Abstract
Alzheimer's disease is characterized by progressive cognitive disturbances and neurotransmitter dysfunction. Previous studies targeting the adrenergic A1 pathway suggest that this plays a role in cognitive impairment in Alzheimer's disease. Previous studies have reported that acute treatment with A1 antagonists appears to improve behavioral deficits in rodent models of memory and behavioral impairment. In this study, we addressed whether the chronic administration of 8-cyclopentyl-1,3-dipropylxanthine, a potent and selective adenosine A1 antagonist, could reverse the memory deficits found in aged APPswe/PS1dE9 mice. Chronic treatment did not improve memory in the APPswe/PS1dE9 mouse model and resulted in reduced exploratory behavior, suggestive of reduced anxiety, and a worsening of long-term memory in nontransgenic mice. These results have important implications for understanding the mechanisms of A1 receptor modulation as a target in Alzheimer's disease therapy.
Keywords: Alzheimer’s disease, Adenosine A1 antagonist, Chronic treatment, Impaired learning, Anxiolytic
1. INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disorder resulting in progressive cognitive impairment and the loss of short term memory. The development of AD is characterized by significant alterations in neurotransmitter pathways. In the CNS, adenosine acts as a neuromodulator, controlling the release of several neurotransmitters including, acetylcholine and glutamate that regulate synaptic transmission and neuronal excitability [7]. The effect of adenosine is mediated by four main G protein coupled receptors (GPCRs), namely A1, A2A, A2B and A3, which have been cloned and pharmacologically characterized. A1 and A3 receptors couple mainly to the inhibitory GI family of proteins, whereas A2A and A2B receptors activate adenylate cyclase by coupling to stimulatory Gs proteins [5]. In AD, GPCR function in amyloid precursor protein (APP) processing and tau phosphorylation is compromised [10].
A number of studies have suggested that adenosine receptors are a potential pharmacological target for the treatment of AD. In neuropathological studies of patients with late-onset AD, there is a clustering of A1 receptors in association with the degenerating neurons containing neurofibrillary tangles and within dystrophic neurites [3]. In this study, the involvement of A1 receptors in the production of soluble secreted form of APP as well as tau phosphorylation and its translocation to the cytoskeleton was demonstrated; Albasanz et al [1] similarly demonstrated an up-regulation of A1 receptors in the frontal cortex of AD patients.
In rodent models, short-term activation of the A1 receptor has been associated with disruptions of learning and memory, whereas selective blockade of the receptor has shown improvement in various behavioral tasks [6, 11, 12, 15, 17, 18, 21, 22, 25]. Treatment of mice with N6-Cyclopentyladenosine (CPA), a highly selective agonist for the A1 receptor, lead to deficits in retention performance. The CPA-elicited deficits in retention performance were, however, blocked by pretreatment with 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), a selective A1 receptor antagonist [16]. In studies conducted by Homayoun et al. [13] to determine the effects of adenosine receptor ligands on amnesia induced by pentylenetetrazole in mice, acute administration of adenosine receptor antagonists, theophylline and 8-phenyltheophylline resulted in a dose dependent reduction in the amnestic effect of pentylenetetrazole.
Although inhibition of A1 receptors is suggested to be a potent target for treatment of memory loss in AD and other cognitive disorders, all of the reported studies on pharmacological modulation of A1 receptors have employed acute pharmacological manipulation to study effects on cognitive behavior. The present study was designed to assess whether chronic administration of a selective adenosine receptor antagonists in an aged mouse model of Alzheimer’s disease had a positive impact on learning and memory.
2. MATERIALS AND METHODS
2.1. Animals
This study used 15-month old APPswe/PS1dE9 (APdE9) double transgenic mice with a C57BL/6J background that were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Genotypes were confirmed by PCR. Treatment groups and number of mice were as follows: non-transgenic littermates (control; n = 12), non-transgenic DPCPX (N=5), transgenic APdE9 (n = 7), APdE9/DPCPX (n = 7). Male mice and female mice were randomized across groups. All mice used in the present study were housed in a room maintained at 23 °C ± 1 °C with a 12 h light–dark cycle and free access to food and water.
2.2. Drug treatment
Animals were injected with 0.9% sterile saline solution with 5% DMSO or 1 mg/kg DPCPX (Tocris Bioscience) dissolved in 5% DMSO once daily intra-peritoneally (IP) for 60 days, prior to conducting behavioral tests. Animals received daily injections during the course of the behavioral studies.
2.3. Open Field test
Anxiety-like and non-cognitive behavior was assessed by the open field test. Each mouse was allowed to explore a novel environment in a clear chamber for 30 min while being monitored (OptoMax, Columbus Instruments) with standard room lighting conditions [23].
2.4. Morris Water Maze test
Spatial learning and memory acquisition was assessed by a modified Morris Water Maze (MWM) paradigm, as previously described [8]. On the 5th day, 24 hours after the last trial, a second probe test was used to assess long-term spatial memory.
2.5. Rotarod test
Motor coordination and balance were evaluated by a motarized rotarod. Each mouse was placed on a horizontal accelerating rod (4–40 rpm) and subjected to 4 trials (5-min/trial) for one day with 15 min. intervals between each trial. A trial ended when the mouse either fell off the rod or time elapsed 5 minutes.
2.6. Statistical analyses
Quantitative results were expressed as mean ± standard error of mean (SEM). Data was analyzed using one-way ANOVA and Fisher LSD post hoc test to determine significant differences between groups. p≤0.05 was considered statistically significant.
3. RESULTS
3.1. Effect of DPCPX on mouse weight
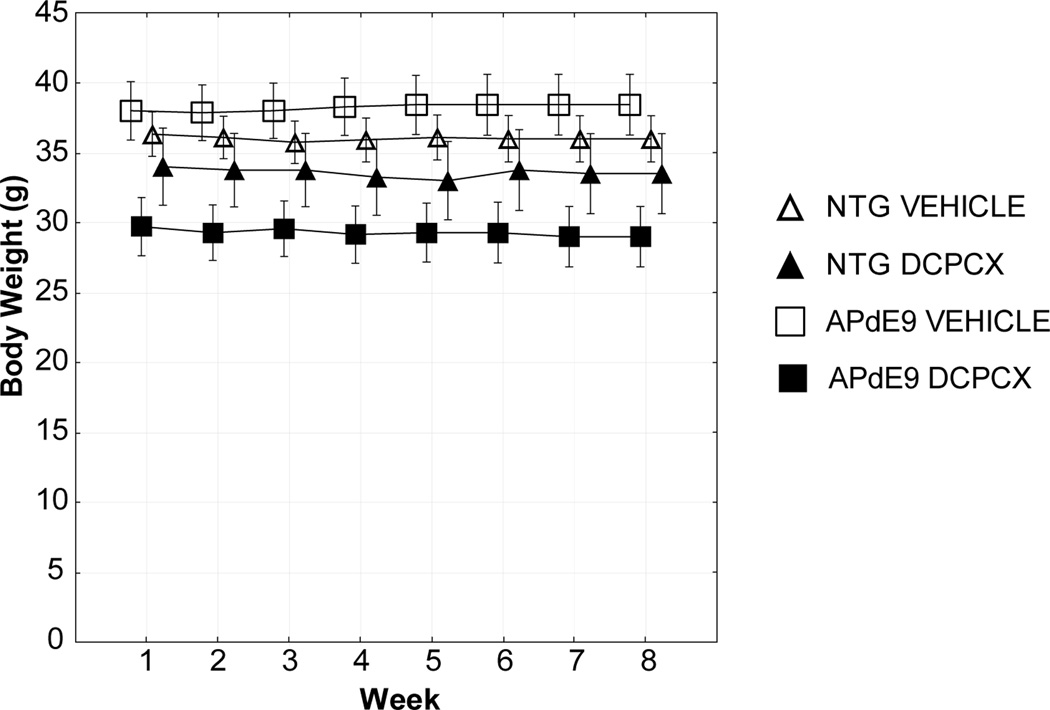
Mouse weight was measured each week during treatment to determine if chronic DPCPX treatment might be toxic. No significant difference in mice weight was observed during treatment in APdE9 and nontransgenic mice (Fig. 1A).
Fig. 1. Effect of DPCPX treatment on weight.
Mice were weighed on a weekly basis; no change in weight in any group was seen over the 8 week treatment period. Mean ± SEM.
3.2. Effect of DPCPX on non-cognitive behavior
Because impaired performance in learning tests can be affected by non-cognitive functions emotional-motivational and sensory-motor processes were assessed. Measures of open field performance, DPCPX treatment had no significant effect on exploratory stereotypic and resting behavior (Fig. 2A.). DPCPX treatment caused no significant difference in motor coordination, and balance on a rotarod (Fig. 2B).
Fig. 2. Effect of DPCPX treatment on non-cognitive behavior.
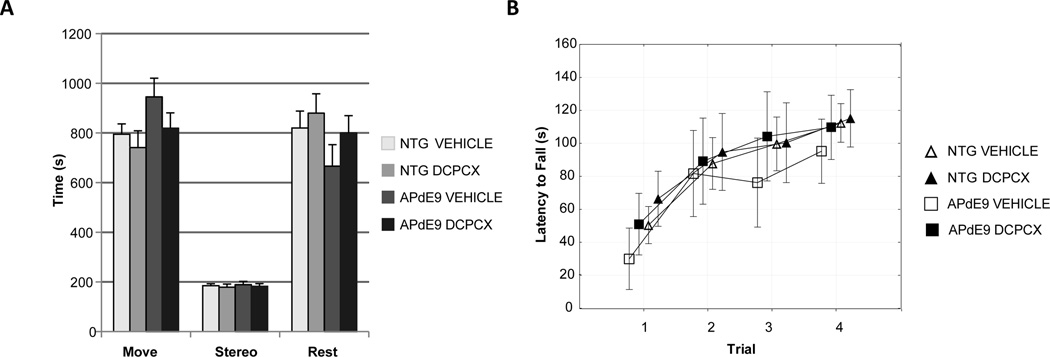
(A) Exploratory behavior measured by the open field activity was unaffected by treatment. (Move: Total activity time; Stereo: time moving in stereotypic fashion; Rest: Time at rest). (B) Motor coordination and balance with accelerating rotarod were unchanged with DCPCX treatment. * p < 0.05; mean ± SEM.
3.3. Analysis of anxiety-like behavior in mice treated with DPCPX
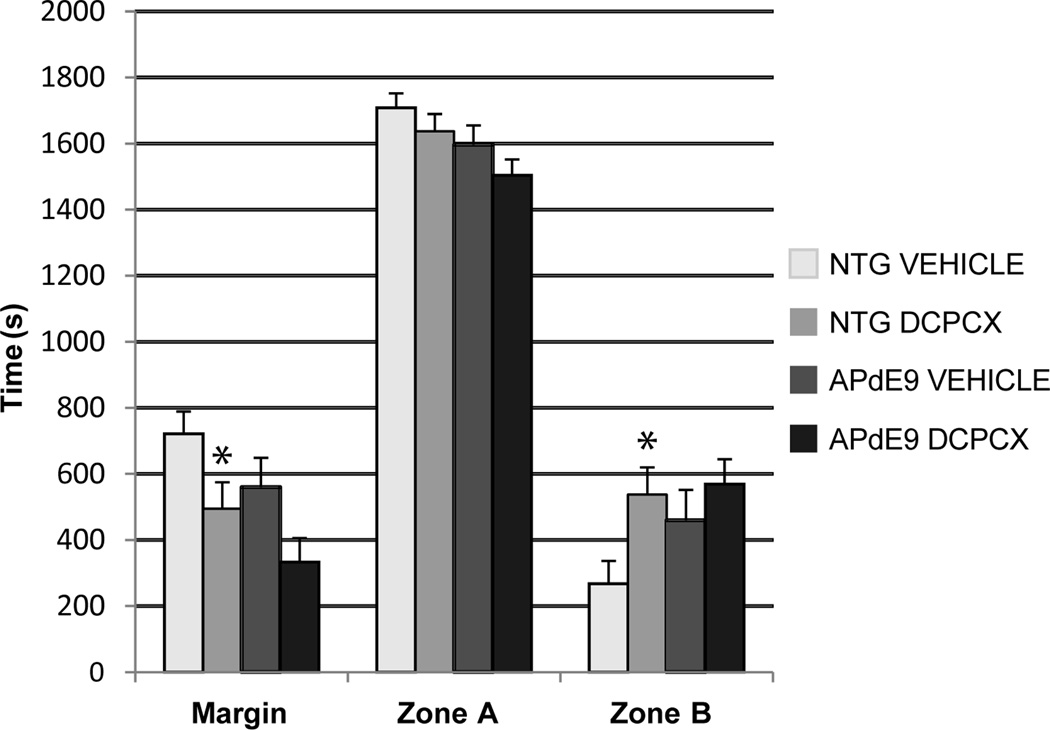
To assess anxiety-like behavior in mice time spent in the center and in the margins was measured. DPCPX treatment had a significant increase in time spent in the center of the open field (Zone B), and in the margins (Zone A) in the nontransgenic, but not the APdE9, mice (Fig. 3A, p < 0.05).
Fig. 3. Effect of DPCPX on anxiety-like behavior.
Nontransgenic mice treated with DCPCX spent significantly less time in the margin and more time in the center of the open field, compared to nontransgenic vehicle-treated mice. APP mice showed a similar trend, although the results were non-significant. (Zone A: perimeter of the open field; Zone B: center of the open field). * p < 0.05; mean ± SEM.
3.4. Effect of DPCPX on Morris water maze test
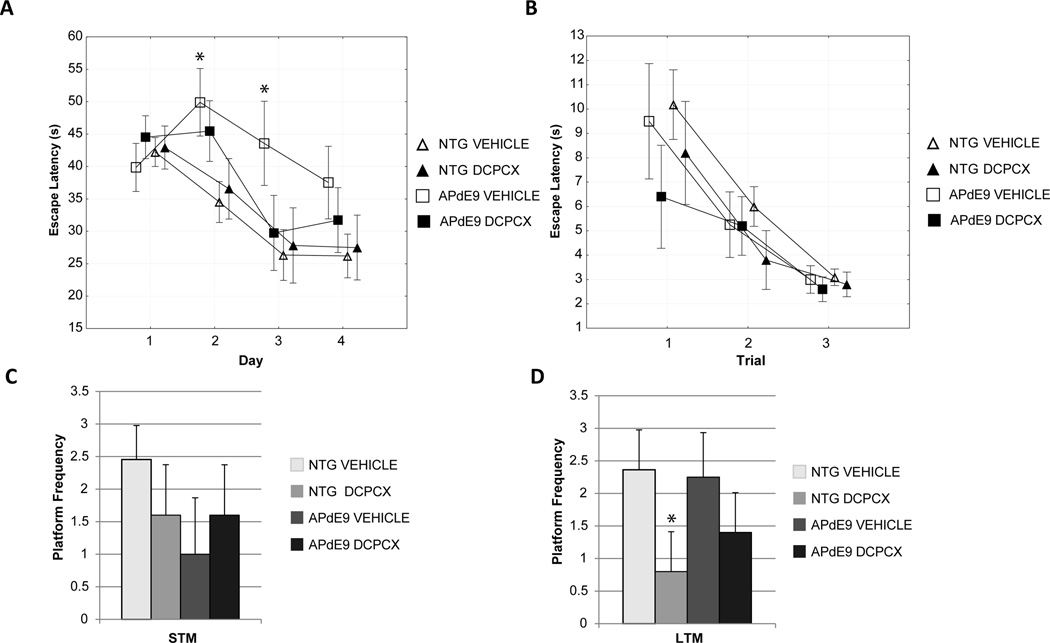
In acquisition trials of the Morris water maze test from days 1 to 4, DPCPX treatment had no significant effect on escape latency between groups. APdE9 mice showed a significantly increased escape latency on days 2 and 3 when compared to nontransgenic vehicle treated animals, indicating impaired learning (Fig. 3A, p < 0.05). DPCPX had no effect on escape latency during the visual acuity trials ruling out visual impairment as a confounding variable (Fig. 3B). In the probe trial to assess short-term memory (STM), 1 hour after the last acquisition trial, there was no significant difference between groups in platform frequency (Fig. 3C). However, in the probe trial to assess long-term memory (LTM), 24 hours after the last acquisition trial, DPCPX treatment significantly decreased platform frequency in nontransgenic mice, indicating an impairment in long-term memory (Fig. 3D, p < 0.05).
4. DISCUSSION
The present paper describes the effect of chronic administration of DPCPX, a selective A1 receptor antagonist on cognitive and non-cognitive properties as well as motor functions in wild type and in a transgenic mice model of AD. Studies that were conducted to test for changes in the behavioral patterns of the mice included the rotarod for effects on general motor performance, open field to monitor non-cognitive and anxiety-like behaviors, and the Morris water maze for spatial learning and memory. Animals treated during the course of the study did not appear to show detectable signs of toxicity from DPCPX treatment; there were no statistically significant changes in weight, movement in the open field and appearance in the treated animals. Treated mice did not exhibit any signs of toxicity or significant difference in motor deficits as was measured by motor coordination and balance on a motorized rotarod.
We observed, however, that DPCPX treatment did have significant effects on non-cognitive behavior. In the open field test, treated mice showed a reduced degree of exploration, suggestive that chronic administration of DPCPX may have an anxiolytic effect. This finding is in contrast to previous literature that showed acute treatment exerted an anxiogenic effect. A study conducted in mice showed that exposure to ethanol produced anxiolytic effects with pronounced hangover-induced anxiety between 12 and 18 hours after ethanol administration. The hangover-induced anxiety-like effect of ethanol could be reduced after acute administration of the selective A1 receptor agonist 2-chloro-N(6)-cyclopentyladenosine. However, the anxiolytic property of CCPA was reversed by pretreatment with DPCPX.[19] Similarly, selective A1 receptor blockade with cyclopentyltheophylline, as well as targeted disruption of the second coding exon of the A1 receptor (A1R−/−), also reduce anxiety in mice [9, 14].
In this study, chronic administration of DPCPX also had an impact on learning and memory. In the Morris water maze (MWM) test, which is designed to test hippocampal-based spatial learning, we found that nontransgenic mice treated with the drug demonstrated statistically significant reductions in crosses over the target platform in the long-term memory component of the MWM, suggesting that there was a mild cognitive impairment in long-term spatial memory with chronic DPCPX treatment. Previous studies using acute blockade of the A1 receptor reported that drug treatment resulted in a significant improvement in learning and memory. For instance, in a study on the role of transient hypoxia in synaptic function impacting on spatial learning and memory, it was observed that brief hypoxia impaired Morris water maze performance, an effect that could be rescued with acute treatment with DPCPX [20]. Another study provided evidence that in an animal model of learning and memory, caffeine a nonspecific adenosine receptor antagonist, enhances memory retention [2]. Our results, however, are consistent with a previous study by Von Lubitz and colleagues [24] that demonstrated that C57BL/6 mice, tested for spatial memory acquisition in Morris water maze, failed to develop spatial preference after chronic inhibition of the A1 receptor using 8-cyclopentyl-1,3-dipropylxanthine.
In summary, chronic administration of DPCPX exerts effects that are substantially different from those in acute treatment. Our data suggest that the effect of acute and chronic inhibition of the A1 receptor produce significantly different behavioral patterns. The opposing effects of acute versus chronic dosing have also been observed in other diseases such as congestive heart failure and asthma [4]. Given the divergence between the short and long-term effects, the development of therapeutic approaches to chronically target the A1 receptor for management of neurodegenerative diseases must be approached with care.
Fig. 4. Effect of DPCPX treatment on long-term memory in the Morris Water Maze.
(A) APdE9 mice had significantly increased escape latency compared to nontransgenic mice on day 2 and 3 during acquisition trials. (B) Escape latency during visual acuity trials. (C) Platform frequency during short-term memory (STM) probe test. (D) DCPCX treated nontransgenic mice had significantly fewer platform crosses than nontransgenic vehicle indicating impaired memory in the long-term memory (LTM) probe test. * p < 0.05; mean ± SEM.
HIGHLIGHTS.
APPswe/PS1dE9 transgenic mice were treated with the A1-antagonist DPCPX for 60 days.
Treated mice did not show overt signs of toxicity or demonstrate impaired motor performance.
DPCPX reduced exploratory behavior, consistent with a general anxiolytic effect.
Treatment worsened long-term memory in nontransgenic mice.
ACKNOWLEDGEMENTS
This work was supported by funding from the National Institutes of Health (1R15AG039008; JE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Albasanz JL, Perez S, Barrachina M, Ferrer I, Martin M. Up-regulation of adenosine receptors in the frontal cortex in Alzheimer's disease. Brain Pathol. 2008;18:211–219. doi: 10.1111/j.1750-3639.2007.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelucci ME, Cesario C, Hiroi RH, Rosalen PL, Da Cunha C. Effects of caffeine on learning and memory in rats tested in the Morris water maze. Braz J Med Biol Res. 2002;35:1201–1208. doi: 10.1590/s0100-879x2002001000013. [DOI] [PubMed] [Google Scholar]
- 3.Angulo E, Casado V, Mallol J, Canela EI, Vinals F, Ferrer I, Lluis C, Franco R. A1 adenosine receptors accumulate in neurodegenerative structures in Alzheimer disease and mediate both amyloid precursor protein processing and tau phosphorylation and translocation. Brain Pathol. 2003;13:440–451. doi: 10.1111/j.1750-3639.2003.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond RA. Is paradoxical pharmacology a strategy worth pursuing? Trends Pharmacol Sci. 2001;22:273–276. doi: 10.1016/s0165-6147(00)01711-9. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G, Fredholm BB, Verkhratsky A. Adenosine and ATP receptors in the brain. Curr Top Med Chem. 2011;11:973–1011. doi: 10.2174/156802611795347627. [DOI] [PubMed] [Google Scholar]
- 6.Corodimas KP, Tomita H. Adenosine A1 receptor activation selectively impairs the acquisition of contextual fear conditioning in rats. Behav Neurosci. 2001;115:1283–1290. doi: 10.1037//0735-7044.115.6.1283. [DOI] [PubMed] [Google Scholar]
- 7.Cunha RA, Almeida T, Ribeiro JA. Parallel modification of adenosine extracellular metabolism and modulatory action in the hippocampus of aged rats. J Neurochem. 2001;76:372–382. doi: 10.1046/j.1471-4159.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 8.Elhardt M, Martinez L, Tejada-Simon MV. Neurochemical, behavioral and architectural changes after chronic inactivation of NMDA receptors in mice. Neurosci Lett. 2010;468:166–171. doi: 10.1016/j.neulet.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florio C, Prezioso A, Papaioannou A, Vertua R. Adenosine A1 receptors modulate anxiety in CD1 mice. Psychopharmacology (Berl) 1998;136:311–319. doi: 10.1007/s002130050572. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Jimenez A, Cowburn RF, Ohm TG, Lasn H, Winblad B, Bogdanovic N, Fastbom J. Loss of stimulatory effect of guanosine triphosphate on [(35)S]GTPgammaS binding correlates with Alzheimer's disease neurofibrillary pathology in entorhinal cortex and CA1 hippocampal subfield. J Neurosci Res. 2002;67:388–398. doi: 10.1002/jnr.10125. [DOI] [PubMed] [Google Scholar]
- 11.Gimenez-Llort L, Masino SA, Diao L, Fernandez-Teruel A, Tobena A, Halldner L, Fredholm BB. Mice lacking the adenosine A1 receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse. 2005;57:8–16. doi: 10.1002/syn.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauber W, Bareiss A. Facilitative effects of an adenosine A1/A2 receptor blockade on spatial memory performance of rats: selective enhancement of reference memory retention during the light period. Behav Brain Res. 2001;118:43–52. doi: 10.1016/s0166-4328(00)00307-7. [DOI] [PubMed] [Google Scholar]
- 13.Homayoun H, Khavandgar S, Zarrindast MR. Effects of adenosine receptor agonists and antagonists on pentylenetetrazole-induced amnesia. Eur J Pharmacol. 2001;430:289–294. doi: 10.1016/s0014-2999(01)01376-0. [DOI] [PubMed] [Google Scholar]
- 14.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mioranzza S, Costa MS, Botton PH, Ardais AP, Matte VL, Espinosa J, Souza DO, Porciuncula LO. Blockade of adenosine A(1) receptors prevents methylphenidate-induced impairment of object recognition task in adult mice. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:169–176. doi: 10.1016/j.pnpbp.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Normile HJ, Barraco RA. N6-cyclopentyladenosine impairs passive avoidance retention by selective action at A1 receptors. Brain Res Bull. 1991;27:101–104. doi: 10.1016/0361-9230(91)90288-u. [DOI] [PubMed] [Google Scholar]
- 17.Ohno M, Watanabe S. Working memory failure by stimulation of hippocampal adenosine A1 receptors in rats. Neuroreport. 1996;7:3013–3016. doi: 10.1097/00001756-199611250-00043. [DOI] [PubMed] [Google Scholar]
- 18.Pereira GS, Mello e Souza T, Vinade ER, Choi H, Rodrigues C, Battastini AM, Izquierdo I, Sarkis JJ, Bonan CD. Blockade of adenosine A1 receptors in the posterior cingulate cortex facilitates memory in rats. Eur J Pharmacol. 2002;437:151–154. doi: 10.1016/s0014-2999(02)01307-9. [DOI] [PubMed] [Google Scholar]
- 19.Prediger RD, da Silva GE, Batista LC, Bittencourt AL, Takahashi RN. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- 20.Sun MK, Xu H, Alkon DL. Pharmacological protection of synaptic function, spatial learning, and memory from transient hypoxia in rats. J Pharmacol Exp Ther. 2002;300:408–416. doi: 10.1124/jpet.300.2.408. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki F, Shimada J, Shiozaki S, Ichikawa S, Ishii A, Nakamura J, Nonaka H, Kobayashi H, Fuse E. Adenosine A1 antagonists. 3. Structure-activity relationships on amelioration against scopolamine- or N6-((R)-phenylisopropyl)adenosine-induced cognitive disturbance. J Med Chem. 1993;36:2508–2518. doi: 10.1021/jm00069a009. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- 23.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Von Lubitz DK, Paul IA, Bartus RT, Jacobson KA. Effects of chronic administration of adenosine A1 receptor agonist and antagonist on spatial learning and memory. Eur J Pharmacol. 1993;249:271–280. doi: 10.1016/0014-2999(93)90522-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang DS, Ren LM. Effect of adenosine A1 receptor antagonist on learning and memory and analysis of its mechanism. Yao Xue Xue Bao. 2003;38:416–419. [PubMed] [Google Scholar]