Abstract
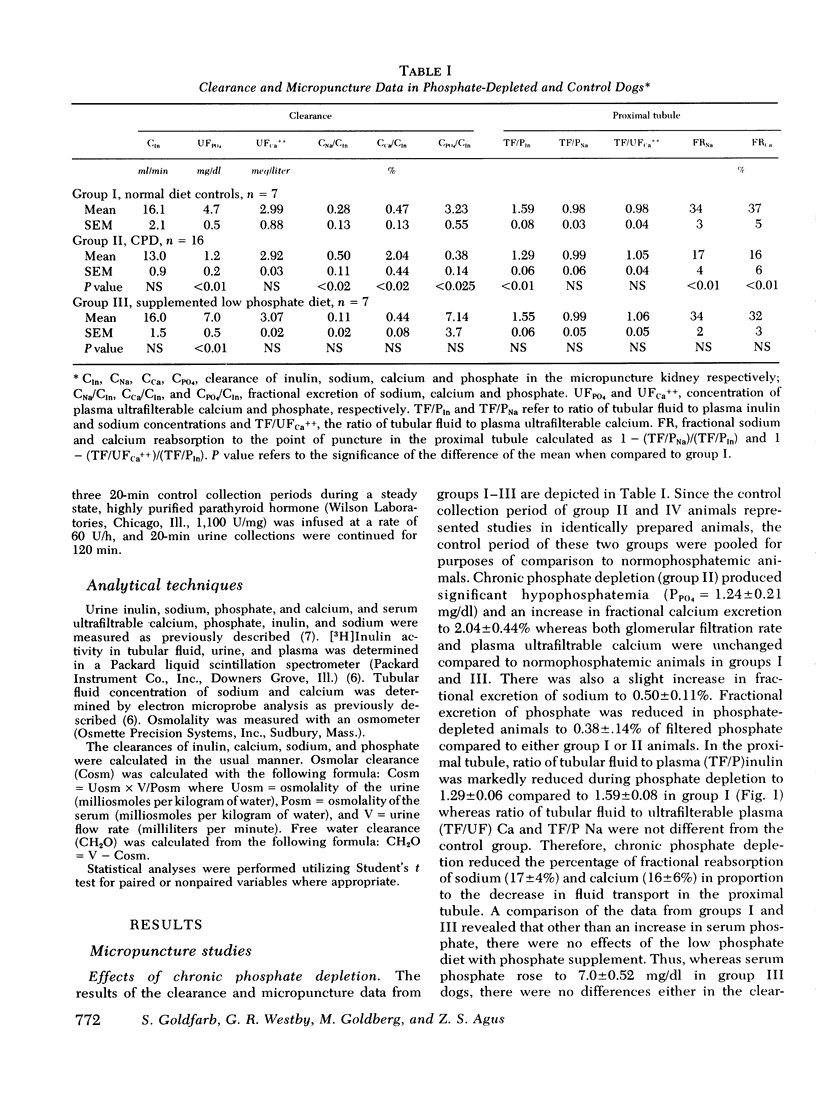
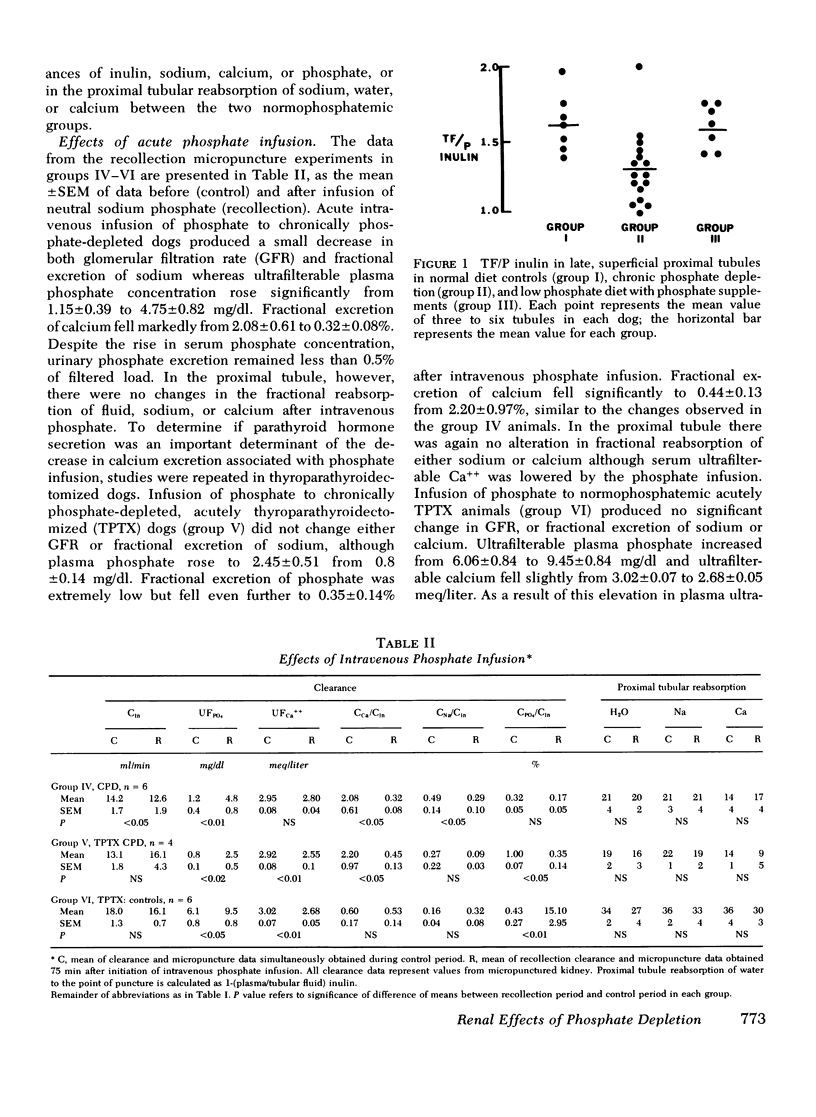
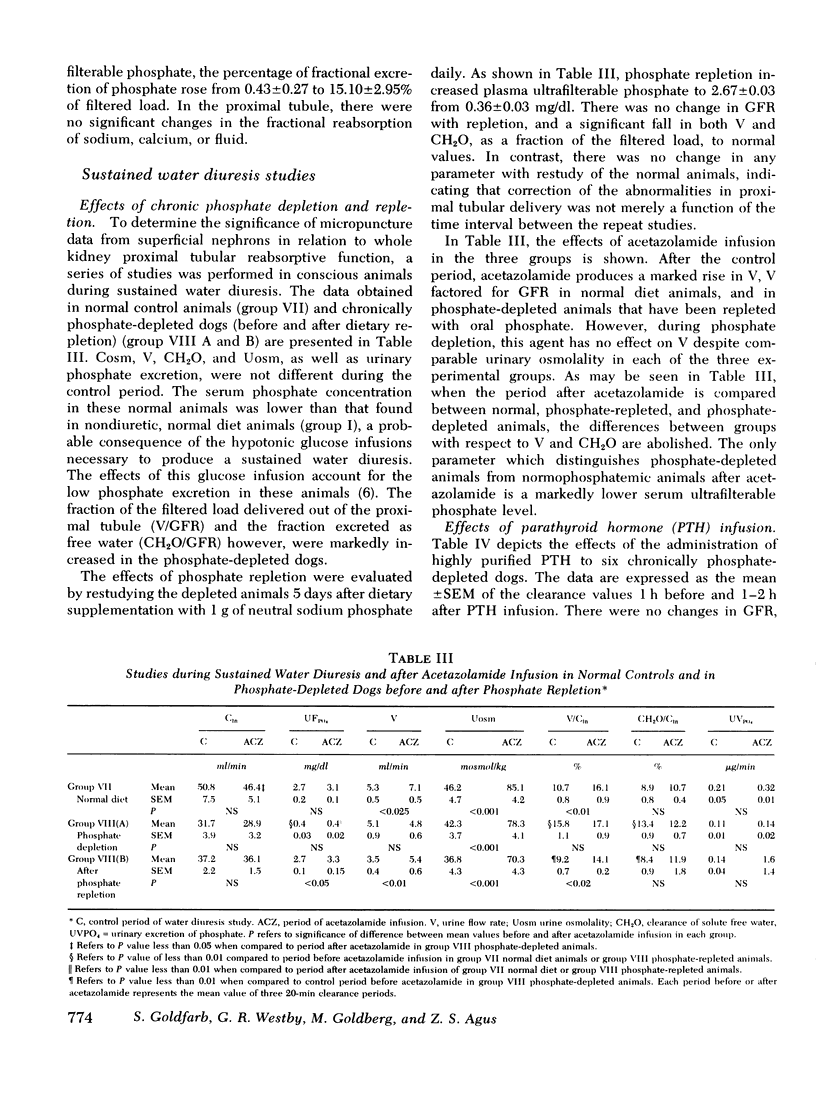
The effects of chronic phosphate depletion on renal tubular function were evaluated by micropuncture and free water clearance studies in the dog. Proximal tubular punctures demonstrated that chronic hypophosphatemia led to a reduction in ratio of tubular fluid to plasma inulin in late superficial tubular from 1.59+/-0.08 in control animals to 1.29+/-0.06 in phosphate-depleted dogs, with proportional inhibition of calcium and sodium reabsorption. The chronic decrease in proximal tubular fluid reabsorption was confirmed by the analysis of sustained water diuresis in conscious, phosphate-depleted dogs, before and after repletion of body PO4 stores, and in control animals. Urine flow rate/100 ml glomerular filtration rate (V/GFR) was significantly higher in PO4 DEPLETION THAN CONTROL (15.8+/-1.1 VS. 10.7+/-0.82). In addition, acetazolamide infusion did not increase V/GFR in phosphate-depleted dogs (15.8+/-1.1 vs. 17.16+/-0.9), supporting the conclusion that inhibition of proximal tubular fluid reabsorption was responsible for the elevated urine flow rate. PO4 repletion over 5 days reduced V/GFR to 9.2+/-0.7 despite no change in urine osmolality and no change in GFR, further suggesting a specific reversible alteration in proximal tubular reabsorption in phosphate depletion. Although hypercalciuria was a constant finding in phosphate depletion (fractional excretion of calcium of 2.04+/-0.4% vs. 0.47+/-0.13% in controls), the enhanced distal delivery of calcium was not a crucial factor; acute phosphate infusion reduced urinary calcium excretion to control values without affecting the reduced proximal tubular reabsorption in either intact or thyroparathyroidectomized phosphate-depleted dogs the change in distal nephron calcium reabsorption was independent of parathyroid hormone (PTH) levels since infusion of PTH failed to alter urinary calcium excretion. We conclude that chronic phosphate depletion leads to a reversible, sustained inhibition in proximal tubular reabsorptive fuction as well as a specific decrease in distal nephron calcium reabsorption. This latter reabsorptive defect is sensitive to phosplate infusion but not corrected by PTH.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Puschett J. B., Senesky D., Goldberg M. Mode of action of parathyroid hormone and cyclic adenosine 3',5'-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest. 1971 Mar;50(3):617–626. doi: 10.1172/JCI106532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, McCurdy D. K., Ludwig G. D., Chaykin L. B., Elkinton J. R. Incomplete renal tubular acidosis. Physiologic studies in three patients with a defect in lowering urine pH. Am J Med. 1968 Jul;45(1):32–42. doi: 10.1016/0002-9343(68)90005-3. [DOI] [PubMed] [Google Scholar]
- Coburn J. W., Hartenbower D. L., Massry S. G. Modification of calciuretic effect of extracellular volume expansion by phosphate infusion. Am J Physiol. 1971 Feb;220(2):377–383. doi: 10.1152/ajplegacy.1971.220.2.377. [DOI] [PubMed] [Google Scholar]
- Coburn J. W., Massry S. G. Changes in serum and urinary calcium during phosphate depletion: studies on mechanisms. J Clin Invest. 1970 Jun;49(6):1073–1087. doi: 10.1172/JCI106323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Yawata Y., VanSanten L., Gilberstadt S., Silvis S., Jacob H. S. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N Engl J Med. 1974 Jun 20;290(25):1403–1407. doi: 10.1056/NEJM197406202902504. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett M., Goldfarb S., Agus Z. S., Narins R. G. The pathophysiology of acid-base changes in chronically phosphate-depleted rats: bone-kidney interactions. J Clin Invest. 1977 Feb;59(2):291–298. doi: 10.1172/JCI108640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller T. J., Carter N. W., Barcenas C., Knochel J. P. Reversible changes of the muscle cell in experimental phosphorus deficiency. J Clin Invest. 1976 Apr;57(4):1019–1024. doi: 10.1172/JCI108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. W., Massry S. G., Arieff A. I., Coburn J. W. Renal bicarbonate wasting during phosphate depletion. A possible cause of altered acid-base homeostasis in hyperparathyroidism. J Clin Invest. 1973 Oct;52(10):2556–2561. doi: 10.1172/JCI107447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter H. R., Mercado A., Rutherford W. E., Rodriguez H., Slatopolsky E., Klahr S. Effects of phosphate depletion and parathyroid hormone on renal glucose reabsorption. Am J Physiol. 1974 Dec;227(6):1422–1427. doi: 10.1152/ajplegacy.1974.227.6.1422. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Amsden T. Acute hemolytic anemia with rigid red cells in hypophosphatemia. N Engl J Med. 1971 Dec 23;285(26):1446–1450. doi: 10.1056/NEJM197112232852602. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R., Kokko J. P. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976 Apr;57(4):818–825. doi: 10.1172/JCI108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klock J. C., Williams H. E., Mentzer W. C. Hemolytic anemia and somatic cell dysfunction in severe hypophosphatemia. Arch Intern Med. 1974 Aug;134(2):360–364. [PubMed] [Google Scholar]
- Kurtzman N. A., White M. G., Rogers P. W., Flynn J. J., 3rd Relationship of sodium reabsorption and glomerular filtration rate to renal glucose reabsorption. J Clin Invest. 1972 Jan;51(1):127–133. doi: 10.1172/JCI106782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemann J., Litzow J. R., Lennon E. J. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967 Aug;46(8):1318–1328. doi: 10.1172/JCI105624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman M. A., Miller D. R., Cohen J., Waterhouse C. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med. 1971 Apr;74(4):562–568. doi: 10.7326/0003-4819-74-4-562. [DOI] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Seldin D. W., Rosin J. M., rector F. C., Jr Evidence against bicarbonate reabsorption in the ascending limb, particularly as disclosed by free-water clearance studies. Yale J Biol Med. 1975 Sep;48(4):337–347. [PMC free article] [PubMed] [Google Scholar]
- Steele T. H., DeLuca H. F. Influence of dietary phosphorus on renal phosphate reabsorption in the parathyroidectomized rat. J Clin Invest. 1976 Apr;57(4):867–874. doi: 10.1172/JCI108363. [DOI] [PMC free article] [PubMed] [Google Scholar]



