should probably concentrate on effects on early healing, rather than using the time to cortical continuity. This study was not designed to show clinical benefit.
The other clinical trial on PTH comprised 65 osteoporotic women with fractures of the pelvic rami.45 Twenty-one patients were allocated to injections with PTH 1-84, and 44 served as untreated controls and received no injections. Computerized tomography exams of the pelvis were made every 4 weeks until radiographic healing. The results were striking: All of the PTH-treated patients had healed at 8 weeks, but only 4 of the controls. Moreover, on a visual analogue scale for pain and in a ‘timed up and go' test, there were also striking and statistically significant differences, which are unlikely to be due entirely to placebo effects. The study is described as a prospective randomized, controlled trial (level 2). However, there is no mention of ethical approval, the study was not reported in a clinical trials registry, and the authors mention as a weakness that ‘the information was abstracted after the study was finished'. Moreover, the treatment allocation process and blinding procedure were not clearly described. I, therefore, contacted the authors, who told me that every second patient coming to one hospital received PTH, and for each control patient at that hospital, another control patient was recruited at another hospital, where no PTH treatment was given. Because of this lack of formal randomization, the study would probably be better described as a case–control study. The treating physicians made the radiographic review themselves, although some kind of blinding is mentioned. In spite of these shortcomings, the results are dramatic, and it is hard to believe that the differences between the groups are entirely due to bias. I believe that some of the unclarities in the article could have been avoided in the editing process.
These two trials give good hope that PTH will emerge as a powerful tool for acceleration of fracture healing, but the perfect study remains to be done. The study on pelvic fractures has the advantage of using a functional variable (timed up-and-go) and a simple and straight forward design. If the results could be reproduced with proper blinding and randomization, that would be good news for future fracture patients.
Footnotes
The author has received research grants or reimbursements from Eli Lilly and Amgen, and owns stock in AddBIO AB, a company working with drug coatings on bone implants.
References
- Henle P, Zimmermann G, Weiss S. Matrix metalloproteinases and failed fracture healing. Bone 2005;37:791–798. [DOI] [PubMed] [Google Scholar]
- Bhandari M, Tornetta P III, Sprague S, Najibi S, Petrisor B, Griffith L et al. Predictors of reoperation following operative management of fractures of the tibial shaft. J Orthop Trauma 2003;17:353–361. [DOI] [PubMed] [Google Scholar]
- Claes L, Ignatius A, Lechner R, Gebhard F, Kraus M, Baumgartel S et al. The effect of both a thoracic trauma and a soft-tissue trauma on fracture healing in a rat model. Acta Orthop 2011;82:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 2012;8:133–143. [DOI] [PubMed] [Google Scholar]
- Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 2011;26:1517–1532. [DOI] [PubMed] [Google Scholar]
- Claes L, Reusch M, Gockelmann M, Ohnmacht M, Wehner T, Amling M et al. Metaphyseal fracture healing follows similar biomechanical rules as diaphyseal healing. J Orthop Res 2011;29:425–432. [DOI] [PubMed] [Google Scholar]
- Goldhahn J, Scheele WH, Mitlak BH, Abadie E, Aspenberg P, Augat P et al. Clinical evaluation of medicinal products for acceleration of fracture healing in patients with osteoporosis. Bone 2008;43:343–347. [DOI] [PubMed] [Google Scholar]
- Hammer RR, Hammerby S, Lindholm B. Accuracy of radiologic assessment of tibial shaft fracture union in humans. Clin Orthop Relat Res 1985;(199):233–238. [PubMed] [Google Scholar]
- Hammer RR. Strength of union in human tibial shaft fracture. A prospective study of 104 cases. Clin Orthop Relat Res 1985;(199):226–232. [PubMed] [Google Scholar]
- Selvik G. Roentgen stereophotogrammetry. A method for the study of the kinematics of the skeletal system. Acta Orthop Scand Suppl 1989;232:1–51. [PubMed] [Google Scholar]
- Pijls BG, Nieuwenhuijse MJ, Schoones JW, Middeldorp S, Valstar ER, Nelissen RG. RSA prediction of high failure rate for the uncoated interax TKA confirmed by meta-analysis. Acta Orthop 2012;83:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MM, Dulai S, Godfrey C, Amanat N, Sztynda T, Little DG. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone 2008;43:653–662. [DOI] [PubMed] [Google Scholar]
- Kim TY, Ha YC, Kang BJ, Lee YK, Koo KH. Does early administration of bisphosphonate affect fracture healing in patients with intertrochanteric fractures? J Bone Joint Surg Br 2012;94:956–960. [DOI] [PubMed] [Google Scholar]
- Colon-Emeric C, Nordsletten L, Olson S, Major N, Boonen S, Haentjens P et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporo Int 2011;22:2329–2336. [DOI] [PubMed] [Google Scholar]
- Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br 2002;84:1093–1110. [DOI] [PubMed] [Google Scholar]
- Dhert WJ, Thomsen P, Blomgren AK, Esposito M, Ericson LE, Verbout AJ. Integration of press-fit implants in cortical bone: a study on interface kinetics. J Biomed Mater Res 1998;41:574–583. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Schilcher J, Fahlgren A. Histology of an undisplaced femoral fatigue fracture in association with bisphosphonate treatment. Frozen bone with remodelling at the crack. Acta Orthop 2010;81:460–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011;364:1728–1737. [DOI] [PubMed] [Google Scholar]
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008;19:733–759. [DOI] [PubMed] [Google Scholar]
- Burr DB. Why bones bend but don't break. J Musculoskelet Neuronal Interact 2011;11:270–285. [PubMed] [Google Scholar]
- Wermelin K, Suska F, Tengvall P, Thomsen P, Aspenberg P. Stainless steel screws coated with bisphosphonates gave stronger fixation and more surrounding bone. Histomorphometry in rats. Bone 2008;42:365–371. [DOI] [PubMed] [Google Scholar]
- Tengvall P, Skoglund B, Askendal A, Aspenberg P. Surface immobilized bisphosphonate improves stainless-steel screw fixation in rats. Biomaterials 2004;25:2133–2138. [DOI] [PubMed] [Google Scholar]
- Wermelin K, Tengvall P, Aspenberg P. Surface-bound bisphosphonates enhance screw fixation in rats—increasing effect up to 8 weeks after insertion. Acta Orthop 2007;78:385–392. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Wermelin K, Tengwall P, Fahlgren A. Additive effects of PTH and bisphosphonates on the bone healing response to metaphyseal implants in rats. Acta Orthop 2008;79:111–115. [DOI] [PubMed] [Google Scholar]
- Hilding M, Aspenberg P. Local peroperative treatment with a bisphosphonate improves the fixation of total knee prostheses: a randomized, double-blind radiostereometric study of 50 patients. Acta Orthop 2007;78:795–799. [DOI] [PubMed] [Google Scholar]
- Hilding M, Aspenberg P. Postoperative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop 2006;77:912–916. [DOI] [PubMed] [Google Scholar]
- Friedl G, Radl R, Stihsen C, Rehak P, Aigner R, Windhager R. The effect of a single infusion of zoledronic acid on early implant migration in total hip arthroplasty. A randomized, double-blind, controlled trial. J Bone Joint Surg Am 2009;91:274–281. [DOI] [PubMed] [Google Scholar]
- Abtahi J, Tengvall P, Aspenberg P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 2012;50:1148–1151. [DOI] [PubMed] [Google Scholar]
- Adolphson P, Abbaszadegan H, Boden H, Salemyr M, Henriques T. Clodronate increases mineralization of callus after Colles' fracture: a randomized, double-blind, placebo-controlled, prospective trial in 32 patients. Acta Orthop Scand 2000;71:195–200. [DOI] [PubMed] [Google Scholar]
- Dimitriou R, Carr IM, West RM, Markham AF, Giannoudis PV. Genetic predisposition to fracture non-union: a case control study of a preliminary single nucleotide polymorphisms analysis of the BMP pathway. BMC Musculoskelet Disord 2011;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471–491. [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Ghanayem AJ, Weiner BK, Rothman DJ, Bono CM. A challenge to integrity in spine publications: years of living dangerously with the promotion of bone growth factors. Spine J 2011;11:463–468. [DOI] [PubMed] [Google Scholar]
- Laursen M, Hoy K, Hansen ES, Gelineck J, Christensen FB, Bunger CE. Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur Spine J 1999;8:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 2002;84-A:2123–2134. [DOI] [PubMed] [Google Scholar]
- Aro HT, Govender S, Patel AD, Hernigou P, Perera de Gregorio A, Popescu GI et al. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J Bone Joint Surg Am 2011;93:801–808. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 2007;40:1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 1999;14:960–968. [DOI] [PubMed] [Google Scholar]
- Holzer G, Majeska RJ, Lundy MW, Hartke JR, Einhorn TA. Parathyroid hormone enhances fracture healing. A preliminary report. Clin Orthop Relat Res 1999;(366):258–263. [DOI] [PubMed] [Google Scholar]
- Skripitz R, Andreassen TT, Aspenberg P. Parathyroid hormone (1-34) increases the density of rat cancellous bone in a bone chamber. A dose-response study. J Bone Joint Surg Br 2000;82:138–141. [DOI] [PubMed] [Google Scholar]
- Skripitz R, Andreassen TT, Aspenberg P. Strong effect of PTH (1-34) on regenerating bone: a time sequence study in rats. Acta Orthop Scand 2000;71:619–624. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 2010;25:404–414. [DOI] [PubMed] [Google Scholar]
- Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int 2011;22:357–362. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–1441. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop 2010;81:234–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am 2011;93:1583–1587. [DOI] [PubMed] [Google Scholar]