Abstract
Bisphosphonates are approved for treating malignant bone disease from advanced cancer because they are effective inhibitors of osteoclast-mediated bone resorption. However, there may be a greater role for the use of bisphosphonates than has previously been considered. There is a large body of preclinical evidence showing that bisphosphonates exert a variety of direct and indirect anticancer activities that affect both tumor cells and the surrounding microenvironment, and that stimulate immune reactions. Recent data from clinical trials have shown that adding the bisphosphonate zoledronate to endocrine therapy or chemotherapy improves disease-free survival of patients with endocrine-responsive early breast cancer in a low estrogen environment (that is, following ovarian suppression therapy or in women with established menopause at diagnosis). Adjuvant treatment with the bisphosphonate clodronate also improves disease-free survival in postmenopausal breast cancer. Additionally, zoledronate was found to prolong survival in patients with symptomatic multiple myeloma or other advanced cancers. Here, we present an overview of preclinical and clinical studies that demonstrate anticancer benefits of bisphosphonates, and we discuss potential mechanisms of action that might be responsible for the anticancer activity of bisphosphonates in the clinic.
Introduction
Bisphosphonates are degradation-resistant structural analogs of pyrophosphates that bind avidly to bone and are ingested by osteoclasts, resulting in inhibition of osteoclast-mediated bone resorption.1 Bisphosphonates are classified on the basis of whether or not they contain a nitrogen atom, with nitrogen-containing bisphosphonates (N-BPs) being more potent than non-N-BPs at inhibiting osteoclast activity.1 Non-N-BPs (for example, etidronate, clodronate) cause the intracellular accumulation of nonhydrolyzable cytotoxic analogs of adenosine triphosphate that subsequently induce osteoclast apoptosis.1 New-generation N-BPs (for example, pamidronate, alendronate, risedronate, ibandronate, zoledronate, minodronate) specifically interfere with farnesyl pyrophosphate synthase (FPPS), a key enzyme in the mevalonate pathway.1 As a consequence, the covalent attachment of isoprenyl chains to small guanosine triphosphatases is blocked, thereby inhibiting their intracellular localization and functions in osteoclasts. Moreover, the disruption of the mevalonate pathway by N-BPs results in the accumulation of isopentenyl pyrophosphate (IPP), which is then converted to a cytotoxic adenosine triphosphate analog called ApppI (triphosphoric acid I-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester) that can induce apoptosis.1 The inhibitory effect of N-BPs on FPPS activity in osteoclasts and the consequences on protein prenylation represent a very important step forward in understanding how these drugs work.
Solid tumors (breast cancer, prostate cancer, lung cancer) and multiple myeloma are prone to develop bone diseases.2 Once tumor cells are in the bone marrow, they do not, on their own, destroy bone. Instead, they alter the functions of osteoclasts and osteoblasts, and hijack signals coming from the bone matrix.2 Specifically, tumor cells enhance bone resorption and inhibit bone formation, which leads to skeletal destruction and subsequent occurrence of skeletal complications.2 These skeletal complications can be fatal or may rapidly impede the quality of life of patients by causing pathological fractures, hypercalcemia, spinal cord compression and loss of mobility.1 Because of their potent antiresorptive activity, bisphosphonates (especially N-BPs) are therefore used to treat malignant bone diseases; they prevent or delay skeletal morbidity associated with bone metastasis.3 However, there may be a greater role for the use of bisphosphonates than has previously been considered.
Preclinical and clinical translational evidence supporting antitumor activity of bisphosphonates
Targeting osteoclasts
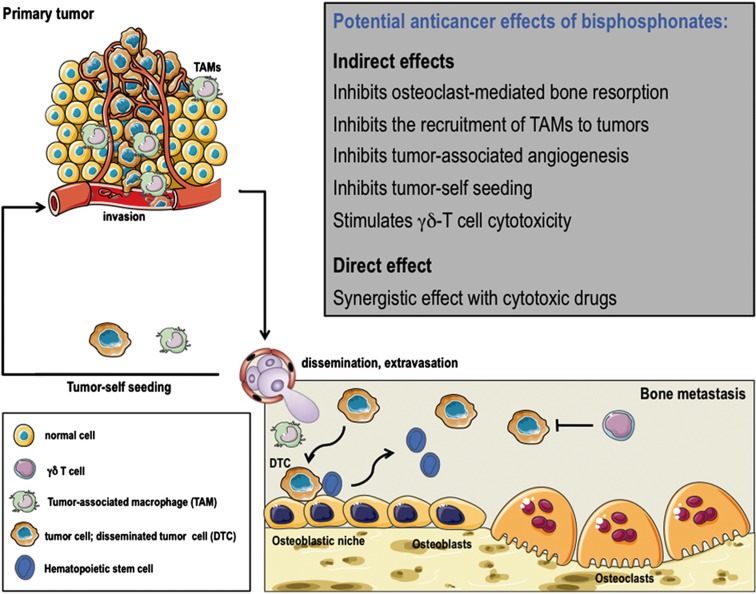
In vivo, N-BPs reduce skeletal tumor burden in a variety of mouse models of bone metastasis from solid tumors (breast, prostate, lung, ovarian, bladder and renal cell carcinomas) and this reduction has been attributed primarily to the antiresorptive activity of bisphosphonates.1,3 This contention is supported by experiments we conducted with a structural analog of the bisphosphonate risedronate, NE-58051, which has a bone mineral affinity similar to that of risedronate, but a 3000-fold lower bone antiresorptive activity in vivo.4 In vitro, both compounds inhibit breast cancer cell proliferation.4 In vivo, we found that NE-58051 (in contrast to risedronate) did not inhibit breast cancer bone metastasis formation or skeletal tumor burden, indicating that the antitumor effects of N-BPs is achieved mainly through inhibition of osteoclast-mediated bone resorption.4 Indeed, the skeleton is a rich source of growth factors including transforming growth factor-β and insulin-like growth factor that are released during bone resorption.2 By inhibiting bone resorption, N-BPs may therefore deprive tumor cells of these bone-derived factors that are required for tumor growth (Figure 1).
Figure 1.
Potential anticancer effects of bisphosphonates in vivo. The figure depicts the primary tumor microenvironment, the blood dissemination of tumor cells, the bone marrow metastatic environment with the osteoblastic niche and osteoclasts, and the recruitment of bone marrow-derived monocytes (TAM) and DTC to the site of the primary tumor. Bisphosphonates render the bone marrow a less hospitable microenvironment for tumor cell colonization, inhibiting osteoclast-mediated bone resorption and stimulating γδ-T cell cytotoxicity. They also interfere with the tumor self-seeding and TAM infiltration of primary tumors. The drawings were produced using Servier Medical Art (www.servier.com).
Targeting cancer cells
In vitro, bisphosphonates (especially N-BPs) inhibit tumor cell adhesion, migration, invasion and proliferation, and induce tumor cell apoptosis, when these compounds are used as single agents or in combination with chemotherapeutic agents.1 In vivo, there are experimental studies providing evidence that N-BPs inhibit the growth of soft tissue tumors and visceral metastases in animals. For example, the treatment of animals bearing 4T1 breast tumor with zoledronate reduces the spontaneous formation of distant metastases to visceral organs (lung, liver), by inhibiting 4T1 cell invasion.1 Similarly, alendronate inhibits Caov-3 ovarian cancer cell invasion in visceral organs in vivo.1 Additional mechanisms through which N-BPs could exhibit direct antitumor activity in vivo are those associated with programmed cell death. For example, zoledronate and minodronate block cancer cell cycle progression of non-small-cell lung carcinomas or induce cancer cell apoptosis in mesothelioma, melanoma, osteosarcoma, and in breast and bladder carcinomas.1 However, high doses of N-BPs have been used in most of these studies, and such high doses are incompatible with approved bisphosphonate-dosing regimens for patients with metastatic disease.1,3 Conversely, it has been shown that a sequential treatment with doxorubicin followed 24 h later by zoledronate (used at clinically relevant dosage) reduces subcutaneous growth of MDA-MB-436 breast tumors in animals.1,5 Importantly, accumulation of unprenylated Rap1A could be only detected in MDA-MB-436 tumors from animals treated with doxorubicin followed by zoledronate, which was indicative of the cellular uptake of zoledronate within subcutaneous tumors.5 Mechanisms responsible for this synergy between zoledronate and doxorubicin are poorly understood. It has been proposed that administering chemotherapy before zoledronate provides more effective anticancer activity because there is a higher uptake of the N-BP in tumor cells in vivo when chemotherapy is given first.5 Results obtained with this sequential treatment are reminescent of those obtained in the neoadjuvant cohort of the AZURE trial, showing that in breast cancer patients receiving neoadjuvant chemotherapy, the addition of zoledronate further reduced the residual invasive tumor size and improved by twofold the complete pathological response, when compared with chemotherapy alone, suggesting an antitumor effect of zoledronate.3
Targeting the premetastatic niche
Studies of the biology underlying the pathogenesis of cancer metastasis support the notion that bone marrow-derived cells (mesenchymal cells, monocytes, macrophages) enable the formation of specific microenvironments in distant organs that are sites of future metastasis, the so-called ‘premetastatic niches'.6 Additionally, bone marrow-derived endothelial progenitor cells are mobilized by tumor-derived growth factors and contribute to the vascularization of these premetastatic niches, thereby preparing the arrival of tumor cells.6 Interestingly, N-BPs (zoledronate being the most extensively studied bisphosphonate) exhibit antiangiogenic properties in vitro and in vivo, and they reduce tumor-associated angiogenesis in different animal models of cancer (myeloma, melanoma, and breast, ovarian and cervical carcinomas),1,3,7 suggesting they could interfere with the vascularization of the premetastatic niche. In some models, N-BP treatment (pamidronate, zoledronate) of animals with mammary carcinomas induces a profound reduction in CD11b+ macrophages infiltrating these tumors, which is accompanied by decreased VEGF and matrix metalloprotease-9 levels in the tumor microenvironment.8 This may be explained by the fact that matrix metalloprotease-9 produced by CD11b+ macrophages regulates the mobilization of VEGF from the extracellular matrix. Moreover, zoledronate treatment results in M2 (anti-inflammatory, proangiogenic) to M1 (antitumor) reversion of CD11b+ macrophages infiltrating mammary tumors in vivo.9 The bone marrow being a reservoir for proangiogenic CD11b+ myelomonocytic cells,1,3 N-BPs could therefore block the recruitment of bone marrow-derived CD11b+ myelomonocytic cells to the site of the premetastatic niche (Figure 1).
Targeting the bone marrow microenvironment
Beside the contribution of bone marrow-derived myeloid and endothelial progenitor cells to the formation of premetastatic niches in distant organs, the bone marrow itself might be a sanctuary for disseminated tumor cells (DTCs).2,7 DTCs from solid tumors can colonize bone early in the disease course (that is, before overt metastases develop). They directly compete with hematopoietic stem cells for occupancy of osteoblastic (also called endosteal) niches in the bone marrow, allowing these DTCs to evade systemic anticancer therapies and lay dormant for extended periods of time before becoming active and metastasizing to secondary sites.10 Indeed, the presence of DTCs in the bone marrow correlates with increased risk of disease recurrence in patients.7 Additionally, an early clinical study showed that adjuvant clodronate treatment reduces the risk of metastasis in women with breast cancer having DTCs in the bone marrow.11 Thus, there is a strong rationale to believe that the use of bisphosphonates early in the disease course might alter the levels of growth factors and other modulators of DTC survival in the bone marrow microenvironment (Figure 1). This contention is supported by preliminary clinical evidence showing that zoledronate in combination with standard anticancer therapy significantly reduces the prevalence of DTCs in the bone marrow from patients with early-stage breast cancer, when compared with standard therapy alone.7,12 Further studies are however required to determine whether the reduction of DTCs by zoledronate provides clinical benefit.
Targeting the immune system
Increased cancer surveillance via activation of γδT cells may represent another potential mechanism through which N-BPs could exhibit anticancer activity. Human Vγ9Vδ2T cells are a subset of human T cells that straddles the border between innate and adaptive immunity, and exhibits anticancer activity.1,7 Evidence for the stimulation of Vγ9Vδ2T cells by N-BPs was first found when increased numbers of γδT cells were observed in patients who had flu-like acute-phase reactions after their first intravenous infusion of pamidronate.1 N-BPs are indeed internalized by peripheral blood mononuclear cells, such as monocytes and dendritic cells, where they inhibit the mevalonate pathway, leading to the intracellular accumulation of IPP which, in turn, activates Vγ9Vδ2T cells and the release of inflammatory cytokines (tumor necrosis factor-α and interferon-γ, thereby contributing to the acute-phase reaction.1 N-BPs also induce intracellular accumulation of IPP/ApppI in a wide variety of human tumor cell lines in vitro and these mevalonate metabolites can be sensed by Vγ9Vδ2T cells as tumor phosphoantigens.1,7 We recently provided in vivo evidence that N-BPs (zoledronate and risedronate) induce IPP/ApppI accumulation in human breast tumors implanted subcutaneously in animals and that human Vγ9Vδ2T-cell infiltrate and inhibit growth of these tumors producing high IPP/ApppI levels, but not those expressing low IPP/ApppI levels.13,14 Additionally, we showed that estrogen receptor (ER)-positive breast tumors are more likely to produce IPP/ApppI after bisphosphonate treatment compared with ER-negative breast tumors. Moreover, the ability of risedronate and zoledronate to activate Vγ9Vδ2T-cell anticancer activity not only depends on IPP/ApppI accumulation in ER-positive tumors but also on expression of tumor cell surface receptor ICAM-1 (intercellular adhesion molecule-1), which triggers the recognition of bisphosphonate-treated breast cancer cells by Vγ9Vδ2T cells in vivo.13,14 These findings suggest therefore that N-BPs can have an adjuvant role in cancer therapy by activating Vγ9Vδ2T-cell cytotoxicity in patients with ER-positive breast cancer that produces high IPP/ApppI levels after N-BP treatment. Indeed, a few phase-I clinical studies reported that zoledronate (+ low-dose interleukin-2) activated Vγ9Vδ2T cells in patients with early or advanced breast cancer, hormone-refractory prostate cancer or advanced non-small-cell lung cancer.15 Notably, there was a significant correlation between clinical outcomes and peripheral blood γδT-cell numbers for each of these studies.15
Clinical evidence supporting antitumor activity of bisphosphonates in the metastatic setting
How do these experimental findings1,3,4,5,7,8,9,10,11,12,13,14 relate to the clinical situation in the metastatic setting? In patients with advanced-stage solid tumors, bisphosphonates (alongside specific anticancer treatments) delay skeletal morbidity associated with bone metastasis.3 However, no benefit in overall survival with bisphosphonates clodronate, pamidronate, ibandronate and zoledronate was observed in the full populations of large randomized clinical trials in breast cancer, prostate cancer and other solid tumors. Thus, these data did not support results obtained from preclinical studies showing bisphosphonates' ability to reduce skeletal tumor burden in animals. Nevertheless, a restrospective analysis of zoledronate phase-III trials demonstrated that in patients with elevated baseline levels of the bone resorption marker N-telopeptide of type I collagen (NTX), the rapid NTX normalization within 3 months of bisphosphonate treatment correlated with a median survival longer by 9 months for breast cancer and 12 months for hormone-refractory prostate cancer, compared with patients who had persistently elevated NTX levels.3 A survival advantage of 6 months was also observed in lung cancer patients with bone metastases who normalized their elevated baseline NTX levels after zoledronate treatment.3 Given the association between pathological fractures and risk of death, it is proposed that these survival benefits with zoledronate may be explained by the reduction of skeletal morbidity (especially pathological fractures), rather than a direct anticancer effect.3 In contrast, clinical evidence is emerging for a direct anticancer effect of zoledronate in patients with newly diagnosed multiple myeloma. Data from the Medical Research Council Myeloma IX trial (n=1960) showed that adding zoledronate to antimyeloma therapies significantly improved overall survival of patients with symptomatic multiple myeloma by 5.5 months compared with clodronate.3 Importantly, the survival benefit with zoledronate was independent of the effects of this bisphosphonate on skeletal morbidity, and thus was consistent with clinically meaningful anticancer activity. The mechanistic basis of this anticancer activity is unknown. In animal models of multiple myeloma, N-BPs have been shown to prevent the development of osteolytic lesions and to indirectly reduce myeloma burden.1 A direct antitumor effect of zoledronate has been also reported in the INA-6.Tu1 myeloma model. Accumulation of unprenylated Rap1A could be detected in these myeloma tumors ex vivo.1 Rap1A is a small GTPase. The accumulation of the unprenylated form of Rap1A within tumors is indicative of the cellular uptake of zoledronate and the subsequent inhibition of FPPS activity.1 By contrast, clodronate does not interfere with FPPS activity.1 Thus, these differences in the mechanisms of action of zoledronate and clodronate might explain the differences in therapeutic effects in the MRC myeloma IX trial. Interestingly, N-BP inhibition of the mevalonate-dependent signaling pathway in myeloma cells leads to IPP/ApppI intracellular accumulation, and these mevalonate metabolites are recognized by human Vγ9Vδ2T cells as tumor phosphoantigens.1 Another potential mechanism for the anticancer effect of zoledronate in the MRC myeloma IX trial might therefore also relate to its capacity to stimulate host anticancer immune responses. Consistent with this hypothesis, it has been shown that pamidronate treatment of the bone marrow from patients with multiple myeloma reduced myeloma cell survival, especially in cultures in which γδT-cell number was increased.16 Additionally, γδT-cell depletion from bone marrow cultures completely abrogated the cytotoxicity effect of pamidronate against myeloma cells.16
Clinical evidence supporting antitumor activity of bisphosphonates in the adjuvant setting
As aforementionned, a wealth of experimental data and clinical translational data support an anticancer role for adjuvant bisphosphonate (especially zoledronate) in the clinic (Figure 1). In this context, the landmark ABCSG-12 trial (n=1803) offered the most compelling data on potential anticancer activity of bisphosphonates, showing that the addition of zoledronate to hormone therapy (anastrozole or tamoxifen) for 3 years reduced the risk of disease progression by 36% in premenopausal women with endocrine-responsive stage I or II breast cancer, who were also receiving goserelin to induce artificial menopause (Table 1).17 Importantly, this reduction in disease progression included reductions not just in bone metastases but also in metastases at other distant sites, as well as in locoregional recurrences.17 Furthermore, women who received zoledronate maintained improvements in relapse-free survival at 62 months' follow-up and there was a significant reduction in the risk of death at 76 months' follow-up, while their treatment lasted 3 years ago (Table 1).7,18 Of note, these benefits in relapse-free survival and overall survival were restricted to patients older than 40 years on study entry (n=1390).7
Table 1. Summary of large phase-III clinical trials evaluating the adjuvant use of a bisphosphonate in early breast cancer.
| Clinical trial | |||||
|---|---|---|---|---|---|
|
Characteristics |
ABCSG-12a |
ZO-FASTb |
Z-FASTc |
AZUREd |
NSABP B-34e |
| Population | 1803 Premenopausal women with stage I/II, endocrine-receptor-positive breast cancer, receiving goserelin (3.6 mg, every 28 days) to induce artificial menopause | 1065 Postmenopausal women with stage I–IIIa, endocrine-receptor-positive breast cancer receiving letrozole (2.5 mg daily) for 5 years | 602 Postmenopausal women with stage I–IIIa, endocrine-receptor-positive breast cancer receiving letrozole (2.5 mg daily) for 5 years | 3360 Pre- and postmenopausal women with stage II/III breast cancer receiving standard chemotherapy and/or endocrine therapy | 3323 Pre- and postmenopausal women with stage I–III breast cancer receiving standard chemotherapy and/or endocrine therapy |
| Treatment | Patients were randomly assigned to receive anastrozole (1 mg daily) or tamoxifen (20 mg daily) with or without zoledronate (4 mg every 6 months) for 3 years | Patients were randomly assigned to receive immediate zoledronate (4 mg every 6 months for 5 years) or delayed zoledronate (initiated only for fracture or high risk thereof) | Patients were randomly assigned to receive immediate zoledronate (4 mg every 6 months for 5 years) or delayed zoledronate (initiated only for fracture or high risk thereof) | Patients were randomly assigned to receive zoledronate 4 mg every 4 weeks for 6 doses, then every 3 months for 8 doses, then every 6 months for 5 doses until 5 years | Patients were randomly assigned to receive oral clodronate (1600, mg daily) for 3 years |
| Outcomes | Endocrine therapy+zoledronate resulted in a 36% reduction in the risk of disease progression (HR=0.64; 95% CI 0.46–0.91; P=0.01) at 36 months' follow-up and continued to reduce the risk at 62 months' follow-up (HR=0.68; 95% CI, 0.51–0.91; P=0.009). In addition, there was an overall survival benefit at 76 months' follow-up (HR=0.59; P=0.04). Benefits were restricted to patients older than 40 years on study entry (n=1390) | The immediate-zoledronate group had a 41% reduction in the risk of disease progression (HR=0.59; 95% CI 0.36–0.96; P=0.0314) at 36 months' follow-up and continued to reduce the risk at 60 months' follow-up (HR=0.66; P=0.0375) | Disease progression rates at 61 months' follow-up were similar between the immediate- and delayed-zoledronate groups (9.8 (95% CI 6–10.3) versus 10.5 (95% CI 6.6–14.4), P=0.628) | No significant difference between the two groups at 59 months' follow-up. In women who were postmenopausal for at least 5 years before study entry, the zoledronate group had a 25% reduction in the risk of disease progression (HR=0.75; 95% CI, 0.59–0.96; P=0.02) and a 26% reduction in the risk of death (HR=0.74; 95% CI, 0.55–0.98; P=0.04) | No significant difference between the two groups at 90.7 months' follow-up. In women who were 50 years or older on study entry, the clodronate group had a 25% reduction in the risk of disease progression (HR=0.75; 95% CI, 0.57–0.99; P=0.045), but not for overall survival (HR=0.80; 95% CI, 0.61–1.04; P=0.094) |
Designed primarily to investigate the bone-preserving activity of zoledronate during adjuvant therapy with aromatase inhibitors, the european Zometa-Femara Adjuvant Synergy Trial ZO-FAST (n=1065) showed after 60 month's follow-up that the immediate addition of zoledronate to adjuvant letrozole therapy reduced the risk of disease progression by 34% in postmenopausal women with endocrine-responsive, stage I–III breast cancer, when compared with patients in the ‘delayed' group who received zoledronate only if bone mineral density declined or non-traumatic fracture occurred (Table 1).19 In the companion North American trial to ZO-FAST (the Zometa-Femara Adjuvant Synergy Z-FAST study; n=602), immediate zoledronate also reduced disease recurrence, but did not result in a statistically significant difference in disease-free survival compared with the delayed-zoledronate group (16 vs 21 at 61 months' follow-up) (Table 1).20 However, taken together these findings17,18,19,20 suggested that zoledronate may improve disease-free survival in breast cancer patients.
Therefore, there was a general disappointment in the scientific community when results from the largest phase-III adjuvant bisphosphonate trial (AZURE) were reported, showing that zoledronate did not improve overall survival or prevent cancer recurrences in women with breast cancer (Table 1).21 In this study, over 3000 pre- or postmenopausal women with stage II or III breast cancer were randomized to receive either standard therapy (mainly chemotherapy) or standard therapy with zoledronate for a duration of 5 years (Table 1). A preplanned subgroup analysis of patients according to their menopausal status revealed, however, that the risk of disease progression in postmenopausal patients was reduced by 25% in the zoledronate group compared with the control group (Table 1).21 The difference in disease recurrences according to menopausal status also translated into a survival benefit with postmenopausal patients showing a 26% reduction in the risk of death (Table 1).21 Interestingly, while using adjuvant clodronate (alongside standard therapy) in women with stage I–III endocrine-responsive breast cancer (NSABP B-34 trial; n=3323), Paterson et al..22 reported results similar to those obtained in the AZURE trial, showing no overall effect on disease-free survival in all patients but a significant reduction on disease progression in those older than 50 years (Table 1).
The reasons why adjuvant zoledronate treatment significantly improved disease-related outcomes in the ABCSG-12 and ZO-FAST trials are unknown. In these trials, the bisphosphonate was administered over long treatment intervals (every 6 months) and, in the ABCSG-12 trial, a persistent benefit in reductions of disease progression and risk of death was maintained 3 years after completion of the treatment.7,18 Although bisphosphonates exhibit direct antitumor activities in animal models of cancer and metastasis, the long-lasting effect of zoledronate in ABCSG-12 with respect to disease-free survival and overall survival militate in favor of indirect antitumor mechanisms. Bisphosphonates bind avidly to bone mineral.1,23 Once bound in the skeleton, they can be embedded in the bone during bone formation then released during subsequent resorption, explaining the very slow and long elimination of bisphosphonates from the skeleton.23 The ‘carryover' effect of zoledronate in the ABCSG-12 trial may therefore be explained by the pharmacological properties of this class of drugs. The slow release of zoledronate from the skeleton enables continuous impregnation of the bone marrow with low iterative bisphosphonate doses that impede the retention of cancer cells in the bone marrow. Preclinical evidence suggests that dDTCs in distant organs can recolonize their tumors of origin.24 This process called ‘tumor self-seeding' could explain, for instance, the local recurrences in breast cancer.24 In the ABCSG-12 study, patients receiving zoledronate experienced fewer locoregional recurrences compared with patients who received hormonal therapy alone (15 vs 29 at 62 months' follow-up).17 Similarly, in the ZO-FAST trial, fewer recurrences were reported in the immediate-zoledronate group compared with the delayed-zoledronate group (5 vs 12 at 60 months' follow-up).19 Although not statistically significant, locoregional recurrences in the Z-FAST trial also occurred in slightly more patients in the delayed-zoledronate group compared with the immediate-zoledronate group (4 vs 2 at 61 months' follow-up).20 Thus, zoledronate released from bone might therefore block tumor self-seeding. Additionally, zoledronate could block mechanisms of secondary spread from bone by inhibiting the recruitment of bone marrow-derived cells to sites of premetastatic niches.
The data obtained in the ABCSG-12 and ZO-FAST/Z-FAST trials are challenged by results obtained in the AZURE trial and by those obtained in the NSABP B-34 trial. The population of patients in AZURE and NSABP B-34 trials were essentially at intermediate or high risk (stage II and III), whereas the ABCSG-12 and ZO-FAST trial populations were primarily at lower risk. The different patients' characteristics, regarding the histological grade of tumors, may probably account for the differences in the results obtained in these trials. Intriguingly, however, data obtained with the postmenopausal AZURE subgroup, the NSABP B-94 subgroup of women older than 50 years (a surrogate for postmenopausal status), and the ZO-FAST, Z-FAST and ABCSG-12 studies are all converging on the same observation that zoledronate or clodronate may prevent breast cancer recurrences at multiple sites, when endogenous levels of estrogens are low (that is, following ovarian suppression therapy or in women with menopause at diagnosis). Thus, adjuvant zoledronate and clodronate can potentially exert antitumor activity in the clinic. The question is to understand why these bisphosphonates work better in an estrogen-poor environment rather than an estrogen-rich environment. There is a general agreement that the reduction of endogenous estrogen levels increases osteoclast-mediated bone resorption in postmenopausal women, and that the use of anti-hormonal drugs (for example, aromatase inhibitors) in pre- or postmenopausal breast cancer also accelerates this bone resorption process. Additionally, preclinical studies provide evidence that skeletal tumor burden in animals is aggravated following ovariectomy, because there is a much higher bone destruction and subsequent release of bone-derived growth factors that, in turn, support tumor growth.1,2,3 Thus, one hypothesis may be that, by preventing enhanced bone destruction induced by the lack of estrogen, zoledronate and clodronate interfere with the tumor-growth-supportive functions of bone-derived growth factors and bone marrow-derived mesenchymal cells (Figure 1). Additionally, there is preclinical evidence that estrogens can influence lung metastasis formation in animals, by mobilizing bone marrow-derived endothelial progenitor cells to the premetastatic niche.25 In the absence of estrogens, endothelial progenitor cells and possibly other cell types, including bone marrow mesenchymal cells and cancer cells, could be more sensitive to the inhibitory effects of zoledronate or clodronate, therefore maximizing indirect antitumor effects of these bisphosphonates to a clinically detectable beneficial level (Figure 1).
Cancer prevention studies
Another line of evidence supporting antitumor activity of bisphosphonates comes from epidemiological studies showing that bisphosphonates for osteoporosis treatment may prevent breast cancer in healthy postmenopausal women.7 For instance, a population-based, case-controlled study (n=5911) in Wisconsin found that current bisphosphonate use for osteoporosis treatment was associated with a 33% reduction in breast cancer risk (hazard ratio (HR)=0.67, 95% confidence interval (CI)=0.51–0.89; P=0.01) compared with subjects who never received bisphosphonate therapy.26 Another database study (n=4039) reported that receiving bisphosphonates for more than 1 year reduced the risk of postmenopausal breast cancer (HR=0.72, 95% CI 0.57–0.90) compared with nonusers.7 Overall, these results support the adjuvant use of bisphosphonates in early breast cancer. Similar findings were observed in colorectal cancer. A population-based, case-controlled study (n=1866) in Israel found a substantial risk reduction in colon cancer incidence (HR=0.50, 95% CI=0.35–0.71) in postmenopausal women receiving oral bisphosphonate treatment, compared with control subjects.7 However, this benefit of oral bisphosphonates on reduction in the risk of colorectal cancer was not observed among women enrolled in a large prospective cohort, the Nurses Health Study (n=86 277), when compared with nonusers of oral bisphosphonates (HR=0.92, 95% CI 0.73–1.14).27 It has been suggested that inadequate control for healthy behaviors associated with the primary indication for use of bisphosphonates (osteoporosis) could account for the opposite results observed in the two studies.27 Conversely, a Danish national register-based cohort study of 30 606 women with osteoporosis taking a bisphosphonate (primarily alendronate) and 124 424 matched control subjects not taking a bisphosphonate documented a 38% reduction in colon cancer death (HR=0.62, 95% CI 0.5–0.76; P<0.01) in bisphosphonate users compared with controls.28 Oral bisphosphonates are poorly absorbed. High local concentrations of bisphosphonates in the colon might therefore exert direct anticancer effects on early cancer lesions of the colonic mucosa.
Conclusion
Bisphosphonates become attractive candidates to prevent cancer metastasis. In addition to their established bone-protective activities, a wealth of preclinical studies has demonstrated these compounds exhibit direct and indirect anticancer activities, and these preclinical findings have been translated to the bedside in several large phase-III clinical trials in patients with early breast cancer (ABCSG-12, Z/ZO-FAST) or symptomatic multiple myeloma (MRC Myeloma IX), showing disease-free survival and overall survival benefits. Additionally, subgroup analyses of postmenopausal women with early breast cancer in AZURE and NSABP B-34 phase-III clinical trials further indicate that there is a potential antitumor activity of bisphosphonates (zoledronate and clodronate) in the adjuvant setting, when these drugs are used in an estrogen-poor environment. Finally, epidemiological studies showed that current use of bisphosphonates in healthy postmenopausal women for the treatment of osteoporosis was associated with a 30% reduced risk of breast cancer and colon cancer. For a research point of view, these findings highlight the importance of identifying mechanisms which, under estrogen deprivation, are responsible for the anticancer activity of zoledronate and clodronate in breast cancer. Similarly, the reasons for which zoledronate improves cancer-related outcomes in patients with newly diagnosed multiple myeloma must be understood. They are likely to be distinct from those responsible for the antitumor activity of zoledronate in breast cancer, since clodronate was only effective on skeletal health outcomes in patients with multiple myeloma, whereas it also improves cancer-related outcomes in patients with breast cancer. Understanding these mechanisms will help to better define patients who could benefit from bisphosphonate therapy.
Footnotes
Philippe Clézardin has served on advisory boards or as a consultant for Novartis and Amgen.
References
- Clézardin P. Bisphosphonates' antitumor activity: an unravelled side of a multifaceted drug class. Bone 2011;48:71–79. [DOI] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2012;11:411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R, Gnant M, Morgan G, Clézardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst 2012;104:1059–1067. [DOI] [PubMed] [Google Scholar]
- Fournier PG, Stresing V, Ebetino FH, Clézardin P. How do bisphosphonates inhibit bone metastases in vivo? Neoplasia 2010;12:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottewell PD, Lefley DV, Cross SS, Evans CA, Coleman RE, Holen I. Sustained inhibition of tumor growth and prolonged survival following sequential administration of doxorubicin and zoledronic acid in a breast cancer model. Int J Cancer 2009;126:522–532. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009;9:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnant M, Clézardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev 2012;38:407–415. [DOI] [PubMed] [Google Scholar]
- Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-bisphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res 2007;67:11438–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C et al. Zoledronic acid repolarizes tumor-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med 2010;14:2803–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 2011;121:1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med 1998;339:357–363. [DOI] [PubMed] [Google Scholar]
- Solomayer EF, Gebauer G, Hirnle P, Janni W, Lück HJ, Becker S et al. Influence of zoledronic acid on disseminated tumor cells in primary breast cancer patients. Ann Oncol 2012;23:2271–2277. [DOI] [PubMed] [Google Scholar]
- Benzaïd I, Mönkkönen H, Stresing V, Bonnelye E, Green J, Mönkkönen J et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res 2011;71:4562–4572. [DOI] [PubMed] [Google Scholar]
- Benzaïd I, Mönkkönen H, Bonnelye E, Mönkkönen J, Clezardin P. In-vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger Vγ9Vδ2 T-cell antitumor cytotoxicity through ICAM-1 engagement. Clin Cancer Res 2012;18:6249–6259. [DOI] [PubMed] [Google Scholar]
- Hamilton E, Clay TM, Blackwell KL. New perspectives on zoledronic acid in breast cancer: potential augmentation of anticancer immune response. Cancer Invest 2011;29:533–541. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδT cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000;96:384–392. [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C et al. ABCSG-12 trial investigators, Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009;360:679–691. [DOI] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 2011;12:631–641. [DOI] [PubMed] [Google Scholar]
- Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 2013;24:398–405. [DOI] [PubMed] [Google Scholar]
- Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C et al. Final 5-year results of Z-FAST trial. Cancer 2012;118:1192–1201. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011;365:1396–1405. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol 2012;13:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers S, Papapoulos S. Pharmacology of bisphosphonates. Bone 2011;49:42–49. [DOI] [PubMed] [Google Scholar]
- Suriano R, Chaudhuri D, Johnson RS, Lambers E, Ashok BT, Kishore R et al. 17Beta-estradiol mobilizes bone marrow-derived endothelial progenitor cells to tumors. Cancer Res 2008;68:6038–6042. [DOI] [PubMed] [Google Scholar]
- Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br J Cancer 2010;102:799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili H, Huang ES, Ogino S, Fuchs CS, Chan AT. A prospective study of bisphosphonate use and risk of colorectal cancer. J Clin Oncol 2012;30:3229–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RG. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate-Danish National Register Based Cohort Study. Osteoporos Int 2012;23:2693–2701. [DOI] [PubMed] [Google Scholar]