Abstract
Background
Older patients are at particular risk for adverse drug reactions. In older people, interventions targeting potentially inappropriate prescriptions (PIPs) are considered important measures to minimise drug-related harm, especially in the general practice setting where most prescriptions for older patients are issued.
Aim
To study the effects of a multifaceted educational intervention on GPs’ PIPs for older patients.
Design and setting
This was a cluster randomised, educational intervention study in Norwegian general practice. Pre-study data were captured from January 2005 to December 2005 and post-study data from June 2006 to June 2007. The educational intervention was carried out from January 2006 to June 2006.
Method
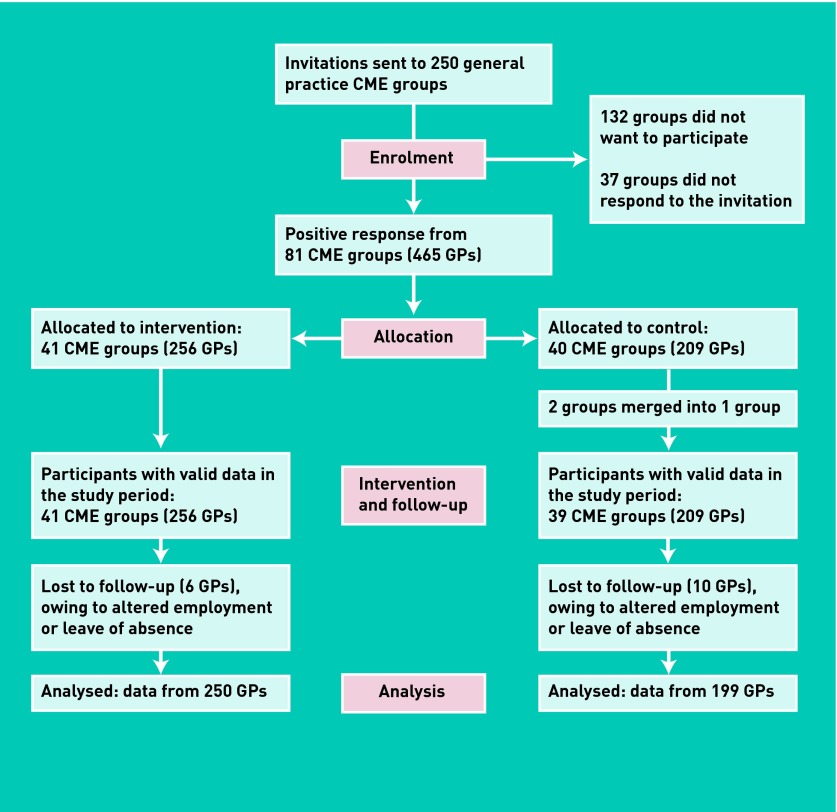
Eighty continuing medical education (CME) groups (465 GPs) were randomised to receive the educational intervention on GPs’ PIPs for older patients (41 CME groups; 256 GPs) or another educational intervention (39 CME groups; 209 GPs); these two groups acted as controls for each other. GPs’ prescription data from before and after the intervention were assessed against a list of 13 explicit PIP criteria for patients aged ≥70 years. In the CME groups, trained GPs carried out an educational programme, including an audit, focusing on the 13 criteria and their rationale.
Results
A total of 449 GPs (96.6%) completed the study; 250 in the intervention group and 199 in the control group. After adjusting for baseline differences and clustering effects, a reduction relative to baseline of 10.3% (95% confidence interval = 5.9 to 15.0) PIPs per 100 patients aged ≥70 years was obtained.
Conclusion
Educational outreach visits with feedback and audit, using GPs as academic detailers in GPs’ CME groups, reduced PIPs for older patients aged ≥70 years in general practice.
Keywords: aged, older people, general practice, inappropriate prescribing, randomised controlled trial, Norway
INTRODUCTION
In older people, adverse drug reactions (ADRs) may be characterised as a modern epidemic commonly impairing peoples’ quality of life, and contributing to about one in 10 hospital admissions.1–5 ADRs cause premature deaths in older people, both in hospitals,6 and in the community.7 The economic burden of ADRs is considerable. In the US, annual hospitalisation costs for ADRs in 2001 were estimated to be $121.8 billion.8 ADRs are commonly caused by inappropriate prescriptions,9 in particular for drugs that are contraindicated or unnecessary.10 A large share of ADRs in older people are preventable.11–14 Interventions targeting potentially inappropriate prescriptions (PIPs) therefore represent important measures to minimise drug-related harm in older people,15 particularly in the general practice setting where the vast majority of prescriptions for older patients are issued. PIPs have been reported to make up between 5% and 34% of all prescriptions to older patients.16–19
Continuing medical education (CME) is essential for maintaining and improving quality in clinical practice,20,21 and may also include various outreach methods like academic detailing. Academic detailing implies a personal visit by a trained person to health professionals in their own setting.22 Typically, it includes assessment of clinicians’ practice against some recommended quality indicators aiming to improve their practice. Academic detailing may be individual visits or group visits of variable duration and may be one out of several components in a multifaceted educational intervention. Academic detailing is found to be superior to, for example, distribution of printed educational materials,23 conferences, or local opinion leaders.24
Studies of educational interventions addressing GPs’ prescribing to older patients are frequently limited to some selected drugs or diagnoses.25
This article from the Prescription Peer Academic Detailing (Rx-PAD) study reports the effects of a multifaceted educational intervention in general practice aiming to reduce the prevalence of a set of listed PIPs for older patients.
METHOD
Participants and setting
In Norway, GP specialists have to renew their specialty every 5 years. Recertification demands participation in a number of peer CME group meetings. Typically, a peer CME group comprises seven or eight GPs who set up their own educational programme for monthly evening meetings. Based on lists provided by the Norwegian Medical Association, peer CME groups in south-eastern parts of Norway (n = 250), were invited by mail to participate in one of two educational interventions. They were informed that the intervention would be decided by randomisation and the two groups would serve as controls for each other. The GPs’ participation would be awarded with CME credits corresponding to a 2-day course.
How this fits in
Most studies from general practice reporting effects of educational interventions to improve physicians’ prescribing quality are limited to a few drugs or therapeutic areas. Reviews have underlined a need for more optimal study designs when assessing the effects of educational interventions. The cluster randomised multifaceted intervention study reported here included about 10% of all GPs in Norway. The intervention, carried out by trained GPs in peer continuous medical education groups, targeted potentially inappropriate prescriptions for older patients across a wide spectrum of therapeutic areas. Significant improvements were obtained for 12 out of 13 predefined explicit criteria for inappropriate prescriptions.
The randomisation was stratified by five geographical regions, in order to reconcile the number of available tutors needed for each arm of the study within each region. Within each stratum, the CME group number and the type of intervention was allocated separately and randomly by staff not engaged in the project. Based on this randomisation, the CME groups were allocated to either the study intervention group or the control group (Figure 1). The study intervention consisted of an educational package on safer prescribing practice for older patients.26 The control groups were assigned to another educational intervention targeting antibiotic prescribing practice for respiratory tract infections.27
Figure 1.
Flow diagram of the trial.
Criteria for inappropriate prescription to older people
Inspired by the US Beers’ criteria,28,29 and recommendations by The Swedish National Board of Health and Welfare,30 a set of 13 explicit PIP criteria, assumed to be relevant for the Norwegian general practice setting, were developed for this study. The criteria comprised 18 different drugs and six drug–drug combinations to be avoided whenever possible for patients aged ≥70 years (Box 1).
Box 1. Thirteen explicit criteria for potentially inappropriate prescriptions used for assessing the appropriateness of GPs’ prescriptions to older patients (≥70 years)
Single drugs
|
Combination of drugs
|
ACE = angiotensin-converting enzyme. ARB = angiotensin receptor blocker. NSAID = non-steroidal antiinflammatory drug. COX = cyclooxygenase. SSRI = selective serotonin reuptake inhibitor.
The educational study intervention
Thirteen GPs were recruited as tutors, denoted as peer academic detailers (PADs). The PADs were either GPs working part time at the Department of General Practice, University of Oslo, or GPs known by the authors from previous CME activities. Each tutor participated in two 2-day pre-study training sessions, focusing on: (1) safety issues in relation to pharmacological treatment in older people; (2) the rationale for the 13 listed PIPs; and (3) how to facilitate learning within a group setting. To avoid conflicts of interests, neither the researchers nor the PADs could have any economic relationships with pharmaceutical companies. With the exception of the statistician, all authors of this paper also served as PADs. Each tutor was responsible for visiting an average of three CME groups.
During a 6-month period (January to June 2006), the CME groups were visited twice by a PAD (Figure 1).
Data-extraction procedures and intervention logistics were piloted in one peer CME group before the intervention, resulting in only minor technical modifications.
Visits in the CME groups, feedback and audit
In the first visit, the main teaching elements of the intervention were presented, including general issues related to drugs in older people. Informed consent was obtained from each GP to get access to their prescription data as recorded in the Norwegian Prescription Database (NorPD).31
Based on baseline data from the period 1 January 2005 to 31 December 2005, individual prescription reports were made and sent to each GP shortly after the first academic detailing visit. The report included individual pre-study (baseline) prevalences of the 13 PIP criteria, as compared to average figures for all participating GPs. The reports also included some suggestions for alternative and safer therapeutic options.
A second academic detailing visit took place about 2 months after the first one and focused on the GPs’ individual prescription patterns in relation to the recommendations given in the first visit and as disclosed in the feedback reports. The PADs facilitated the discussion within the CME group, where each GP exposed their own prescription patterns as presented in his or her report, and potentials for improvements were discussed within the group.
Regional workshop
Three months after the second academic detailing visit, all GPs were gathered at a regional full-day workshop, chaired by two of the authors. Here, rational pharmacological treatment for older patients aged ≥70 years was outlined in more depth, including the evidence for using the 13 explicit criteria as outcome measures in this study.
One year after the intervention, GPs’ post-study prescription data were captured from NorPD for the period 1 July 2006 to 30 June 2007. New personal reports were generated and sent to the participating GPs. Here, improvements in individual GPs’ prescription patterns were presented in comparison to overall averages.
Potentially inappropriate prescribing
PIPs were measured as the number of new instances of inappropriate prescribing for each of the 1-year observation periods according to the list of explicit criteria (Box 1). For each of the single drug criteria (criteria 1 to 7, Box 1), a prescription for that medication within 1 year was defined as one ‘hit’ for the patient. Repeat prescription during the observation period did not add up more hits. For the drug–drug combination criteria (criteria 8 to 12, Box 1), prescription of a listed drug–drug combination within the same month, or estimated concurrent use over a period of 1 month or more, was defined as one ‘hit’. Duration of use was estimated based on prescribed amounts, in terms of defined daily doses (DDDs).32 Concomitant uses of three or more psychotropic drugs (criterion 13, Box 1) was defined as one ‘hit’ when the prescribed amounts (DDDs) of the drugs indicated concurrent use over a period of 3 months or more.
Outcome measures
Primary outcome measures were changes in prescription patterns after the tailored educational intervention according to the listed criteria (Box 1), and as compared with corresponding figures for the control group.
Sample size and statistics
It was assumed that the average number of GPs per CME group was 7.5, the average number of listed patients aged ≥70 years per GP was 165,33 and an intracluster correlation coefficient (ICC) was set to be 0.085, based on a previous Norwegian academic detailing study in general practice.34 The sample size calculation was based on the objective to detect, with 80% power, a clinically significant change in the proportions of patients that were given potentially inappropriate prescriptions, from an assumed proportion of 25% (based on a pilot study involving 13 physicians) to 17%, at a 5% significance level. With an assumed ICC of treatment choices of 0.085, for clustering of patients within peer groups, and an assumed number of 165 patients aged ≥70 years per practice and 7.5 practices per peer group, the total design effect would be 106.1; thus the number of patients needed in each arm of the study after accounting for intracluster correlation was 43 077. Dividing this again among practices and then peer groups, it was calculated that 35 peer groups were required in each trial arm. Allowing for varying group sizes and some withdrawals, it was decided to recruit a total of about 80 peer groups.
RESULTS
A total of 81 CME groups responded positively and were randomised to either an intervention group (41 CME groups) or a control group (40 CME groups). For reasons of feasibility, two small CME groups in the control group were merged into one (Figure 1).
Sixteen GPs (six in the intervention group and 10 in the control group) dropped out, owing to altered employment or leave of absence; 449 GPs (96.5%) completed the trial (250 in the intervention group and 199 in the control group). Characteristics of the participating GPs and their prescriptions to older patients (1 034 034 prescriptions for 81 810 patients before and 1 104 391 for 80 521 after the intervention) are listed in Table 1.
Table 1.
Characteristics of participating continous medical education (CME) groups and GPs
| Characteristics | Intervention group | Control group |
|---|---|---|
| CME groups, n | 41 | 39 |
|
| ||
| GPs participating, n | 250 | 199 |
|
| ||
| GPs’ sex, % male | 70 | 67 |
|
| ||
| GP specialist, % | 89 | 82 |
|
| ||
| GPs in urban practice, % | 52 | 56 |
|
| ||
| GPs’ age, mean years | 51 | 49 |
|
| ||
| Patients aged ≥70 years receiving any prescriptions, n | ||
| Before intervention | 46 737 | 35 073 |
| After intervention | 45 310 | 35 211 |
|
| ||
| Prescriptions to patients aged ≥70 years, n | ||
| Before intervention | 598 492 | 435 542 |
| After intervention | 626 690 | 477 701 |
Effects of the intervention
Unadjusted pre- and post-study prescriptions to the more than 80 000 patients aged ≥70 years are presented in Table 2. For all indicators except ‘concomitant use of three or more psychotropic drugs’, the reduction of PIPs was apparent in the intervention group.
Table 2.
Potentially inappropriate prescriptions (PIPs) issued by 449 GPs to 81 810 patients aged ≥70 years before and 80 521 patients after an educational intervention
| Potentially inappropriate prescriptions | Intervention group, number of prescriptions | Control group, number of prescriptions | Change, % (95% CI) | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Patients aged ≥70 years issued a prescription | 46 737 | 45 310 | 35 073 | 35 211 | |
| Total number of PIPs | 12 750 | 10 149 | 9048 | 8521 | −12.0 (−12.6 to −11.48) |
| Tricyclic antidepressants | 1136 | 900 | 757 | 750 | −17.1 (−19.3 to −14.9) |
| Amitryptyline | 604 | 452 | 391 | 404 | −25.3 (−28.8 to −21.8) |
| Doxepin | 146 | 123 | 107 | 96 | −2.7 (−5.3 to −0.1) |
| Trimipramine | 312 | 265 | 216 | 204 | − 6.9 (−9.7 to −4.1) |
| Clomipramine | 74 | 60 | 43 | 46 | −21.4 (30.7 to 12.1) |
| Antihistamines | 1277 | 963 | 877 | 875 | −21.6 (−23.9 to −12.4) |
| Dexchlorpheniramine | 188 | 86 | 134 | 119 | −41.8 (−48.8 to −34.7) |
| Promethazine | 86 | 78 | 88 | 88 | −5.9 (−10.9 to− 0.9) |
| Alimemazine | 562 | 463 | 379 | 396 | −18.7 (−21.9 to −15.5) |
| Hydroxyzine | 441 | 336 | 276 | 272 | −19.9 (−23.6 to −16.2) |
| Antipsychotics | 767 | 603 | 420 | 455 | −24.7 (−27.7 to −21.7) |
| Chlorpromazine | 67 | 43 | 45 | 35 | −13.6 (−21.8 to −5.4) |
| Chlorprotixene | 155 | 136 | 104 | 126 | −28.0 (−35.1 to −20.9) |
| Levomepromazine | 545 | 424 | 271 | 294 | −25.1 (−28.7 to −21.5) |
| Long-acting benzodiazepines | 2312 | 1716 | 1763 | 1461 | −5.7 (−6.7 to −4.7) |
| Nitrazepam | 1834 | 1378 | 1348 | 1142 | −10.1 (−11.5 to −8.7) |
| Flunitrazepam | 478 | 338 | 415 | 319 | 0.5 (−0.1 to 1.1) |
| Carisoprodol | 451 | 316 | 370 | 284 | −2.0 (−3.3 to −0.7) |
| Strong analgesics | 680 | 494 | 337 | 258 | −9.4 (−11.6 to −7.2) |
| Propoxyphene | 604 | 423 | 312 | 226 | −8.6 (−10.8 to −6.4) |
| Pethidine | 42 | 49 | 11 | 19 | −4.8 (−11.3 to 1.7) |
| Opioid + spasmolytic | 34 | 22 | 14 | 13 | −29.1 (−44.4 to −13.8) |
| Theophylline, tablets | 284 | 196 | 205 | 143 | 0.5 (−0.3 to 1.3) |
| Beta-blocker + unselective calcium antagonist | 166 | 133 | 123 | 111 | −7.4 (−11.4 to −3.4) |
| NSAID + warfarin | 362 | 211 | 208 | 188 | −32.2 (−36.6 to −32.2) |
| NSAID + ACE inhibitor | 1841 | 1565 | 1289 | 1194 | −5.1 (−6.1 to −4.1) |
| NSAID + SSRI | 434 | 299 | 310 | 270 | −16.3 (−19.8 to −12.8) |
| NSAID + diuretic | 1588 | 1255 | 1384 | 1350 | −15.2 (−17.0 to −13.4) |
| Concomitant use of ≥3 psychotropic drugs | 1244 | 1288 | 990 | 1049 | 0.9 (0.4 to 1.4) |
ACE = angiotensin-converting enzyme. NSAID = non-steroidal antiinflammatory drug. SSRI = selective serotonin reuptake inhibitor.
Adjusted for changes in the control group, the share of all prescriptions that were considered to be potentially inappropriate went down by 13% relative to baseline (0.3% in absolute terms, meaning the difference between rates before and after the intervention). Prior to the intervention, 9278 out of 46 737 patients in the intervention group received one or more PIP. This was reduced to 7655 out of 45 310 (Table 3). Adjusted for both baseline differences and intra-cluster effects, the prevalence of PIPs was reduced by 3.3% or 12.1% relative to baseline (Table 4). The largest reductions as compared with baseline were seen for drugs with strong anticholinergic properties: tricyclics, ‘old’ antihistamines and ‘old’ antipsychotics (18.9%), and for the combination of a non-steroidal antiinflammatory drug with warfarin (33.3%) (Table 4).
Table 3.
Effects of an educational intervention in general practice on the number of older patients being exposed to ≥PIP, mean number of PIPs per 100 older patients, and mean number of PIPs per 100 prescriptions
| Group | Patients (%) exposed to one or more PIPs | PIPs per 100 patients (95% CI)a | PIPs per 100 prescriptionsb (95% CI) |
|---|---|---|---|
| Intervention group | |||
| Baseline | 9278 (19.9) | 27.3 (24.9 to 29.6) | 2.3 (2.2 to 2.4) |
| Follow-up | 7655 (16.9) | 22.4 (21.2 to 23.8) | 1.8 (1.7 to 1.9) |
| Reduction | −1623 (−3.0) | −4.9 (−5.8 to − 3.9) | −0.5 (−0.6 to −0.4) |
|
| |||
| Control group | |||
| Baseline | 6427 (18.6) | 25.8 (23.4 to 28.1) | 2.2 (2.0 to 2.2) |
| Follow-up | 5977 (17.2) | 24.2 (22.8 to 25.6) | 1.9 (1.8 to 2.0) |
| Reduction | −450 (−1.4) | −1.6 (−2.6 to −0.6) | −0.3 (−0.4 to −0.2) |
|
| |||
| Change due to interventionc | |||
| Absolute | −1173 (−1.6) | −3.3 (−4.6 to −1.9) | −0.3 (−0.4 to−0.2) |
| Relative (%) | −8.1 | −12.1 (−16.8 to −6.9) | −13.0 (−17.3 to −8.6) |
PIP = potentially inappropriate prescription.
Adjusted for baseline differences and intracluster effect.
Total number of prescriptions issued for patients aged ≥70 years during baseline and follow-up periods, respectively.
Absolute change denotes differences between figures from before and after the intervention, while relative change represents this difference relative to the baseline figures.
Table 4.
Prevalence of potentially inappropriate prescriptions (PIPs) for patients aged ≥70 years at baseline by the various PIP criteria and changed prescribing due to the educational intervention
| Intervention group, n = 250 GPs | Control group, n = 199 GPs | Changes due to the interventionb | ||||
|---|---|---|---|---|---|---|
| Absolute change, | Relative change, | |||||
|
| ||||||
| Baseline % | Change %a | Basline % | Change %a | % (95% CI) | % (95% CI) | |
| Tricyclic antidepressants | 2.4 | −0.4 | 2.1 | 0.0 | −0.4 (−0.8 to −0.0) | −16.7 (−32.8 to 0.0) |
| Antihistamines | 2.6 | −0.4 | 2.4 | 0.0 | −0.4 (−0.9 to 0.1) | −15.3 (−34.5 to 3.8) |
| Antipsychotics | 2.9 | −0.8 | 2.5 | 0.0 | −0.7 (−1.2 to −0.3) | −24.1 (−41.3 to −10.3) |
| Long-acting benzodiazepines | 4.7 | −1.2 | 4.7 | −0.7 | −0.4 (−1.1 to 0.2) | −8.5 (−23.4 to 4.3) |
| Carisoprodol | 1.0 | −0.3 | 1.1 | −0.3 | −0.1 (−0.3 to 0.2) | −10.0 (−30.0 to 20.0) |
| Strong analgesics | 1.4 | −0.4 | 0.9 | −0.2 | −0.2 (−0.5 to 0.1) | −14.3 (−35.7 to 7.1) |
| Theophylline by mouth | 0.5 | −0.1 | 0.6 | −0.2 | 0.1 (−0.1 to 0.2) | 20.0 (−20.0 to 40.0) |
| Beta-blocker + verapamil or diltiazem | 0.3 | −0.1 | 0.3 | −0.1 | 0.0 (−0.3 to 0.1) | − |
| NSAID + warfarin | 0.6 | −0.3 | 0.5 | −0.0 | −0.2 (−0.4 to −0.1) | −33.3 (−66.6 to −16.7) |
| NSAID + ACE inhibitorc | 3.5 | −0.4 | 3.5 | −0.2 | −0.3 (−0.8 to 0.3) | −8.5 (−15.44 to 5.8) |
| NSAID + SSRI | 0.8 | −0.2 | 0.9 | −0.1 | −0.1 (−0.3 to 0.1) | −12.5 (−37.5 to 0.0) |
| NSAID + diuretic | 3.9 | −0.5 | 3.8 | −0.0 | −0.5 (−1.1 to 0.1) | −12.8 (−28.2 to 2.6) |
| ≥3 psychotropic psychotropic drugsd | 2.5 | 0.2 | 2.7 | 0.2 | 0.1 (−0.4 to 0.2) | −4.0 (−16.0 to 8.0) |
| Total PIPs per 100 patients aged ≥70 years | 27.3 | −4.9 | 25.8 | −1.6 | −3.3 (−5.6 to −0.9) | −12.1 (−16.8 to−6.9) |
ACE = angiotensin-converting enzyme. COX = cyclooxygenase. NSAID = non-steroidal antiinflammatory drug. SSRI = selective serotonin reuptake inhibitor.
The changes of prescribing are adjusted for baseline differences and clustering effects.
Absolute change denotes the difference between proportions before and after the intervention, while relative change denotes the absolute change as % of baseline proportion. Figures are adjusted for baseline differences and clustering effects on the peer CME group level.
Also including COX-2 inhibitors and angiotensin receptor blockers.
Concurrent use of three or more of opiate-containing analgesics, hypnotics, tranquillisers, antipsychotics, or antidepressants.
Intracluster correlation coefficient
With regard to the total PIP rates, the estimated ICCs for baseline data were 0.26 (95% confidence interval [CI] = 0.15 to 0.42) for the intervention groups and 0.23 (95% CI = 0.12 to 0.41) for the controls. For the post-intervention data, the ICCs were 0.21 (95% CI = 0.10 to 0.38) (intervention group) and 0.20 (95% CI = 0.09 to 0.37) (controls). When including both intervention/control and pre/post intervention as explanatory variables, the estimated residual ICC was 0.27 (95% CI = 0.20 to 0.36).
From a covariance analysis including the baseline PIP rates as the explanatory variable, the residual ICCs for the post-intervention data were 0.025 (95% CI = 0.001 to 0.500) for the intervention arm and 0.059 (95% CI = 0.001 to 0.300) for the control arm. When the intervention group was included as a covariate (intervention versus controls), the estimated residual ICC became 0.045 (95% CI = 0.009 to 0.190).
DISCUSSION
Summary
The multifaceted educational intervention in peer CME groups in this study resulted in fewer PIPs for older patients. Even if moderate, these reductions in inappropriate prescribing may be clinically important at a population level. The 12.1% reduction in PIPs to older patients observed is considered to be clinically relevant.35 In other words, about one in eight inappropriate prescriptions were avoided, which here corresponds to about 1600 patients who were no longer or less exposed to PIPs.
Strengths and limitations
This was a large study including approximately one-tenth of all Norwegian GPs. This, combined with an extraordinarily high completion rate (96.5%), a strong clinical relevance experienced by the participating GPs,36 and the completeness of the prescription data, all strengthen the study results. The fact that an intervention effect was observed for almost all outcome measures contributes further to the validity of the results.
The average CME groups in the control arm included fewer GPs than those in the trial arm. This was mostly caused by some extraordinarily large CME groups randomised to the intervention. This happened by coincidence and did not influence the results. Thirty-two per cent of the invited peer CME groups agreed to participate. To reveal possible differences in prescribing practice between those who agreed to participate and those who did not, a comparison was made between the participating GPs and all other Norwegian GP specialists. No significant differences in their prescribing practice were revealed.37
Therefore, the authors believe that the participating GPs were fairly representative of the Norwegian population of GPs in general. Using GPs as PADs possibly had a positive effect on both GPs’ participation and their perceptions of the relevance of the intervention. Focus group interviews with GPs and PADs after the intervention revealed a positive attitude among GPs to receiving advice and guidance from fellow GPs.36 The PADs did not consider themselves to be experts, but rather as ‘members of the same family’, and this, combined with their independence from both the pharmaceutical industry and health authorities, was probably important in obtaining GPs’ trust and acceptance. The extra benefits gained by using GPs instead of non-physicians as academic detailing visitors have also been reported in a Dutch study.38
GPs in the control group also improved their prescribing practice for older patients; this may partly be due to a Hawthorne effect in response to the fact that they knew they were being studied.39 The design of this study and others with a similar design may thus underestimate the effects of the intervention.
The effect of the intervention is likely to be influenced by the choice of PIP criteria, not only because of the different prevalences of the various criteria, but maybe also as a result of different levels of agreement among the GPs, in relation to the criteria. For example, the proportion of GPs prescribing tricyclic antidepressants went down by 16.7% (0.4% in absolute terms), while no corresponding effect was seen on simultaneous prescription of three or more different psychotropic drugs. Less effect on the latter may also reflect an expanding market for newer psychopharmaceutical drugs, but also that for some drugs, or in some contexts, barriers to improved prescribing may be more difficult to identify and overcome.
The ICC accounts for the relatedness of clustered data by comparing the variance within clusters with the variance between clusters.40 Based on another Norwegian cluster randomised educational intervention trial in general practice,34 the sample size needed for this trial was calculated based on the ICC factor (0.085) of the earlier trial. However, that study was randomised at the level of GP practices and focused on antibiotic prescribing for urinary tract infections, which is a clinical procedure that is likely to be standardised at practice level. Therefore, it is not surprising that a lower ICC (0.045) was found in the present study, which also implied that the sample size was more than sufficient.
Comparison with existing literature
No other studies with a comparable design and based in general practice have been found that investigate the effects of a broad spectrum of PIP criteria, as seen in this study. A review from 2009 included 24 intervention studies on reducing inappropriate prescriptions to older patients.25 Only four studies were educational interventions, two of which targeted limited therapeutic areas such as reduction of prescribing long-acting benzodiazepines to older people,41 or of inappropriate prescribing of proton pump inhibtors.42 Only one study examined the effect of interventions on overall prescribing quality,43 but the study lacked a control group. Owing to its size (large numbers of participating GPs) and scope (addressing prescribing quality across multiple therapeutic areas), the present study represents an important contribution to the knowledge of educational interventions in general practice.
Implications for research and practice
In a three-round Delphi process among Norwegian specialists in clinical pharmacology, geriatrics and general practice, the PIP criteria used in this trial have subsequently been rated along with 23 other criteria to be ‘clinically highly relevant’.41 The output of that exercise was the Norwegian General Practice criteria for assessing PIPs to older patients.44 An updated version of the Beers’ criteria has been published recently,45 illustrating the continuous need for updating the PIP criteria.
However, it is not known how the intervention effects found in this study will progress. A long-term follow-up is therefore planned. Furthermore, the effects reported here are averages for a large sample of GPs, some of whom may have changed their own prescribing for older patients substantially, while others may not have done so. Therefore, it will be relevant to identify factors predicting different effect sizes. This will be explored in more detail in a forthcoming paper. Studies including clinical patient outcomes related to interventions towards PIPs are also required, as well as health-economic analyses assessing cost–benefit issues related to this kind of intervention.
Acknowledgments
We thank the peer academic detailers and all the participating GPs, with special thanks to Svein Gjelstad for data file management. Finally, we thank the Norwegian Ministry of Health, the Norwegian Medical Association, and the Research Council of Norway for making this study possible.
Funding
The study was carried out with grants from the Norwegian Medical Association, the Norwegian Ministry of Health and the Research Council of Norway.
Ethical approval
Extraction of data from the Norwegian Prescription Database was based on written, informed consent from all physicians. The regional committee approved the project for research ethics and the Norwegian Social Science Data Service. The Directorate for Health and Social Affairs accepted a dispensation from the Health-Professional Secrecy regulations. However, all patient data were anonymised before analyses. The study was approved by the Regional Ethics Committee (region South) in October 2005 (reference S-05272), and by the Norwegian Social Science Data Services in October 2005 (reference 200500838 SM/RH).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Gosney M, Tallis R. Prescription of contraindicated and interacting drugs in elderly patients admitted to hospital. Lancet. 1984;2(8402):564–567. doi: 10.1016/s0140-6736(84)90775-x. [DOI] [PubMed] [Google Scholar]
- 2.Hallas J. Drug related hospital admissions in subspecialities of internal medicine. Dan Med Bull. 1996;43(2):141–155. [PubMed] [Google Scholar]
- 3.Mjorndal T, Boman MD, Hagg S, et al. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002;11(1):65–72. doi: 10.1002/pds.667. [DOI] [PubMed] [Google Scholar]
- 4.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneeweiss S, Hasford J, Gottler M, et al. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol. 2002;58(4):285–291. doi: 10.1007/s00228-002-0467-0. [DOI] [PubMed] [Google Scholar]
- 6.Buajordet I, Ebbesen J, Erikssen J, et al. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250(4):327–341. doi: 10.1046/j.1365-2796.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- 7.Wester K, Jonsson AK, Spigset O, et al. Incidence of fatal adverse drug reactions: a population based study. Br J Clin Pharmacol. 2008;65(4):573–579. doi: 10.1111/j.1365-2125.2007.03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001;41(2):192–199. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- 9.Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116(6):394–401. doi: 10.1016/j.amjmed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Lindley CM, Tully MP, Paramsothy V, et al. Inappropriate medication is a major cause of adverse drug reactions in elderly patients. Age Ageing. 1992;21(4):294–300. doi: 10.1093/ageing/21.4.294. [DOI] [PubMed] [Google Scholar]
- 11.Atkin PA, Veitch PC, Veitch EM, et al. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging. 1999;14(2):141–152. doi: 10.2165/00002512-199914020-00005. [DOI] [PubMed] [Google Scholar]
- 12.Goettler M, Schneeweiss S, Hasford J. Adverse drug reaction monitoring--cost and benefit considerations. Part II: cost and preventability of adverse drug reactions leading to hospital admission. Pharmacoepidemiol Drug Saf. 1997;6(suppl 3):S79–S90. doi: 10.1002/(sici)1099-1557(199710)6:3+<s79::aid-pds294>3.3.co;2-f. [DOI] [PubMed] [Google Scholar]
- 13.Howard R, Avery A, Bissell P. Causes of preventable drug-related hospital admissions: a qualitative study. Qual Saf Health Care. 2008;17(2):109–116. doi: 10.1136/qshc.2007.022681. [DOI] [PubMed] [Google Scholar]
- 14.Howard RL, Avery AJ, Slavenburg S, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63(2):136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmader KE, Hanlon JT, Landsman PB, et al. Inappropriate prescribing and health outcomes in elderly veteran outpatients. Ann Pharmacother. 1997;31(5):529–533. doi: 10.1177/106002809703100501. [DOI] [PubMed] [Google Scholar]
- 16.Aparasu RR, Sitzman SJ. Inappropriate prescribing for elderly outpatients. Am J Health Syst Pharm. 1999;56(5):433–439. doi: 10.1093/ajhp/56.5.433. [DOI] [PubMed] [Google Scholar]
- 17.Brekke M, Rognstad S, Straand J, et al. Pharmacologically inappropriate prescriptions for elderly patients in general practice: how common? Baseline data from The Prescription Peer Academic Detailing (Rx-PAD) study. Scand J Prim Health Care. 2008;26(2):80–85. doi: 10.1080/02813430802002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyborg G, Straand J, Brekke M. Inappropriate prescribing for the elderly--a modern epidemic? Eur J Clin Pharmacol. 2012;68(7):1085–1094. doi: 10.1007/s00228-012-1223-8. [DOI] [PubMed] [Google Scholar]
- 19.Straand J, Rokstad KS. Elderly patients in general practice: diagnoses, drugs and inappropriate prescriptions. A report from the More & Romsdal Prescription Study. Fam Pract. 1999;16(4):380–388. doi: 10.1093/fampra/16.4.380. [DOI] [PubMed] [Google Scholar]
- 20.Brown CA, Belfield CR, Field SJ. Cost effectiveness of continuing professional development in health care: a critical review of the evidence. BMJ. 2002;324(7338):652–655. doi: 10.1136/bmj.324.7338.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughn HT, Rogers JL, Freeman JK. Does requiring continuing education units for professional licensing renewal assure quality patient care? Health Care Manag (Frederick) 2006;25(1):78–84. doi: 10.1097/00126450-200601000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549–556. [PubMed] [Google Scholar]
- 23.Farmer AP, Legare F, Turcot L, et al. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2008;(3):CD004398. doi: 10.1002/14651858.CD004398.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Flodgren G, Parmelli E, Doumit G, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011;(8):CD000125. doi: 10.1002/14651858.CD000125.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur S, Mitchell G, Vitetta L, et al. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging. 2009;26(12):1013–1028. doi: 10.2165/11318890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Straand J, Fetveit A, Rognstad S, et al. A cluster-randomized educational intervention to reduce inappropriate prescription patterns for elderly patients in general practice — The Prescription Peer Academic Detailing (Rx-PAD) study [ NCT00281450] BMC Health Serv Res. 2006;6:72. doi: 10.1186/1472-6963-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gjelstad S, Fetveit A, Straand J, et al. Can antibiotic prescriptions in respiratory tract infections be improved? A cluster-randomized educational intervention in general practice — the Prescription Peer Academic Detailing (Rx-PAD) Study [ NCT00272155] BMC Health Serv Res. 2006;6:75. doi: 10.1186/1472-6963-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med. 1991;151(9):1825–1832. [PubMed] [Google Scholar]
- 29.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157(14):1531–1536. [PubMed] [Google Scholar]
- 30.Swedish National Board of Health and Welfare [Socialstyrelsen] Indicators for evaluation of the quality of drug therapy in the elderly. 2003. In Swedish. Report 2003-110-20. http://www.socialstyrelsen.se/publikationer2003/2003-110-20 (accessed 4 Jul 2013).
- 31.Norwegian Prescription Database. www.fhi.no (accessed 29 May 2013).
- 32.WHO Collaborating Centre for Drugs Statistics Methodology Definition and general considerations [definition of the defined daily dose] http://www.whocc.no/ddd/definition_and_general_considera/ (accessed 28 May 2013).
- 33.The Norwegian Labour and Welfare Service Statistics for The Regular General Practionar Sceme(GP Scheme) in Norway. http://www.nav.no/Om+NAV/Tall+og+analyse/Annen+statistikk/Helsetjenester/FastlegeordningenT (accessed 29 May 2013).
- 34.Flottorp S, Oxman AD, Havelsrud K, et al. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ. 2002;325(7360):367. doi: 10.1136/bmj.325.7360.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higashi T, Shekelle PG, Adams JL, et al. Quality of care is associated with survival in vulnerable older patients. Ann Intern Med. 2005;143(4):274–281. doi: 10.7326/0003-4819-143-4-200508160-00008. [DOI] [PubMed] [Google Scholar]
- 36.Frich JC, Hoye S, Lindbaek M, et al. General practitioners and tutors’ experiences with peer group academic detailing: a qualitative study. BMC Fam Pract. 2010;11:12. doi: 10.1186/1471-2296-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gjelstad S, Straand J, Dalen I, et al. Do general practitioners’ consultation rates influence their prescribing patterns of antibiotics for acute respiratory tract infections? J Antimicrob Chemother. 2011;66(10):2425–2433. doi: 10.1093/jac/dkr295. [DOI] [PubMed] [Google Scholar]
- 38.Van den Hombergh P, Grol R, van den Hoogen HJ, et al. Practice visits as a tool in quality improvement: mutual visits and feedback by peers compared with visits and feedback by non-physician observers. Qual Health Care. 1999;8(3):161–166. doi: 10.1136/qshc.8.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarney R, Warner J, Iliffe S, et al. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Ann Fam Med. 2004;2(3):204–208. doi: 10.1370/afm.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Midlov P, Bondesson A, Eriksson T, et al. Effects of educational outreach visits on prescribing of benzodiazepines and antipsychotic drugs to elderly patients in primary health care in southern Sweden. Fam Pract. 2006;23(1):60–64. doi: 10.1093/fampra/cmi105. [DOI] [PubMed] [Google Scholar]
- 42.Batuwitage BT, Kingham JG, Morgan NE, et al. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007;83(975):66–68. doi: 10.1136/pgmj.2006.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wessell AM, Nietert PJ, Jenkins RG, et al. Inappropriate medication use in the elderly: results from a quality improvement project in 99 primary care practices. Am J Geriatr Pharmacother. 2008;6(1):21–27. doi: 10.1016/j.amjopharm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Rognstad S, Brekke M, Fetveit A, et al. The Norwegian General Practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients. Scand J Prim Health Care. 2009;27(3):153–159. doi: 10.1080/02813430902992215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Geriatrics Society 2012 Beers Criteria Update Expert Panel American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]