Abstract
Bisphenol A (BPA), is a well-known endocrine disruptor compound (EDC) that affects the normal development and function of the female and male reproductive system, however the mechanisms of action remain unclear. To investigate the molecular mechanisms of how BPA may affect ten different nuclear receptors, stable cell lines containing individual nuclear receptor ligand binding domain (LBD)-linked to the β-Gal reporter were examined by a quantitative high throughput screening (qHTS) format in the Tox21 Screening Program of the NIH. The results showed that two receptors, estrogen receptor alpha (ERα) and androgen receptor (AR), are affected by BPA in opposite direction. To confirm the observed effects of BPA on ERα and AR, we performed transient transfection experiments with full-length receptors and their corresponding response elements linked to luciferase reporters. We also included in this study two BPA analogs, bisphenol AF (BPAF) and bisphenol S (BPS). As seen in African green monkey kidney CV1 cells, the present study confirmed that BPA and BPAF act as ERα agonists (half maximal effective concentration EC50 of 10-100 nM) and as AR antagonists (half maximal inhibitory concentration IC50 of 1-2 μM). Both BPA and BPAF antagonized AR function via competitive inhibition of the action of synthetic androgen R1881. BPS with lower estrogenic activity (EC50 of 2.2 μM), did not compete with R1881 for AR binding, when tested at 30 μM. Finally, the effects of BPA were also evaluated in a nuclear translocation assays using EGPF-tagged receptors. Similar to 17β-estradiol (E2) which was used as control, BPA was able to enhance ERα nuclear foci formation but at a 100-fold higher concentration. Although BPA was able to bind AR, the nuclear translocation was reduced. Furthermore, BPA was unable to induce functional foci in the nuclei and is consistent with the transient transfection study that BPA is unable to activate AR.
Keywords: Bisphenol A and related compounds, androgen receptor, qHTS, transfection, imaging analysis
1. Introduction
Endocrine disrupting chemicals (EDC) are environmental compounds that arise from many different sources and which act as hormone mimics to disrupt normal endocrine function, leading to altered sexual development and reproduction in both sexes of humans and wild life animals (reviews and references therein [1,2,3,4]), as well as other endocrine related functions such as thyroid functions [5]. Although EDCs affect a broad range of endocrine functions in different tissues and organs, many studies have focused on the estrogen signaling pathway [6] and fewer on the androgen or other hormonal signaling pathways. Based on epidemiology studies, some pesticides and other environmental chemicals are associated with reduced semen quality [7,8], testicular dysgenesis syndrome [9] and other male reproductive abnormalities [10], and an increased risk for testicular and prostate cancer [11,12]. The normal development and function of the male reproductive system is dependent on the action of endogenous androgens (review and references therein, [10]) and the biological functions of androgens are primarily mediated by the AR [13,14], which is expressed in many organs including the hypothalamus, pituitary, liver, prostate, and testes. While EDCs interfere with androgen-dependent signaling pathways through multiple mechanisms, modulation of AR function is a major mechanism. Therefore, one major goal of the present study was to explore mechanism(s) by which certain EDCs interfere with AR function.
Although a number of mid-throughput screening methods have been developed to detect the effect of EDC on AR activity, the number of compounds screened has been limited [15,16,17,18,19]. Recently, the Tox21 collaboration was formed among several federal agencies (NTP/NIEHS, NCGC/NCATS, EPA, and FDA) to develop and validate the utility of qHTS methods for chemical testing. In Tox21 Phase I, an NTP library of 1408 substances was screened for the ability of these substances to activate or inhibit transcription by a panel of 10 different nuclear receptors (NRs) [20]. This approach provided an opportunity to evaluate chemicals that interact with multiple nuclear receptors and a way to evaluate a broad spectrum of functional cross-talks between the tested compounds and the nuclear receptors. Using this approach, compounds identified in an initial screening can then be validated and analyzed in-depth.
BPA is a high volume production, commercial chemical that is used in polycarbonate plastics and epoxy resins and appears in a wide range of plastic products, flame-retardants, and dental sealants (review and references therein, [21,22]). BPA metabolites were found in more than 90% of the urine samples collected from the general populations of the United States and Italy [23,24]. A recent study found a positive association between BPA excretion in urine and the expression of two estrogen-responsive genes, ESR2 (ERβ) and ESRRA (ERRα), in peripheral blood leukocytes of adults [25], suggesting that BPA is bioactive in humans. While this compound is a well-known EDC that causes a variety of health problems and has been extensively studied, most of the studies on BPA have focused on its estrogenic activity (review and reference therein, [26,27]). In fact, BPA has multiple effects on many endocrine related signaling pathways [26,27]. It functions as an antagonist for the androgen receptor [28,29,30], thyroid receptor [31,32] and aryl hydrocarbon receptor [28]. It potentially binds and activates the glucocorticoid receptor [33] and suppresses aromatase activity [28]. Furthermore, BPA binds estrogen-related receptor gamma (ERRγ) with a strong binding affinity constant (Kd) of 5.5 nM [34]. Since ERRγ is highly expressed in the placenta [35], it can facilitate the accumulation of BPA in the placenta, thus increasing exposure of the developing fetus to this compound.
How BPA antagonizes AR function is not well studied and the molecular mechanisms are unclear. Using in vitro ligand competition assays, it was demonstrated that BPA could displace the radio-labeled synthetic androgen R1881 on AR [16,36,37]. With the yeast two-hybrid system, it was demonstrated that BPA affects AR function in multiple ways [38] and in mammalian cell-based transfection experiments, BPA showed an inhibitory effect on AR transactivation function [39]. However, the possible mechanisms by which BPA antagonizes AR function still needs further clarification. In this study, we examined the effect of BPA on 10 NR transactivation qHTS assays conducted during Tox21 Phase I and found that BPA had a significant effect on ERα and AR mediated activities. We verified these effects by transient transfection with a full-length receptor and the luciferase reporter gene in CV-1 African green monkey kidney cells and examined the inhibitory mode of action of BPA on AR function by competition experiments. In addition, we examined the effects of BPA in ERα and AR redistribution assays. Data from the current study demonstrated that BPA antagonizes AR activity by competition of androgen binding, reducing the AR movement from cytoplasm into nucleus and prevent the formation of functional complexes that are the prerequisite of transcription.
2. Materials and Methods
2.1. Reagents
BPA (Chemical Abstracts Services Registry Number, CASRN: 80-05-7), BPAF (CASRN: 1478-61-1), BPS (CASRN: 80-08-1), hydroxyflutamide (OHF, CASRN:13311-84-7), androstenedione (CASRN:63-05-8), ICI 182,780 (ICI, CASRN:129453-61-8), casodex (CSX, CASRN:90357-06-5), cyproterone acetate (CypAC, CASRN:2098-66-0) and 17β-estradiol (E2, CASRN:50-28-2) were obtained from MRIGlobal under contract to NTP. The synthetic androgen, methyltrienolone (R1881, CASRN: 965-93-5) was purchased from RTI International (Durham, NC), and ERα redistribution assay kit (R04-056-01) and AR redistribution assay kit (R04-043-01) were obtained from Thermo Scientific (Pittsburgh, PA).
2.2. β-Lactamase reporter gene assay and qHTS
GeneBLAzer β-lactamase (bla) HEK293T cell lines that co-express the Gal4-reporter system with Gal4DBD-linked LBD of related human NRs and Gal4 response element-bla reporter were obtained from Invitrogen (Carlsbad, CA). When a compound binds to the LBD of the fusion receptor, it will activate the bla reporter. The experimental procedure for compound formatting, qHTS and data analysis were described previously [20,40]. Briefly, cells stably transfected with receptor-LBD fusion protein and the bla reporter, were dispensed in 1,536-well plates, incubated at 37°C for 6 h before the compounds at 15 different concentrations (1 × 10-10 to 1 × 10-4 M) were added. The bla activity was evaluated 16-18 h after compound addition. Fluorescence intensity (405 nm excitation, 460 nm and 530 nm emission) was measured on an Envision plate reader (PerkinElmer, Shelton, CT). Data were expressed as the ratio of 460 nm to 530 nm emissions.
2.3. Transient transfection and luciferase assay
All plasmids, pSG5-AR, MMTV-Luciferase, Renilla-luc, pRST7-ERα, and 3X-ERE-TATA-Luc used in transient transfection experiments were provided by Integrated Oncology Solutions (IOS, Durham, NC) under contract.
For AR functional assay, monkey kidney CV1 (AR negative) cells were bulk transfected (using Fugene 6 from Roche, IN) with a DNA mixture consisting of pSG5-AR and MMTV-Luciferase (reporter gene to measure the transcriptional activity of AR), and Renilla –Luc (normalization of transfection efficiency) in a 1:10:1 ratio. Following 24-hr transfection, cells were trypsinized, diluted in DMEM cell culture medium containing 10% charcoal/dextran treated fetal bovine serum, and re-plated into 96-well plates containing test ligand pre-diluted in DMSO (0.5% final concentration). Cells were then incubated with either R1881 or testing compounds for an additional 24 h before harvesting in cell lysis buffer and assaying for luciferase (firefly and renilla) activity with Dual-Glo Luciferase Assay system (Promega) according to the manufacturer’s protocol. This assay was performed in both agonist and antagonist modes. In the agonist mode assays, cells were incubated with increasing concentrations (10-8 to 10-4M) of test ligand while in antagonist mode assays, cells were incubated in the presence of an EC80 concentration (5×10-10M) of the synthetic agonist R1881 in addition to increasing concentrations of test ligands. To evaluate the antagonist mode of BPA and related compounds on AR function, we added various concentrations of BPA, BPAF or BPS to the synthetic androgen R1881 at concentrations from 3×10-8 to 1×10-13 M in transient transfection experiments as described above. For ERα functional assay, CV-1 (ERα negative) or HepG2 cells were bulk transfected (using Fugene 6 from Roche) with a DNA mixture consisting of pRST7-ERα, 3X-ERE-TATA-luc (reporter gene to measure the transcriptional activity of ERα), and Renilla –Luc (normalization of transfection efficiency) in a 1:10:1 ratio. Hormone treatments were carried out as described for AR except phenol red free medium was used instead. Each assay consisted of triplicate samples and the experiment was repeated 2-3 times. An EC80 concentration of E2 (2×10-10 M) was present in increasing concentrations of test ligand in ER antagonist mode assay.
2.4. Nuclear receptor redistribution assay
The ERα and AR redistribution assays were carried out according to the manufacturer’s instruction. Briefly, recombinant U2OS cells stably expressing human ERα (GenBank Acc.NM_000125) or human AR (GenBank Acc. NM_000044) fused to the C-terminus of enhanced green fluorescent protein (EGFP) were maintained in phenol red-free DMEM in the presence of 2 mM L-Glutamine, 1% Penicillin-Streptomycin, 0.5 mg/ml G418 and 10% FBS at 37C, 5% CO2, 95% humidity incubator. Cells were plated in 384-well collagen-coated plates with charcoal-stripped and dextran-treated FBS and incubated overnight before treatment. Cells were treated with various concentrations of compounds at different times fixed, stained and permeabilized in 10% Formalin, 0.5% Triton-X100 and 1 μM Hoechst for 20 minutes, then washed twice with PBS. The plates were imaged on the Thermo Scientific Arrayscan VTI high content imager. A minimum of 200 cells per well were analyzed using the Compartmental Analysis Bioapplication.
2.5. Statistical analysis
Data analyses and concentration response Hill curve fitting were performed using Graphpad Prism (La Jolla, CA).
3. Results
3.1. Effect of BPA on ten nuclear receptors
The NTP 1408 compound library was screened against the LBD region of 10 nuclear receptors; AR, ERα, FXRα (farnesoid × receptor alpha), GR (glucocorticoid receptor), LXRβ (liver × receptor beta), PPARγ (peroxisome proliferator-activated receptor gamma), PPARδ (peroxisome proliferator-activated receptor delta), RXRα (retinoid × receptor alpha), TRβ (thyroid hormone receptor beta), and VDR (vitamin D receptor) in both agonist and antagonist modes at 15 concentrations ranging from 0.5 nM to 92 μM along with a DMSO vehicle control as described previously [20]. The screening data showed that BPA stimulated ERα and inhibited AR transactivation function. These observations were confirmed when the effects of BPA on ERα and AR activation were re-tested over the same concentration range (Fig. 1). BPA had no effect on the activation of FXRα, GR, LXRβ, PPARδ, RXRα, TRβ and VDR in tested in either agonist or antagonist mode, while PPARγ was minimally affected (data not shown). In order to validate and further investigate the interaction between BPA and ERα/AR, follow up studies using full-length receptors were performed.
Figure 1.
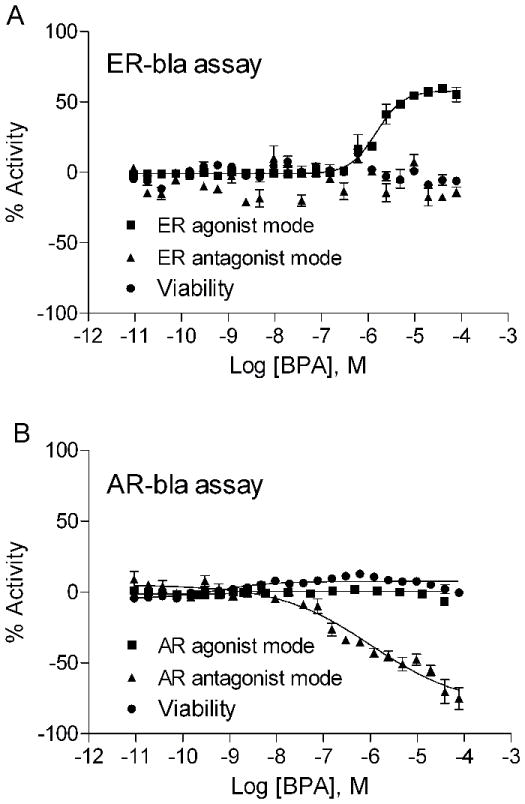
Concentration response curves of BPA on (A) ERα-bla and (B) AR-bla assays. GeneBLAzer β-lactamase HEK293 cell lines that co-express Gal4-reporter system with Gal4DBD-linked ERα-LBD or AR-LBD were tested in 12-24 concentrations from 0.5 nM to 92 μM range in HTS format. Each data point represents mean ± SD of triplicate measurement with 1 representative experiment. The experiment was repeated three times.
3.2. BPA, BPAF and BPS studies with full-length ERα and AR
To confirm the BPA interaction with the ERα- and AR-LBD region in the primary screen, we carried out experiments transiently transfected with the full-length ERα and 3x-ERE-Luc or AR and MMTV-Luc in CV1 monkey kidney cells. In addition to BPA, we also tested the BPA analogues, BPAF and BPS, in these experiments. The results of this study (EC50 and IC50 for both AR and ER) are summarized in Table 1. Both BPA and BPAF demonstrated ER activation but no ER inhibition was observed (Figure 2 A and B). BPS, another BPA analogue, also showed weak estrogenic activity with an EC50 of 2.2 μM, with a potency that was 10 times lower than BPA and BPAF (Figure 2C). Hydroxyflutamide (OHF), an androgen antagonist, and methyltrienolone (R1881), a synthetic androgen [41], had no effect whereas the ER antagonist ICI 182,780 (ICI) completely blocked ERα activity at nM concentrations. Contrary to their agonist activity on ERα, BPA and BPAF demonstrated AR antagonist activity at an IC50 of 1-2 μM which is 10-fold less potent than that of the antiandrogen casodex (CSX) (Table 1 and Fig. 3 B), but showed no agonist activity (Fig. 3A). The positive control, androstenedione and R1881 were found to have AR agonist activity at EC50s of 8.92 nM and 6.98 pM, respectively (Table 1 and Figure 3A). It should be noted that BPAF demonstrated cell toxicity at the highest concentration tested (100μM) whilst BPA did not (data not shown). However, these toxic concentrations are well above their apparent EC50 and IC50 concentrations confirming that both BPA and BPAF are bona fide modulators of both ERα and AR transcriptional activity.
Table 1.
| Compounds | AR | ER | ||
|---|---|---|---|---|
| EC50 | IC50 | EC50 | IC50 | |
| BPA | NA | 2.34E-06 | 2.72E-07 | NA |
| BPAF | NA | 1.29E-06 | 9.69E-08 | NA |
| BPS | NA | NA | 2.20E-06 | NA |
| 17β-Estradiol | NA | NA | 1.26E-10 | NA |
| ICI,182780 | NA | NA | NA | 5.64E-09 |
| R1881 | 6.98E-12 | NA | NA | NA |
| Androstenedione | 8.92E-09 | NA | NA | NA |
| Casodex | NA | 3.27E-07 | NA | NA |
| OH-flutamide | 1.21E-05 | 1.71E-08 | NA | NA |
Figure 2.
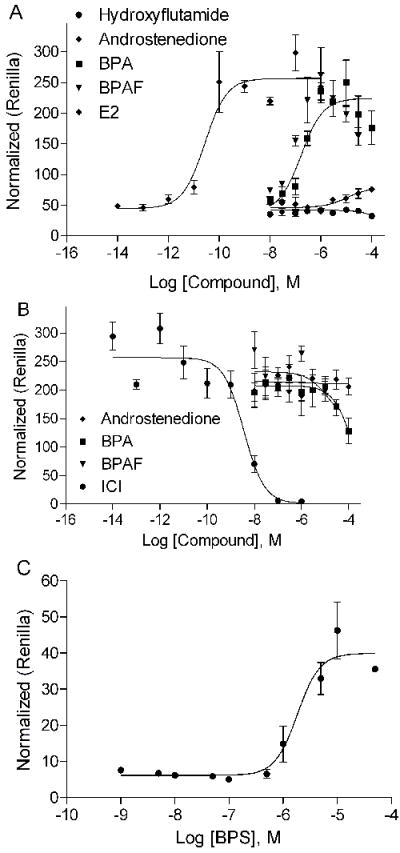
Effect of BPA, BPAF, and BPS on ERα transcriptional activity. A. Concentration response curves of BPA and BPAF on ERα activation. Hydroxyflutamide and androstenedione were negative control. B. BPA and BPAF do not inhibit ERα function. ER antagonist ICI, showed strong inhibition while androstenedione used as negative control has no effect. C. Concentration response curve of BPS on ERα activation. Mean ± SD of representative experiment with triplicate samples are shown. The experiment was repeated two times.
Figure 3.
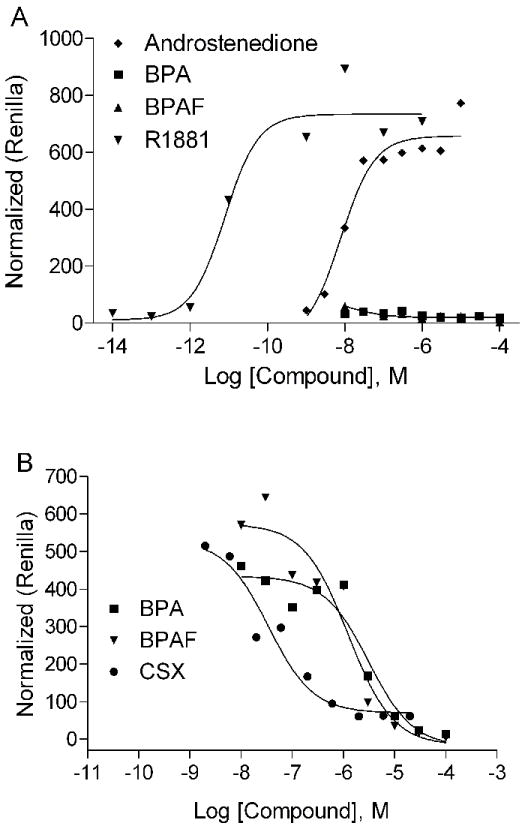
Effect of BPA and BPAF on AR transcriptional activity. A. BPA and BPAF do not activate AR transcriptional activity. R1881 and the androstenedione showed strong activation. B. BPA and BPAF inhibit AR transcriptional activity. The androgen antagonist, CSX strongly inhibits AR activity. Mean ± SD of representative experiment with triplicate samples are shown. The experiment was repeated twice.
To investigate the mechanism(s) by which BPA and BPAF were AR antagonists, we carried out competition experiments. The concentration response curves of R1881 were examined in the presence of various concentrations of BPA and BPAF. As shown in Fig. 4 A and B, both BPA and BPAF caused right parallel shifts for the R1881 concentration-response curve, indicating competitive antagonism. In this study, we also tested BPS for its ability to inhibit transactivation function of the AR because it has been demonstrated to be a weak ERα agonist (Fig. 4C). Interestingly, the presence of BPS at concentrations of 10 and 30 μM did not significantly change the potency of R1881 as demonstrated by the dose-response curve, although the Vmax was decreased by about 17%.
Figure 4.
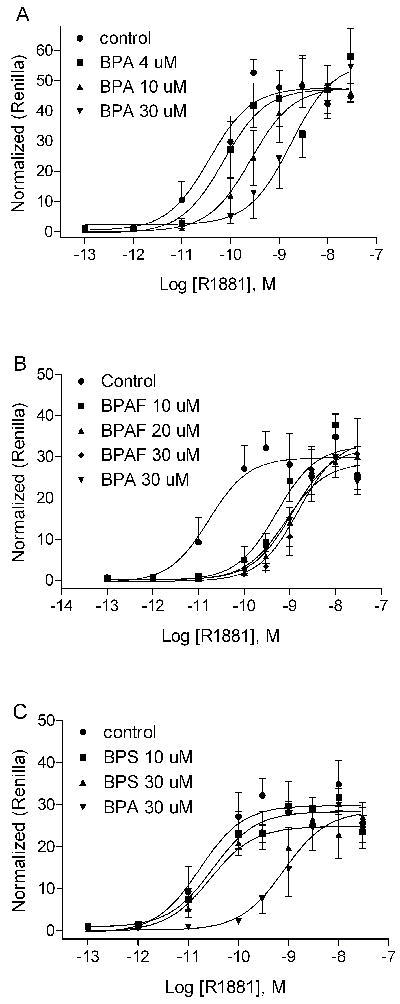
BPA, BPAF, and BPS compete for binding with androgen to the AR. A. BPA competes with R1881 and inhibits AR activity at dose-dependent manner. B. BPAF competes with R1881 and inhibits AR activity at dose-dependent manner. C. BPS does not compete with R1881 nor inhibit AR activity at the highest dose tested. Each data point represents mean ± SD of triplicate samples within 1 representative experiment. The experiment was repeated four times.
3.3. Nuclear receptor redistribution assays
Endogenous ER and AR receptors are highly mobile and dynamic within cells. Upon ligand binding, the receptors accumulate in nuclear compartments (foci) containing coactivators and other proteins required for transactivation function. This event is one of the indispensable and common steps for steroid hormone receptor-mediated transactivation [14,42,43]. The commercially available Enhanced Green Fluorescent Protein (EGFP) tagged ERα and AR redistribution assay systems provide the tools to examine the effect of BPA on the early events in ERα- and AR-mediated transactivation function. The primary output in the ERα redistribution assay is the formation of nuclear foci since the ERα is evenly distributed in the nucleus in the absence of ligand. The ERα-induced foci formation is concentration-dependent and is comparable to the transactivation assay (Fig. 2) with EC50 values of 0.29 nM for E2 and 558 nM for BPA (Fig. 5A). Although BPA induced measurable formation ERα foci, it required an approximate 2000-fold higher concentration to achieve the same level of foci counts as E2 (Fig. 5A). In addition, BPA did not interfere with the E2-induced foci formation until high concentrations (>35 μM) were used (Fig. 5B).
Figure 5.
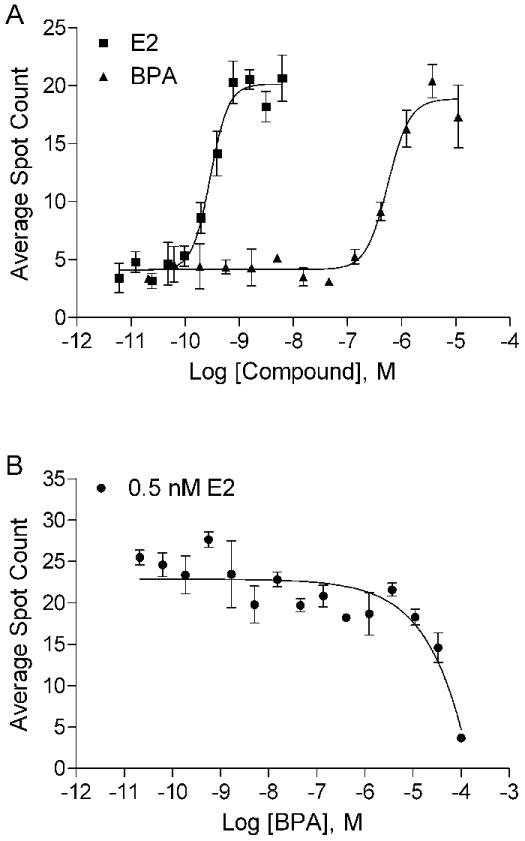
Effect of BPA on ERα redistribution assay. A. BPA induces ERα nuclear foci formation in a dose-dependent manner. B. BPA does not interfere with 0.5 nM E2-induced nuclear foci formation at concentrations less than 1 μM. Mean ± SD of representative experiment with triplet samples are shown. The experiment was repeated twice.
AR is normally located in the cytoplasm and moves into nuclei minutes after ligand binding. The ligand-AR will then reorganize with the nuclear proteins into distinct foci [44]. Therefore, the image based AR redistribution assay is designed for analysis of ligand-induced nuclear translocation and formation of nuclear foci by the EGFP-AR. To determine the proper treatment duration for the experiments, we counted the number of foci in the nucleus of cells treated with R1881 at 15 concentrations for 0.25 to 6 h (Fig. 6). EC50 concentration of R1881-induced strong foci formation as seen at 0.25, 0.5, 1 and 6 h are 4.2, 0.9, 0.4 and 0.1 nM, respectively (Fig. 6). The signal-to-basal ratio of the maximum R1881 treatment was highest after 6 h of incubation (8-9-fold). Therefore, 6 h was chosen as an optimal treatment time for R1881, CypAC, and BPA in the AR redistribution assay. As expected, R1881 induced a high level of foci formation calculated by counting nuclear spots (foci) (Fig. 7A) whereas BPA did not (Fig. 7B). These data were consistent with the transactivation assay (Fig. 3A). The androgen antagonist CypAC was able to induce a small number of nuclear foci at a high concentration (Fig. 7A). Similar to the known AR antagonists such as OHF and CAS [45], CypAC showed nuclear translocation as measured by the increase in the average nuclear foci intensity (Fig. 7C). Unlike the behavior of these known anti-androgens, BPA was ineffective except at very high concentrations (>10 μM) in promoting AR nuclear translocation (Fig. 7D). These data were supported by the imaging of the treated cells (Fig. 7E). In the presence of 0.5 nM R1881, high concentrations of BPA (100 μM) inhibited spot count in the nuclei (Fig. 8A) and showed some intense fluorescence in the nuclei but no foci formation (Fig. 8B) suggesting that under this condition, BPA could compete out the R1881 binding and move the AR into the nucleus. However, the complex was unable to form functional foci as required for the transactivation activity.
Figure 6.
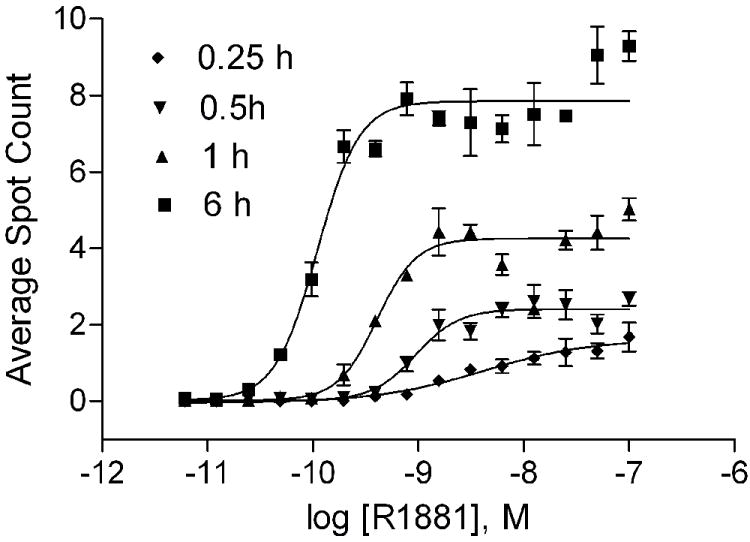
Time course of AR redistribution at various concentrations of R1881. Mean ± SD of representative experiment with triplet samples are shown. The experiment was repeated twice.
Figure 7.
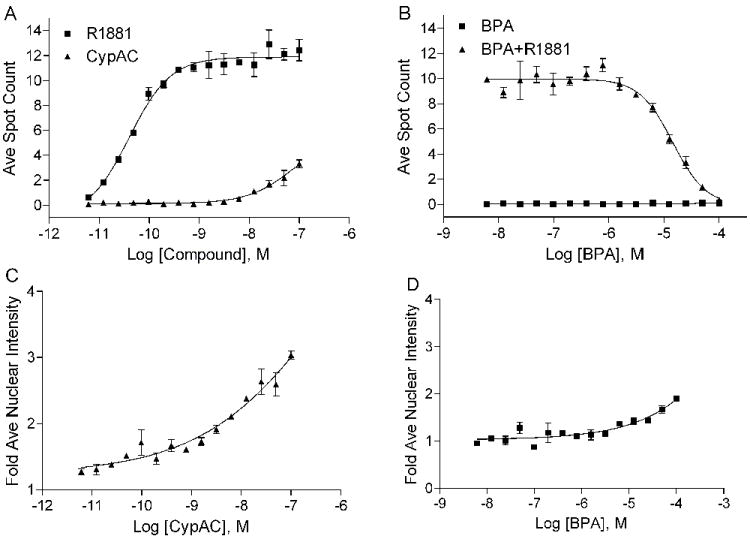
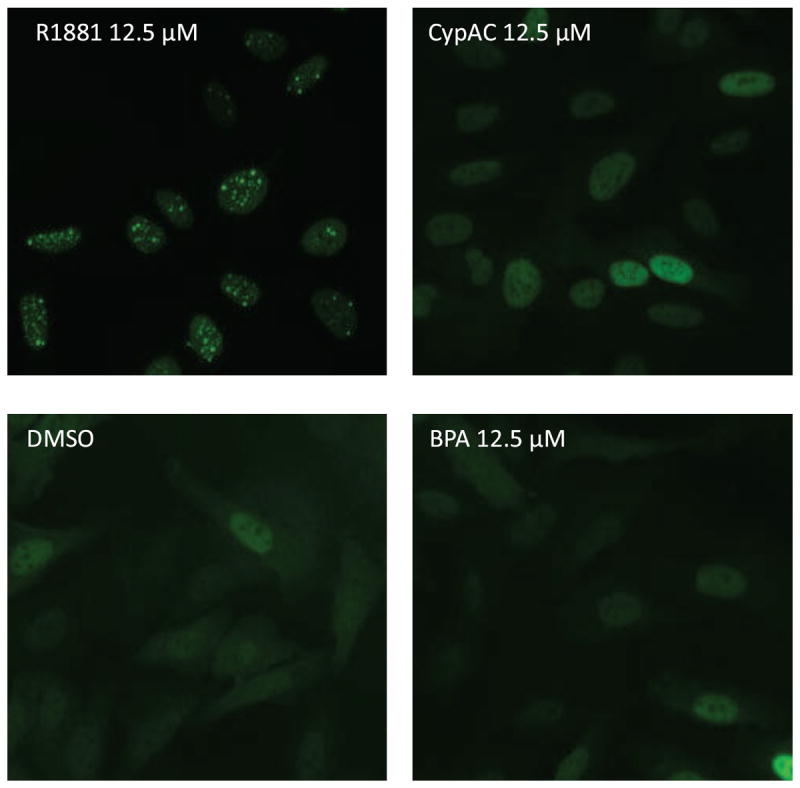
Effect of R1881, CypAC and BPA on AR redistribution assay. A. Nuclear spot counts for agonist and antagonist controls (R1881 and CypAC respectively). B. Nuclear spot counts for BPA alone and in the presence of agonist R1881. C and D. Fluorescence intensity measurement of nuclear to cytoplasmic ratio in cells treated with CypAC (C) and BPA (D). Mean ± SD of representative experiment with triplet samples are shown. The experiment was repeated twice. E. Imaging of cells treated with various compounds.
Figure 8.
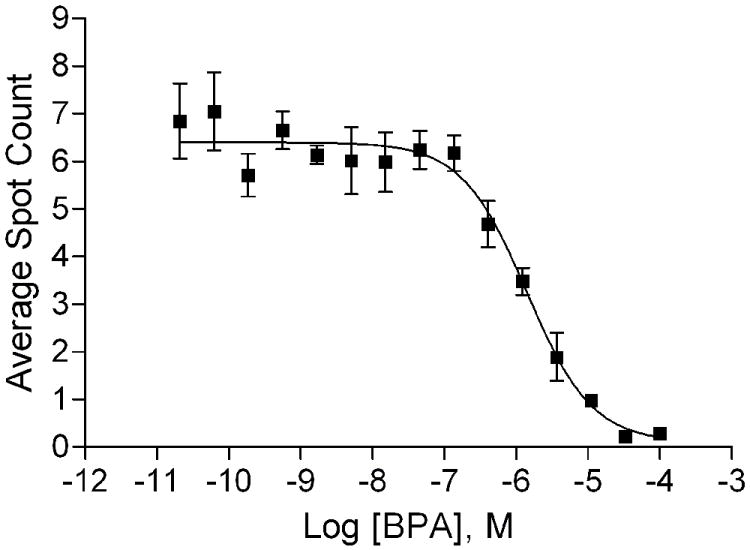
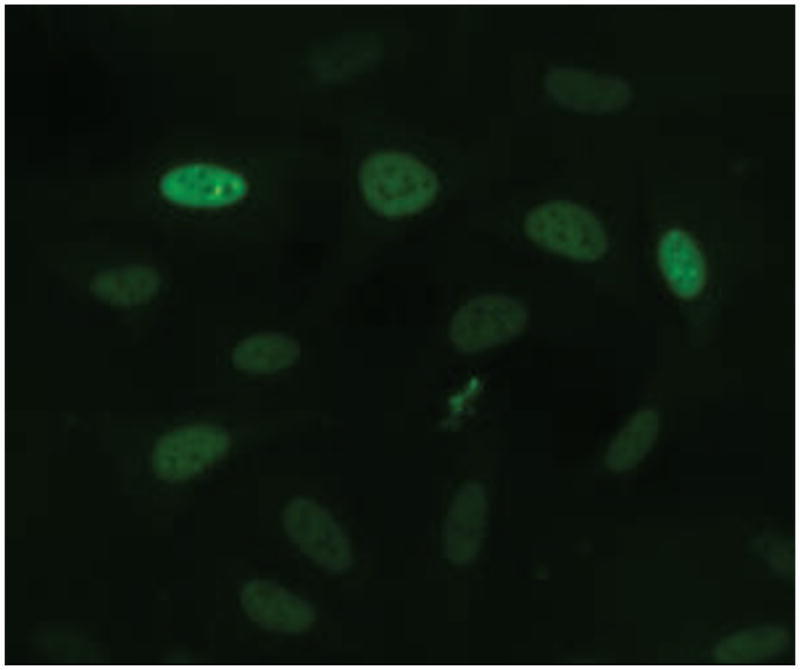
BPA blocked R1881-induced nuclear foci formation. A. Dose-dependent inhibition of R1881-induced nuclear foci formation by BPA. Mean ± SD of representative experiment with triplet samples are shown. The experiment was repeated twice. B. Image shown that a high concentration of BPA may move the AR-EGFP into nuclei but no foci formation.
4. Discussion
Although several studies have shown that BPA exhibits strong anti-androgenic activity both in vitro and in vivo (see review and references therein [10,27,28], there are discrepancies between the results from different research groups, including the failure to demonstrate BPA anti-androgenic activity in HepG2 cells [46] and the observation that BPA did not exert major androgenic effects in the Hershberger assay [47]. In addition, little is known about the mechanism(s) of the AR antagonism by BPA. In this study, we showed that BPA antagonizes AR activity by competing with R1881 binding to the LBD thus confirming our primary qHTS data. BPA by itself did not activate AR in a cell-based reporter assay in agreement with our imaging analysis showing that BPA could not induce functional foci, which is an essential feature of transcriptional activity. Furthermore, BPA-AR nuclear translocation was retarded.
In the competition experiments, both BPA and BPAF antagonized R1881 and shifted the R1881 dose-response curve to the right without affecting the maximal response of the full-length receptor to R1881. This indicated that antagonistic effects of BPA and BPAF on AR activation involve competition between these compounds and the AR agonist for binding at the LBD. These studies revealed one of the mechanisms on how BPA affects AR function. Within the NR superfamily [13] AR contains a unique feature in the amino-terminus (N) which plays an important functional role [48]. Whereas most of the NR cofactor interactions and transactivation functions depend on the activation function 2 domain (AF-2) at the carboxy-terminus (C), the AR contains a major activation function 1 domain (AF-1) at its N terminus that requires interaction between the N/C functional domains for full activity (reviews and references therein [49,50]). It is not known whether BPA or BPAF affects the AR N/C interaction. Based on the results of competition experiments, this appears unlikely because there was no effect on the Vmax of the dose-response curves suggesting that the major region for BPA and BPAF interaction resides in the LBD. Interestingly, the structurally closely related analogue, BPS did not significantly shift the dose-response curve of R1881 at concentrations up to 30 μM, although slight decreases of Vmax were observed. Whether BPS could affect AR function in another way has yet to be determined. Being a weaker estrogen (Fig. 2C) that has no obvious effects on AR, BPS, with a higher stability and heat resistance is being considered as a substitute for BPA, but nonetheless, its environmental impact on the ecosystem and human health still needs more study.
A majority of the functional studies on nuclear receptors are focused on cell-based reporter gene expression assays. This type of assays have limitations as the data represent the accumulative response of all the cells which may be at the different stages of the cell cycles and expressing different levels of the receptors. To complement the cell-based reporter assays we included the image-based ERα and AR assays on nuclear translocation and nuclear patterning changes for the early steps of gene activation. Results on the BPA effect of ERα function are consistent with reports in the literature. The EC50s for reporter assays and nuclear foci formation for both E2 and BPA are similar (table I and Figure 5) suggesting that functional formation of foci is a prerequisite for transcriptional activation. Although the estrogenic activity of BPA is 1,000 times lower than that of endogenous E2, its high production volume and wide spread use in consumer products makes it a potentially important EDC. The other two BPA related compounds, BPAF and BPS were not examined in the image-based analyses. Since the number of foci detected is parallel to the transactivation function, we can predict that the BPAF will induce functional foci similar to BPA wheras BPS would need much higher concentrations to show such an effect. It has been reported that BPA binds ERβ stronger than ERα in a binding assay [51] and recruits different coactivators [52]. Recently, ERα- and ERβ-mediated BPA, BPAF and Zearalenone effects were examined in a cell-based reporter assay [53]. Whether BPA and BPAF act as agonists or antagonists in the estrogen signaling pathway via ERα or ERβ is dependent on the cell-type, concentration and the estrogen response element tested. Based on those data it was possible that the expression level of ERα and ERβ would contribute to the complex tissue-dependent effect of BPA.
To test AR function we used the relatively stable synthetic androgen R1881 as the ligand [41]. Although the length of treatment with R1881 in the transfection assays was longer than in the imaging analysis, the EC50 values obtained in both studies were relatively similar (Table I and Fig. 6 for R1881). Even though AR translocation could happen in minutes after ligand binding [44], more time was required for the reorganization of the AR into distinct intranuclear foci and thus transactivation activity. The 6 h imaging analysis time may be shorter than required for complete foci formation and full functional activation. When combined with R1881, BPA was able to reduce the number of R1881-induced foci, a response pattern similar to several environmental compounds, such as vinclozolin, nitrofen and DDT which have previously been characterized as having anti-androgenic activity [45,54]. Unlike those compounds and several known AR antagonists, including CypAC, which induced a modest nuclear translocation of the receptor (about 45-53% of R1881 response), at concentrations lower than 1 μM, BPA was unable to induce nuclear translocation until it reached the concentration of 10 μM, being about 100x less efficient in AR nuclear translocation than the AR antagonist CypAC (Figure 7). This observation is in contrast to an earlier report showing that BPA did not translocate AR-GFP to the nucleus [54]. It is not known how BPA inhibits AR movement. It could impair the AR dissociation from the molecular chaperones in the cytoplasm [44] or interfere with the proper modification of the nuclear localization signal (NLS) which plays an indispensable role in determining AR intracellular location [55,56]. These subtle differences among the compounds on AR function are indicative of the complexity and the mechanisms that are involved in gene activation.
Although BPA does not have agonist activity on the wild-type AR, it is able to activate the AR (T877A) mutant and promotes prostate cancer cell proliferation [57,58]. This ability of BPA to activate tumor derived AR is conserved across multiple AR mutants; therefore, environmental exposures to BPA might promote tumor growth and tumor recurrence [59]. It is intriguing how a single mutation in AR can turn BPA from a weak antagonist into an agonist in prostate cancer cells. EDCs could modulate AR functions via multiple intracellular targets. Different compounds might either directly or indirectly alter the conformation of AR thereby changing its preference for coactivators or corepressors [60,61] and its androgenic or anti-androgenic activities. Thus far, screening studies compounds that modulate AR function have been limited to AR binding (see review and references therein [10]). Environmental compounds could modulate AR function via non-binding mechanisms such as by targeting heat shock proteins [62,63], histone deacetylases [64], and several kinases, including HER2/neu kinase [65]. Identification of intracellular factors that mediate the effects of these compounds could vastly improve our understanding of nuclear receptor biology and the development of therapeutic interventions to the EDC effects.
5. Conclusion
BPA affects the endocrine system on multiple levels and in multiple pathways. As an established ERα agonist, BPA is also a strong anti-androgenic compound. We explored the mechanisms of BPA inhibition on AR function by using transcriptional activation and AR redistribution assays. We found that BPA binds AR and competes with androgen binding at the LBD region of the receptor. Like known androgen antagonists, BPA is unable to promote the formation of functional AR foci in the nucleus; however, unlike other known antagonists, BPA inhibits the efficient nuclear translocation of AR. BPA binding to AR interferes with nuclear receptor translocation such that AR may require a higher concentration and/or longer time for the translocation process. Based on these data, BPA is a potent endocrine disruptor that interferes with normal endocrine function in multiple ways.
Highlights.
BPA antagonizes AR function by competitive inhibition of androgen binding.
Prevents functional foci formation in the nucleus.
Reduce the AR presence in the nucleus.
BPAF have similar activation function in ERα and inhibition in AR as BPA
BPS is a weaker ERα agonist and does not inhibit AR function
Acknowledgments
We thank Brad Collins as coordinator of NTP for obtaining the compounds from MRIGlobal. We appreciate the valuable comments of John Bucher. This work was supported by the Intramural Research Programs of the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health (NIH).
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cederroth CR, Zimmermann C, Nef S. Soy, phytoestrogens and their impact on reproductive health. Mol Cell Endocrinol. 2012;355:192–200. doi: 10.1016/j.mce.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 2.Fowler PA, Bellingham M, Sinclair KD, Evans NP, Pocar P, Fischer B, Schaedlich K, Schmidt JS, Amezaga MR, Bhattacharya S, Rhind SM, O’Shaughnessy PJ. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol. 2012;355:231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Nordkap L, Joensen UN, Blomberg Jensen M, Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–230. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen HE, Adamsson A. Cryptorchidism and endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355:208–220. doi: 10.1016/j.mce.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Safe S. Environmental estrogens: roles in male reproductive tract problems and in breast cancer. Rev Environ Health. 2002;17:253–262. doi: 10.1515/reveh.2002.17.4.253. [DOI] [PubMed] [Google Scholar]
- 7.Meeker JD, Singh NP, Ryan L, Duty SM, Barr DB, Herrick RF, Bennett DH, Hauser R. Urinary levels of insecticide metabolites and DNA damage in human sperm. Hum Reprod. 2004;19:2573–2580. doi: 10.1093/humrep/deh444. [DOI] [PubMed] [Google Scholar]
- 8.Swan SH. Semen quality in fertile US men in relation to geographical area and pesticide exposure. Int J Androl. 2006;29:62–68. doi: 10.1111/j.1365-2605.2005.00620.x. discussion 105-108. [DOI] [PubMed] [Google Scholar]
- 9.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 10.Luccio-Camelo DC, Prins GS. Disruption of androgen receptor signaling in males by environmental chemicals. J Steroid Biochem Mol Biol. 2011;127:74–82. doi: 10.1016/j.jsbmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alavanja MC, Samanic C, Dosemeci M, Lubin J, Tarone R, Lynch CF, Knott C, Thomas K, Hoppin JA, Barker J, Coble J, Sandler DP, Blair A. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am J Epidemiol. 2003;157:800–814. doi: 10.1093/aje/kwg040. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwan IJ. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer. 2004;11:281–293. doi: 10.1677/erc.0.0110281. [DOI] [PubMed] [Google Scholar]
- 15.Araki N, Ohno K, Nakai M, Takeyoshi M, Iida M. Screening for androgen receptor activities in 253 industrial chemicals by in vitro reporter gene assays using AR-EcoScreen cells. Toxicol In Vitro. 2005;19:831–842. doi: 10.1016/j.tiv.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, Xie Q, Perkins R, Owens W, Sheehan DM. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003;16:1338–1358. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- 17.Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect. 2004;112:524–531. doi: 10.1289/ehp.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanseverino J, Eldridge ML, Layton AC, Easter JP, Yarbrough J, Schultz TW, Sayler GS. Screening of potentially hormonally active chemicals using bioluminescent yeast bioreporters. Toxicol Sci. 2009;107:122–134. doi: 10.1093/toxsci/kfn229. [DOI] [PubMed] [Google Scholar]
- 19.Vinggaard AM, Niemela J, Wedebye EB, Jensen GE. Screening of 397 chemicals and development of a quantitative structure--activity relationship model for androgen receptor antagonism. Chem Res Toxicol. 2008;21:813–823. doi: 10.1021/tx7002382. [DOI] [PubMed] [Google Scholar]
- 20.Huang R, Xia M, Cho MH, Sakamuru S, Shinn P, Houck KA, Dix DJ, Judson RS, Witt KL, Kavlock RJ, Tice RR, Austin CP. Chemical genomics profiling of environmental chemical modulation of human nuclear receptors. Environ Health Perspect. 2011;119:1142–1148. doi: 10.1289/ehp.1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of Environmental Chemicals in Diabetes and Obesity: A National Toxicology Program Workshop Report. Environ Health Perspect. 2012 doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Money C, McCormack P, Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melzer D, Galloway T. Bisphenol A and adult disease: making sense of fragmentary data and competing inferences. Ann Intern Med. 2011;155:392–394. doi: 10.7326/0003-4819-155-6-201109200-00009. [DOI] [PubMed] [Google Scholar]
- 26.Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Bonefeld-Jorgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115(Suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Xu XL, Xu LC, Song L, Hong X, Chen JF, Cui LB, Wang XR. Antiandrogenic activity of pyrethroid pesticides and their metabolite in reporter gene assay. Chemosphere. 2007;66:474–479. doi: 10.1016/j.chemosphere.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 31.Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 32.Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 33.Prasanth GK, Divya LM, Sadasivan C. Bisphenol-A can bind to human glucocorticoid receptor as an agonist: an in silico study. J Appl Toxicol. 2010;30:769–774. doi: 10.1002/jat.1570. [DOI] [PubMed] [Google Scholar]
- 34.Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ Health Perspect. 2008;116:32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poidatz D, Dos Santos E, Brule A, De Mazancourt P, Dieudonne MN. Estrogen-related receptor gamma modulates energy metabolism target genes in human trophoblast. Placenta. 2012;33:688–695. doi: 10.1016/j.placenta.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Kim TS, Yoon CY, Jung KK, Kim SS, Kang IH, Baek JH, Jo MS, Kim HS, Kang TS. In vitro study of Organization for Economic Co-operation and Development (OECD) endocrine disruptor screening and testing methods- establishment of a recombinant rat androgen receptor (rrAR) binding assay. J Toxicol Sci. 2010;35:239–243. doi: 10.2131/jts.35.239. [DOI] [PubMed] [Google Scholar]
- 37.Paris F, Balaguer P, Terouanne B, Servant N, Lacoste C, Cravedi JP, Nicolas JC, Sultan C. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit alpha and beta estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002;193:43–49. doi: 10.1016/s0303-7207(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 38.Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- 39.Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Xia M, Huang R, Witt KL, Southall N, Fostel J, Cho MH, Jadhav A, Smith CS, Inglese J, Portier CJ, Tice RR, Austin CP. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect. 2008;116:284–291. doi: 10.1289/ehp.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doering CH, Leyra PT. Methyltrienolone (R1881) is not aromatized by placental microsomes or rat hypothalamic homogenates. J Steroid Biochem. 1984;20:1157–1162. doi: 10.1016/0022-4731(84)90360-1. [DOI] [PubMed] [Google Scholar]
- 42.Mainwaring WI. The mechanism of action of androgens. Monogr Endocrinol. 1977;10:1–178. [PubMed] [Google Scholar]
- 43.Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem. 2001;276:36865–36868. doi: 10.1074/jbc.R100041200. [DOI] [PubMed] [Google Scholar]
- 44.Marcelli M, Stenoien DL, Szafran AT, Simeoni S, Agoulnik IU, Weigel NL, Moran T, Mikic I, Price JH, Mancini MA. Quantifying effects of ligands on androgen receptor nuclear translocation, intranuclear dynamics, and solubility. J Cell Biochem. 2006;98:770–788. doi: 10.1002/jcb.20593. [DOI] [PubMed] [Google Scholar]
- 45.Szafran AT, Szwarc M, Marcelli M, Mancini MA. Androgen receptor functional analyses by high throughput imaging: determination of ligand, cell cycle, and mutation-specific effects. PLoS One. 2008;3:e3605. doi: 10.1371/journal.pone.0003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–858. [PubMed] [Google Scholar]
- 47.Nishino T, Wedel T, Schmitt O, Schonfelder M, Hirtreiter C, Schulz T, Kuhnel W, Michna H. The xenoestrogen bisphenol A in the Hershberger assay: androgen receptor regulation and morphometrical reactions indicate no major effects. J Steroid Biochem Mol Biol. 2006;98:155–163. doi: 10.1016/j.jsbmb.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995;270:7341–7346. doi: 10.1074/jbc.270.13.7341. [DOI] [PubMed] [Google Scholar]
- 49.Askew EB, Minges JT, Hnat AT, Wilson EM. Structural features discriminate androgen receptor N/C terminal and coactivator interactions. Mol Cell Endocrinol. 2012;348:403–410. doi: 10.1016/j.mce.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clinckemalie L, Vanderschueren D, Boonen S, Claessens F. The hinge region in androgen receptor control. Mol Cell Endocrinol. 2012;358:1–8. doi: 10.1016/j.mce.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 52.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J Biol Chem. 2000;275:35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential Estrogenic Actions of Endocrine-Disrupting Chemicals Bisphenol A, Bisphenol AF, and Zearalenone through Estrogen Receptor alpha and beta in Vitro. Environ Health Perspect. 2012;120:1029–1035. doi: 10.1289/ehp.1104689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomura A, Goto K, Morinaga H, Nomura M, Okabe T, Yanase T, Takayanagi R, Nawata H. The subnuclear three-dimensional image analysis of androgen receptor fused to green fluorescence protein. J Biol Chem. 2001;276:28395–28401. doi: 10.1074/jbc.M101755200. [DOI] [PubMed] [Google Scholar]
- 55.Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121:957–968. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- 56.Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, Roy AK. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14:1162–1174. doi: 10.1210/mend.14.8.0497. [DOI] [PubMed] [Google Scholar]
- 57.Hess-Wilson JK, Webb SL, Daly HK, Leung YK, Boldison J, Comstock CE, Sartor MA, Ho SM, Knudsen KE. Unique bisphenol A transcriptome in prostate cancer: novel effects on ERbeta expression that correspond to androgen receptor mutation status. Environ Health Perspect. 2007;115:1646–1653. doi: 10.1289/ehp.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther. 2002;1:515–524. [PubMed] [Google Scholar]
- 59.Wetherill YB, Hess-Wilson JK, Comstock CE, Shah SA, Buncher CR, Sallans L, Limbach PA, Schwemberger S, Babcock GF, Knudsen KE. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Ther. 2006;5:3181–3190. doi: 10.1158/1535-7163.MCT-06-0272. [DOI] [PubMed] [Google Scholar]
- 60.Hodgson MC, Shen HC, Hollenberg AN, Balk SP. Structural basis for nuclear receptor corepressor recruitment by antagonist-liganded androgen receptor. Mol Cancer Ther. 2008;7:3187–3194. doi: 10.1158/1535-7163.MCT-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sack JS, Kish KF, Wang C, Attar RM, Kiefer SE, An Y, Wu GY, Scheffler JE, Salvati ME, Krystek SR, Jr, Weinmann R, Einspahr HM. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc Natl Acad Sci U S A. 2001;98:4904–4909. doi: 10.1073/pnas.081565498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, Nieto M, Du J, Stegmaier K, Raj SM, Maloney KN, Clardy J, Hahn WC, Chiosis G, Golub TR. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Solit DB, Scher HI, Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin Oncol. 2003;30:709–716. doi: 10.1016/s0093-7754(03)00346-4. [DOI] [PubMed] [Google Scholar]
- 64.Dobosy JR, Roberts JL, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol. 2007;177:822–831. doi: 10.1016/j.juro.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 65.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]


