Abstract
Investigation of the physiologic relevance of bone marrow adipose tissue (BMAT) during growth may promote understanding of the bone-fat axis and confluence with metabolic factors. The objective of this pilot investigation was two-fold: (1) to evaluate the relationships among total body fat, bone mineral content (BMC) and femoral BMAT during childhood and underlying metabolic determinants and (2) to determine if the relationships differ by race. Participants included white and non-Hispanic black girls (n=59) ages 4–10 years. Femoral BMAT volume was measured by magnetic resonance imaging, BMC and body fat by dual-energy X-ray absorptiometry. Metabolic parameters were assessed in the fasted state. Total fat and BMC were positively associated with BMAT; however, simultaneous inclusion of BMC and body fat in the statistical model attenuated the association between BMC and BMAT. Differences in BMAT volume were observed, non-Hispanic black girls exhibiting marginally greater BMAT at age eight (P=0.05) and white girls exhibiting greater BMAT at age ten (P<0.001). Metabolic parameters conferred differential impact by race, such that, a positive association for BMAT and leptin (P=0.02) and adiponectin (P=0.002) in white girls while BMAT and insulin were inversely related in non-Hispanic black girls (P=0.008). Our findings revealed a positive relationship between BMAT, body fat and BMC, although body fat, respective to leptin, contributed partly to the relationship between BMAT and BMC. Despite large differences in total fat between non-Hispanic black and white, the relationship between BMAT and BMC was similar to white girls. However, this relationship appeared to be impacted through different mechanisms according to race.
Introduction
A positive association between bone mineral content (BMC) and weight status has been consistently documented. Thus, greater weight has characteristically been recognized as a protective factor from bone-related injury.1 However, contemporary investigations have revealed overrepresentation in bone fracture incidence at various anatomical sites among obese individuals,2,3,4 suggesting that fat accrual in excess may perturb processes underlying bone integrity. In essence, greater bone size does not necessarily translate into stronger bones,5,6,7 and factors that may compromise bone integrity warrant investigation.
Whereas under normal physiologic conditions, bone mineral deposition and resorption are tightly coupled, during growth, bone formation is favored and these developmental (re)modeling processes ultimately contribute to bone strength. Seemingly counterintuitive, absolute higher BMC with increasing body size may not parallel enhanced properties of bone building.6,7 The extent to which excess adiposity, during the growth period, may have the capacity to disrupt bone (re)modeling and long-term bone health has not been well-described. However, rapid growth secondary to obesity and associated metabolic perturbations (for example, hyperinsulinemia, hyperleptinemia, dyslipidemia) may alter the coupling between bone deposition and resorption leading to imbalance in bone remodeling processes (for example, intertrabecular space infusion at the epiphysis with lamellar bone, intracortical porosity).8,9,10,11 This dysynchrony likely contributes to volume of bone marrow adipose tissue (BMAT), as it has been established that the bone marrow depot is sensitive to metabolic perturbations ensuing pathological conditions, which effect energy balance, including metabolic conditions (for example type 2 diabetes, obesity and anorexia nervosa).12,13,14 Thus, the bone marrow compartment represents a unique niche providing direct interaction between bone and fat cells as it is here that undifferentiated stem cells capable of multiple lineage pathways meet their fate.15 Investigation including assessment of the marrow compartment and systemic interactions may provide some insight into the interrelationship between the bone-fat axis.
A reciprocal relationship exists between the bone and adipose microenvironments, such that energetic pathways directed toward the latter averts cell lineage away from bone remodeling.16 As the foundations of body composition are largely established during growth and development, with actions of metabolic factors largely in play, investigative efforts to characterize bone processes should be targeted early in the life course. In addition, the marrow is converted from red (hematopoietic) to yellow (adipose) in a predictable, progressive manner such that ∼10% is adipose at birth and ∼90% of the marrow is adipose at reproductive maturity.17,18 However, it is speculated that obesity and related metabolic perturbations may accelerate this conversion process. The objective of this investigation was to evaluate the relationships among total body fat, BMC and BMAT during childhood, as well as underlying metabolic determinants, and to determine if the relationships differ by race.
Results
Baseline characteristics
Descriptive characteristics of the total sample population and by race are presented in Table 1. Non-Hispanic black girls were reproductively more mature likely contributing to greater height, weight, adiposity and BMC. However, BMAT was not significantly different between groups. Metabolic parameters of the sample population are illustrated in Table 2. Race-related differences in fasting glucose, low-density lipoprotein cholesterol and parathyroid hormone (PTH) were observed, such that higher low-density lipoprotein cholesterol and lower fasting glucose, and PTH were apparent in non-Hispanic black girls compared with white girls. Additionally, while not statistically different between races, non-Hispanic black girls did exhibit 25-hydroxy vitamin D levels below that which is considered sufficient (30 ng ml−1).
Table 1. Descriptive characteristics of sample population.
| Total (n=59) | Black girls (n=25) | White girls (n=34) | |
|---|---|---|---|
| Age (years) | 7.7±0.3 | 8.0±0.4 | 7.5±0.3 |
| Tanner (I–IV)a | 1.4±0.1 | 1.8±0.2b | 1.2±0.1 |
| Height (inches) | 51.0±0.9 | 53.7±1.4b | 49.1±1.1 |
| Weight (lb) | 88.0±5.9 | 114.7±9.4b | 68.4±5.6 |
| Total fat (kg) | 15.2±1.4 | 22.2±2.2b | 10.1±1.3 |
| Percent fat (%) | 34.6±1.3 | 40.9±1.6b | 29.9±1.5 |
| Total BMC (g) | 1169±59 | 1381±98b | 1013±61 |
| BMAT (cm3) | 88.3±8.3 | 100.4±22.9 | 83.6±7.7 |
Table 2. Fasting metabolic parameters of sample population.
| Total (n=59) | Black girls (n=25) | White girls (n=34) | |
|---|---|---|---|
| Insulin (uU ml−1) | 8.3±0.9 | 9.0±1.6 | 8.0±1.2 |
| Glucose (mg dl−1) | 91.1±1.4 | 86.2±1.7a | 93.2±1.7 |
| Leptin (ng ml−1) | 9.1±1.9 | 8.6±3.3 | 9.2±2.1 |
| Triglycerides (mg dl−1) | 69.3±4.9 | 60.9±5.5 | 74.3±6.9 |
| HDL (mg dl−1) | 50.8±3.0 | 51.7±4.9 | 50.2±3.8 |
| LDL (mg dl−1) | 82.4±3.5 | 92.7±5.1a | 76.2±4.3 |
| Adiponectin (mcg ml−1) | 14.1±1.1 | 15.8±5.9 | 13.8±1.0 |
| PTH (pg ml−1) | 35.1±2.2 | 24.6±4.8a | 37.0±2.3 |
| Vitamin D (ng ml−1) | 30.5±1.7 | 24.2±2.7 | 31.7±1.8 |
Abbreviations: Black, non-Hispanic Black; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; PTH, parathyroid hormone; Vitamin D,25-hydroxy vitamin D.
aIndicates significant difference among Black and White girls (P<0.05).
BMAT and body composition correlations by age and race
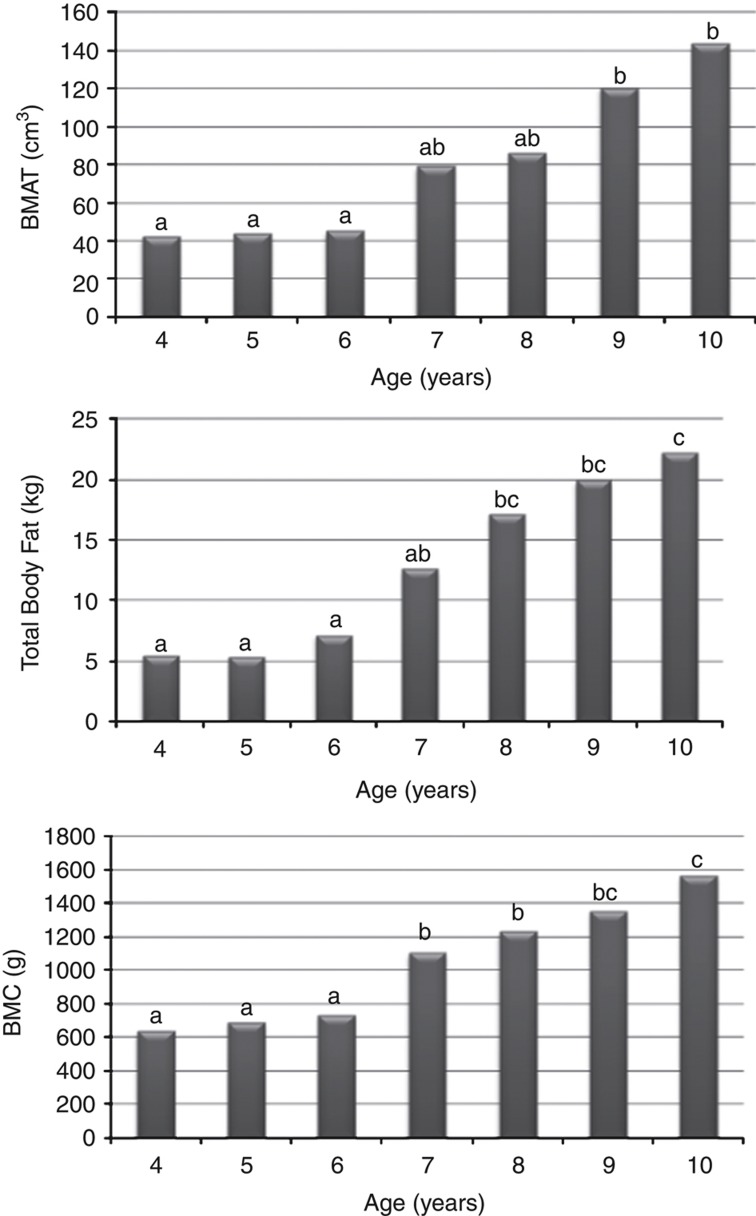
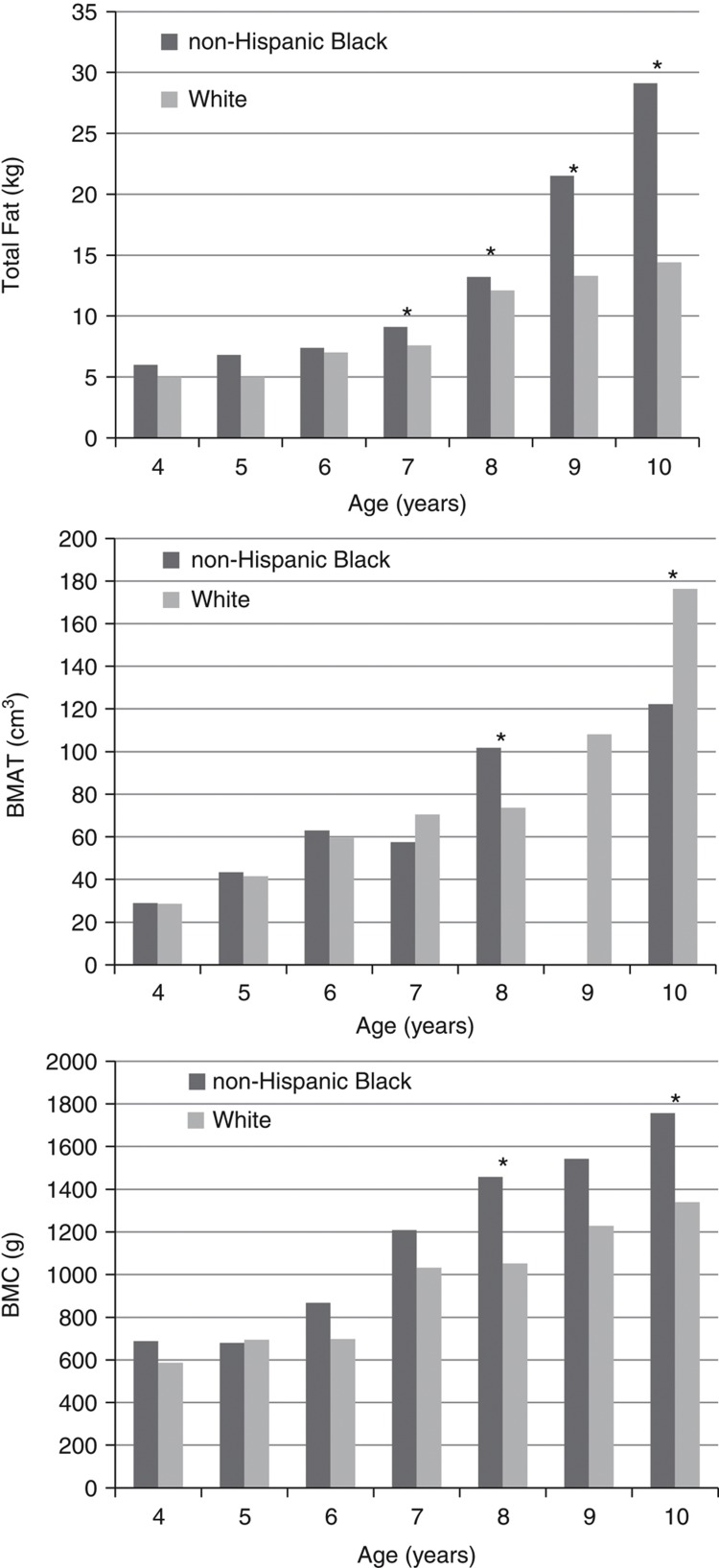
Figure 1 presents BMAT volume, total body fat and BMC by age for the total sample. As age increased, a progressive increase in BMAT volume, total body fat and BMC was observed. Whereas no significant difference was observed from ages four to six, nine-year-old girls had significantly greater BMAT volume than those <9 years of age, total body fat beginning at eight years of age, and BMC beginning at seven years. When assessing differences in BMAT volume by age, non-Hispanic black girls had marginally greater BMAT at age eight (P=0.05), whereas white girls had greater BMAT at age ten (P<0.001; Figure 2). Additionally, when assessing differences in total fat by age, non-Hispanic black girls had greater total fat at ages seven, eight, nine and ten years than white girls.
Figure 1.
BMAT volume, total body fat and BMC by age in sample population. Note: a,b,c denotes significant difference (P<0.05). N-values for each age group are as follows: 4 years=6, 5 years=6, 6 years=7, 7 years=6, 8 years=10, 9 years=14, and 10 years=10.
Figure 2.
Race differences for total fat, BMAT volume and BMC by age. Note: *denotes significant differences. No BMAT data provided for 9-year-old non-Hispanic Black because none were present in the analysis. N-values for each age group for non-Hispanic Black participants are as follows: 4 years=2, 5 years=2, 6 years=3, 7 years=2, 8 years=6, 9 years=0 and 10 years=4. N-values for each age group for white participants are as follows: 4 years=4, 5 years=4, 6 years=4, 7 years=4, 8 years=4, 9 years=8 and 10 years=6.
Independent associations between BMAT and body composition parameters
The independent relationships of BMAT with body composition parameters are presented in Table 3 for the overall sample and by race. BMAT was positively associated with total body fat (P<0.05) and BMC (P<0.001). However, simultaneous inclusion of total body fat and BMC in the statistical model resulted in attenuation of the relationship between BMAT and BMC, but not total body fat (albeit of marginal significance; P=0.07). While body fat accounts for a large proportion of the relationship, our results, support at least a partial involvement in the relationship between BMAT and BMC. When analyzed according to race, the relationship with BMAT was maintained among white girls for both total body fat (P=0.002) and BMC (P=0.01), but only marginally with total body fat in non-Hispanic black girls (P=0.05). Inclusion of both total body fat and BMC in the model resulted in attenuation of the relationship between BMC and BMAT in white girls as it did in the overall sample; however, the relationship between total body fat and BMAT remained significant (P<0.02).
Table 3. The independent relationships of BMAT and total body fat and BMC.
|
Total fata |
BMCa |
Total fatb |
BMCb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | β | P | R2 | β | P | R2 | β | p | β | P | |
| Total (n=59) | 0.79 | 0.02 | 0.008 | 0.78 | 0.08 | <0.001 | 0.84 | 0.01 | 0.07 | 0.10 | 0.13 |
| Black girls (n=25) | 0.82 | 0.02 | 0.05 | 0.78 | 0.07 | 0.10 | 0.84 | 0.02 | 0.32 | 0.06 | 0.09 |
| White girls (n=34) | 0.79 | 0.02 | 0.002 | 0.79 | 0.10 | 0.01 | 0.87 | 0.02 | 0.02 | 0.06 | 0.14 |
Black, non-Hispanic Black; BMC, bone mineral content.
aModel 1: independent association.
bModel 2: simultaneous inclusion of bone and fat in statistical model with BMAT as dependent variable. Values in bold indicate significant associations.
Independent associations between BMAT and metabolic parameters
The independent associations of BMAT with individual metabolic parameters, as well as the interactive effect of these associations with the inclusion of total body fat and BMC are presented in Table 4. When investigating the relationship between BMAT and individual metabolic parameters (that is, insulin, leptin and adiponectin), positive associations were only observed between BMAT and leptin in the total sample (P=0.01). Additionally, when total body fat was included in the model circulating adiponectin contributed to the relationship between BMAT and total fat. However, when BMC was included in the model the relationship between BMAT and BMC was attenuated by leptin. When stratified by race, among white girls a positive relationship was observed for BMAT with leptin (P=0.02), as well as with adiponectin (P=0.002), and a marginal association between BMAT and insulin (P=0.07) was observed. However, the only observed association with metabolic parameters among non-Hispanic black girls was an inverse association between insulin and BMAT (P=0.008) when controlling for both total fat and BMC. Inclusion of adiponectin attenuated the relationship between BMAT and BMC, yet among non-Hispanic black girls the relationship between BMAT and BMC is maintained with the inclusion of insulin.
Table 4. The independent associations of BMAT with individual metabolic parameters with and without the inclusion of total body fat and/or BMC.
| Leptin | Adipo | Insulin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | P-value | R2 | P-value | R2 | P-value | |||||||
| Total (n=59)a | 0.81 | 0.01 | 0.76 | 0.19 | 0.77 | 0.29 | ||||||
| Black girls (n=25)a | 0.76 | 0.47 | 0.71 | 0.12 | 0.81 | 0.38 | ||||||
| White girls (n=34)a | 0.8 | 0.02 | 0.75 | 0.002 | 0.79 | 0.07 | ||||||
| Totfat | Leptin | Totfat | Adipo | Totfat | Insulin | |||||||
| |
R2 |
|
P-value |
R2 |
|
P-value |
R2 |
|
P-value |
|||
| Total (n=41)b | 0.81 | 0.34 | 0.45 | 0.84 | <0.001 | <0.001 | 0.89 | 0.01 | 0.66 | |||
| Black girls (n=12)b | 0.75 | 0.84 | 0.29 | 0.86 | 0.48 | 0.63 | 0.83 | 0.66 | 0.48 | |||
| White girls (n=29)b | 0.8 | 0.48 | 0.001 | 0.89 | <0.001 | <0.001 | 0.87 | 0.09 | 0.80 | |||
| BMC | Leptin | BMC | Adipo | BMC | Insulin | |||||||
| |
R2 |
|
P-value |
R2 |
|
P-value |
R2 |
|
P-value |
|||
| Total (n=41)b | 0.82 | 0.6 | 0.04 | 0.81 | 0.09 | 0.04 | 0.75 | <0.001 | 0.8 | |||
| Black girls (n=12)b | 0.78 | 0.84 | 0.23 | 0.74 | 0.98 | 0.91 | 0.71 | 0.25 | 0.31 | |||
| White girls (n=29)b | 0.80 | 0.32 | 0.13 | 0.8 | 0.07 | 0.05 | 0.81 | 0.05 | 0.10 | |||
| Totfat | BMC | Leptin | Totfat | BMC | Adipo | Totfat | BMC | Insulin | ||||
| |
R2 |
P-value |
R2 |
P-value |
R2 |
P-value |
||||||
| Total (n=41)c | 0.82 | 0.38 | 0.70 | 0.48 | 0.88 | <0.001 | 0.3 | <0.001 | 0.84 | 0.24 | 0.004 | 0.69 |
| Black girls (n=12)c | 0.74 | 0.85 | 0.86 | 0.40 | 0.80 | 0.57 | 0.93 | 0.70 | 0.81 | 0.01 | 0.005 | 0.01d |
| White girls (n=29)c | 0.80 | 0.39 | 0.38 | 0.84 | 0.84 | 0.001 | 0.62 | 0.001 | 0.84 | 0.13 | 0.35 | 0.95 |
Abbreviations: Adipo, adiponectin; Black, non-Hispanic Black; BMAT, bone marrow adipose tissue; BMC, bone mineral content; Totfat, total fat.
aModel 1: independent association.
bModel 2: simultaneous inclusion of Bone or Fat with metabolic parameters.
cModel 3: the contribution of body Fat and BMC as moderators of the association between metabolic parameters and BMAT.
dIndicates a negative association. Values in bold indicate significant associations. Supercripts a, b, c indicate different regression models.
Discussion
During puberty, an understanding of the physiological events occurring in the bone marrow compartment initiated by interactions in the bone-fat axis may provide insight into developmental origins, which directly impact long-term skeletal health. Normal marrow conversion follows an orderly and progressive manner, in which red marrow (predominantly comprised of hematopoietic elements) is replaced by yellow marrow (BMAT; primarily adipogenic), such that by early adulthood the marrow compartment of the appendicular skeleton is comprised of virtually all adipose tissue.16 Accompanying the characteristic accelerated take off demarcating the linear growth spurt is a significant increase in the proportion of red marrow to yellow marrow. Indeed these processes are coupled, yet the amount, timing and distribution of yellow and red marrow may increase in response to metabolic stress.19 Dysregulation, consequential to alterations in energy provision (that is, starvation, obesity and so on.), in the balance between adipogenesis, at the expense of osteblastogenesis, has been shown to compromise bone integrity in adults,20 as well as in pediatrics with anorexia nervosa.13,21 During growth and development, perturbations in this mechanism may exert action on more highly susceptible common progenitor stem cell thereby directing lineage away from osteogenesis.22,23 While assessment of cell lineage is difficult in vivo, imaging techniques allow for the quantification of BMAT volume, and provide information regarding the function and relevance of the conversion of red marrow to BMAT early in the life course.
In adults, marrow adiposity is independently associated with increased fractures.24 Childhood fractures have been associated with alterations in body composition, that is, increased adiposity and impaired bone structure,25 and prevalence of fracture rates have increased ∼20% (Pressley et al.2). Recently, Shen et al.26 reported an inverse relationship between BMD and BMAT in young and old adults, independent of race or total body fat. Speculatively, increased adipose tissue within the marrow compartment could lead to alterations in the stem cell lineage allocation involved in bone remodeling, increasing adipogenesis thereby impairing bone integrity.15 However, despite the reported effects of adipocyte infiltration within the bone marrow compartment,27 during growth the hormonal milieu promotes bone formation (that is, increase in bone size), explaining the increase in BMC. In this pediatric population, we show that although the level of adiposity may contribute to the positive relationship between BMAT and BMC, consistent with the notion that increased adiposity in the marrow compartment may lead to an increase in bone modeling, qualitative exploration encompassing outer and inner surfaces extending beyond BMC is essential. Contrary to findings in adults, our results show positive relationships between BMAT, total body fat, and BMC, which is particularly impactful during growth. The relationship is evident with proximity to puberty and is plausibly related to the additive effects of the metabolic and reproductive hormonal milieu interacting with adiposity during this critical period. Indeed non-Hispanic black girls in this cohort have greater overall adiposity, with greater BMAT at age 8; however, a greater BMAT is identified in white girls at age 10, consistent with their pubertal progression.
Despite no differences in BMAT volume among subjects, a race-dependent differential effect was observed in regard to interacting factors linking the bone-fat axis to BMAT volume. Similarly, a differential contribution of adiponectin and insulin was observed between white and non-Hispanic black girls. While these results were unexpected given differences in adiposity, investigators from our group have recently reported higher leptin in African-American women that was not explained by differences in body composition and fat distribution.28 These findings support a mechanism in which inherent physiologic differences may underlie disparate outcomes in long-term chronic disease risk, particularly the noted impairments in bone integrity despite greater bone volume recently identified among African-American adult women,2 and warrants further investigation.
Whereas the anabolic effects of insulin on bone are well-understood,29 the interactive effects of insulin with adiposity and bone formation on BMAT are less clear. Insulin was not independently associated with BMAT across the overall sample, and although only marginally significant, higher insulin was independently associated with BMAT in white girls only. On the contrary, independent of total body fat and BMC, insulin and BMAT in non-Hispanic black girls displayed a strong inverse association. In this sample, non-Hispanic black girls were more hyperinsulinemic than their white counterparts, aligning with that commonly reported30,31 and suggestive that elevated insulin levels may confer mitogenicity at least during the growth period.32 Additionally, greater insulin utilizing capacity by BMAT may function in upregulation of fatty acid metabolism, whereas in the normal physiologic state insulin's influence within the bone marrow would typically lead to osteoblast proliferation.33 Nonetheless, the divergent findings reported herein lend limited insight to underlying mechanisms, which may lead to preferential proliferation of adipocytes.
Adipokines may mediate the relationship apparent between bone and adiposity, as well as the composition of the marrow compartment. Elevated leptin and low adiponectin levels have been independently associated with increased risk of adverse outcomes (that is, inflammation, cardiovascular disease, type 2 diabetes),1,34 and have a major role in bone remodeling.35,36 Herein, leptin was positively associated with BMAT. In girls followed throughout puberty, leptin was positively associated with change in bone area,37 despite animal models suggesting leptin functions through neural pathways inhibiting bone formation. It is plausible, given leptin's role in reproduction,34 that the action of leptin may depend on current leptin status and the mode of the action (central or peripheral effects), as well as life stage.1,38,39 Although counterintuitive, yet confined only to the white population, adiponectin also associated positively with BMAT. Adiponectin is acknowledged for its potential insulin-sensitizing, anti-inflammatory and anti-atherogenic effects.40 Additionally, the inverse reported relationship of adiponectin and insulin sensitivity is thought to be independent of adiposity levels.34 Thus, the influence of adipokines in bone regulation, particularly adiponectin, may be life cycle-dependent.
The use of robust body composition measures and magnetic resonance imaging (MRI) assessment of BMAT allowed for greater precision beyond that which has been previously used to assess race-related differences in markers of bone turnover and body composition. Although this study generates valuable insight regarding the contemporary bone–fat relationship, limitations must be taken into consideration. Despite the cross-sectional nature of this pilot study, with modest sample sizes in each ethnic and age group, the interesting findings relating to BMAT lay the groundwork for future studies of longer duration and larger sample size. Additionally, in terms of BMAT volume, it must be realized that a statistically significant difference is not necessarily of biological significance; that is, a statistically significant group difference in bone mass may not be transferred to a difference in fracture risk. Further, the independent and interactive effects of adiposity and puberty are difficult to delineate. While the relationship between BMC/D and BMAT in adults may be independent of total body fat, the interplay during growth and development when a large proportion of BMAT conversion occurs has not been fully investigated. However, finding an effect in early-pubertal girls can be regarded as promising in the quest to understand the reciprocal relationship between fat and bone, and the implied bidirectional influence of the bone marrow compartment. Although we examined only the appendicular skeleton, specifically the femur, that of the axial skeleton may also be relevant and warrants investigation. Future studies should include a larger sample size, inclusion of multiple races/ethnicities, as well as inclusion of individuals with a wider range of body habitus.
Whereas in adults the presence of BMAT is associated with impairments in metabolic and skeletal health reminiscent of aging,19,36,41 the timing of conversion of hematopoietic marrow to BMAT is suggestive of physiologic relevance for linear growth, mineralization and osteoblast function. Accordingly, bone-fat interplay has profound implications for developmental origins of health and disease. Fat within bone may acquire different metabolic status dictated by energy metabolism and certain local demands of physiological importance. Our findings revealed a positive relationship between BMAT, fat mass and BMC, although fat mass, respective to leptin, contributed in large part to the relationship between BMAT and BMC. Additionally, despite large differences in total fat mass between non-Hispanic black and white girls, the relationship between fat mass, BMAT and BMC was similar to white girls. However this relationship appeared to be impacted through different mechanisms according to race. Indeed, long-term skeletal and metabolic health is significantly influenced by physiologic changes during the pubertal transition. Accordingly, further investigations are warranted to elucidate the extent to which greater BMAT volume earlier in the prepubertal transition may underlie lifelong interactions among the bone-fat axis.
Materials and methods
Participants
This pilot study represents an ongoing cohort comprised of healthy participants enrolled in clinical studies, including dietary and physical activity interventions, and observational studies aimed at investigating body composition during the pubertal transition42,43 conducted at the University of Alabama at Birmingham (UAB). Participants were recruited through newspaper advertisements, flyers posted at various community partnerships and by word-of-mouth. Irrespective of study enrollment, the present investigation is limited to cross-sectional analyses of baseline data for participants who underwent dual-energy X-ray absorptiometry (DXA) and MRI scans and venipuncture over the period of 2009–2012. The total sample included 59 girls ages 4–10 years (42% non-Hispanic black). All the procedures were approved by the UAB Institutional Review Board and informed consent and assent (where appropriate) were obtained.
Anthropometric assessment
Weight was measured using a digital scale to the nearest 0.1 kg in minimal clothing without shoes. Height was also recorded without shoes using a digital stadiometer. Waist circumference was measured at the narrowest apparent part of the torso between the ribs and iliac crest as described by Lohman et al.44 Waist circumference measures were obtained using a flexible tape measure (Gulick II; Country Technology, Inc., Gays Mills, WI, USA) and recorded to the nearest 0.1 cm.
Body composition
Adipose tissue distribution and body composition were assessed by MRI and DXA, respectively. Whereas DXA measures total tissue content based upon a real estimate, magnetic resonance through delineation and simple signal thresholding allows contrast between tissue compartments, thus providing a more sensitive quantification of fat and bone.
MRI imaging and analysis
Measures for femoral BMAT were acquired using MRI. MRI which involves no ionizing radiation is emerging as a comprehensive tool for fat quantification. For MRI, children were scanned using a Philips 3T system in the UAB Division of Cardiology.45,46 A series of T1-weighted slices (allowing for rapid scans with strong fat-water tissue contrast) were acquired at the upper-leg regions with data acquisition beginning at the iliac crest and continuing to the knee (superior border of the patella).
Scans were analyzed off-site in the laboratory at the University of Southern California using SliceoMatic (Tomovision, Inc., Magog, Quebec, Canada) software. The technique used for analysis has been described elsewhere.47,48 Briefly, the procedure involves the transfer of images to an offline workstation, followed by the use of SliceOMatic software that requires manual intervention.
Dual-energy X-ray absorptiometry
Total body composition (body fat mass and BMC) was measured by DXA using a GE Lunar Prodigy densitometer (GE LUNAR Radiation Corp., Madison, WI, USA). Participants were scanned in light clothing, while lying flat on their backs with arms at their sides. DXA scans were performed and analyzed using pediatric software (enCORE 2002 Version 6.10.029). In our laboratory, the coefficient of variation (CV) for repeated measures of total body fat mass is 6.55%.
Assay of metabolites
The pubertal transition is accompanied by various changes in the metabolic hormonal milieu, which may be disrupted by obesity. Accordingly, the contribution of insulin/glucose homeostasis, lipid profile and circulating adipokines to the relationship between BMAT, BMC and total fat were evaluated. Glucose was measured in 12 μl sera with the glucose oxidase method using a SIRRUS analyzer (inter-assay CV 2.56%). Insulin was analyzed using a TOSOH AIA-600 II Automated Immunoassay Analyzer (TOSOH Bioscience, South San Francisco, CA, USA). Assay sensitivity is 15.42 pmol l−1, mean intra-assay CV is 4.69%, and inter-assay CV is 6.0%. Triglycerides were assessed with the glycerylphosphate method. High-density lipoprotein cholesterol was analyzed using a two-reagent system involving stabilization of low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, and chylomicrons using cyclodextrin and dextrin sulfate, and subsequent enzymatic-colorometric detection of high-density lipoprotein cholesterol. Further, leptin and adiponectin, both integral components of bone remodeling were analyzed by radioimmunoassay as described (ELISA Immuno Assay (EIA); ImmunoDiagnostic Systems (IDS), Fountain Hills, AZ, USA).49,50 Serum 25-hydroxy vitamin D and PTH were assayed either in the UAB Core Laboratory or commercially (Quest Diagnostics, San Juan Capistrano, CA, USA). In the UAB Core Laboratory, serum 25-hydroxy vitamin D was assessed by EIA immunoassay (Immunodiagnostics Systems Inc, Fountain Hills, AZ, USA) and PTH by a two-site immunoradiometric assay. The intra-assay CV for the analysis of PTH and 25-hydroxy vitamin D were 7.76 and 4.83, and the mean inter-assay c.v. were 2.07% and 4.94%, respectively.
Statistical analysis
General linear models were used to evaluate demographic characteristics of the sample population. Multiple linear regression analysis was used to evaluate the relationship between BMAT, body composition and metabolic parameters. All models were adjusted for age, height and race (where appropriate). Statistical significance was set at P<0.05. All data were analyzed using SAS 9.1 software (SAS institute Inc., Cary, NC, USA).
Acknowledgments
This study was supported by Grant no. R00DK8333.
Footnotes
The authors declares no conflicts of interest.
References
- Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressley JC, Kendig TD, Frencher SK, Barlow B, Quitel L, Waqar F. Epidemiology of bone fracture across the age span in blacks and whites. J Trauma 2011;71:S541–S548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Burton AC, Bradburn M, Nielson CM, Orwoll ES, Eastell R. Distribution of bone density in the proximal femur and its association with hip fracture risk in older men: The MrOS study. J Bone Miner Res 2012;27:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock NK, Laing EM, Hamrick MW, Baile CA, Hall DB, Lewis RD. Bone and fat relationships in postadolescent black females: a pQCT study. Osteoporos Int 2011;22:655–665. [DOI] [PubMed] [Google Scholar]
- Backstrom IC, MacLennan PA, Sawyer JR, Creek AT, Rue LWI, Gilbert SR. Pediatric obesity and traumatic lower-extremity long-bone fracture outcomes. J Trauma Acute Care Surg 2012;73:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC et al. Orthopedic complications of overweight in children and adolescents. Pediatrics 2006;117:2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res 2012;27:1–10. [DOI] [PubMed] [Google Scholar]
- Brandi ML. Microarchitecture, the key to bone quality. Rheumatology. (2009;48:iv3–iv8. [DOI] [PubMed]
- Compston J. Bone quality: what is it and how is it measured? Arquivos Brasileiros de Endocrinologia & Metabologia 2006;50:579–585. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone 2004;34:599–604. [DOI] [PubMed] [Google Scholar]
- Seeman E, Delmas PD. Bone quality ΓÇö the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250–2261. [DOI] [PubMed] [Google Scholar]
- Devlin MJ. Why does starvation make bones fat? Am J Hum Biol 2011;23:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res 2010;25:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 2009;122:409–414. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Nuttall ME. The relationship between adipose tissue and bone metabolism. Clin Biochem 2012;45:874–879. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 2009;19:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology 1990;175:219–223. [DOI] [PubMed] [Google Scholar]
- Waitches G, Zawin JK, Poznanski AK. Sequence and rate of bone marrow conversion in the femora of children as seen on MR imaging: are accepted standards accurate? Am J Roentgenol 1994;162:1399–1406. [DOI] [PubMed] [Google Scholar]
- Tuljapurkar SR, McGuire TR, Brusnahan SK, Jackson JD, Garvin KL, Kessinger MA et al. Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J Anatomy 2011;219:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE et al. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab 2012;97:1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 2009;94:2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2006;2:35–43. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 2009;66:236–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di IN, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab 2010;95:2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio S, Greco EA, Fornari R, Donini LM, Lenzi A. Is obesity in women protective against osteoporosis? Diabetes Metab Syndr Obes 2011;4:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D et al. MRI-measured pelvic bone marrow adipose tissue is inversely related to DXA-measured bone mineral in younger and older adults. Eur J Clin Nutr 2012;66:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med 2010;14:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azrad M, Gower B, Hunter G, Nagy T. Racial differences in adiponectin and leptin in healthy premenopausal women. Endocrine 2012. (e-pub ahead of print 15 September 2012; 10.1007/s12020-012-9797-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Motyl KJ. No bones about it: insulin modulates skeletal remodeling. Cell 2010;142:198–200. [DOI] [PubMed] [Google Scholar]
- Casazza K, Goran MI, Gower BA. Associations among insulin, estrogen, and fat mass gain over the pubertal transition in African-American and European-American girls. J Clin Endocrinol Metab 2008;93:2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes 2003;52:1047–1051. [DOI] [PubMed] [Google Scholar]
- Xu L, Wang Q, Wang Q, Lyytikäinen A, Mikkola T, Völgyi E et al. Concerted actions of insulin-like growth factor 1, testosterone, and estradiol on peripubertal bone growth: A 7-year longitudinal study. J Bone Miner Res (2011) 26: 2204–2211 [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Katoh Y, Nakamura K, Itoh S, Iesaki T, Daida H et al. Enhanced accumulation of adipocytes in bone marrow stromal cells in the presence of increased extracellular and intracellular [Ca(2+)]. Biochem Biophys Res Commun 2012;423:672–678. [DOI] [PubMed] [Google Scholar]
- Federico G, Baroncelli GI, Vanacore T, Fiore L, Saggese G. Pubertal changes in biochemical markers of growth. Horm Res 2003;60:46–51. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone 2012;50:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 2012;50:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, Roth CL. A new link between skeletaon, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obes 2010;34:852–858. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT et al. Leptin Inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000;100:197–207. [DOI] [PubMed] [Google Scholar]
- Burguera B, Couce ME. Leptin access into the brain: a saturated transport mechanism in obesity. Physiol Behav 2001;74:717–720. [DOI] [PubMed] [Google Scholar]
- Casazza K, Hanks LJ, Alvarez JA. Role of various cytokines and growth factors in pubertal development. Med Sport Sci. 2010;55:14–31. [DOI] [PubMed] [Google Scholar]
- Wren TAL, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab 2011;96:782–786. [DOI] [PubMed] [Google Scholar]
- Hanks LJ, Newton AL, Casazza K Getting to the height of the matter: the relationship between stature and adiposity in early pubertal children. Ethnicity Dis 2013;23:71–76. [PMC free article] [PubMed] [Google Scholar]
- Casazza K, Cardel M, Dulin-Keita A, Hanks LJ, Gower BA, Newton AL et al. Reduced carbohydrate diet to improve metabolic outcomes and decrease adiposity in obese peripubertal African American girls. J Pediatric Gastroenterol Nutr 2012;54:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman TG, Going SB. Body composition assessment for development of an international growth standard for preadolescent and adolescent children. Food Nutr Bull 2006;27:S314–S325. [DOI] [PubMed] [Google Scholar]
- Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 2007;18:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza K, Hanks LJ, Hidalgo B, Hu HH, Affuso O. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: a pilot study. Bone 2012;50:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity 2010;18:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HH, Nayak KS, Goran MI.. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev 2011;12:e504–e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes 2005;54:2772–2778. [DOI] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Goran MI, Smith A, Kent E. Leptin in postmenopausal women: influence of hormone therapy, insulin, and fat distribution. J Clin Endocrinol Metab 2000;85:1770–1775. [DOI] [PubMed] [Google Scholar]