Abstract
The stimulus complexity of naturally occurring odours presents unique challenges for central nervous systems that are aiming to internalize the external olfactory landscape. One mechanism by which the brain encodes perceptual representations of behaviourally relevant smells is through the synthesis of different olfactory inputs into a unified perceptual experience — an odour object. Recent evidence indicates that the identification, categorization and discrimination of olfactory stimuli rely on the formation and modulation of odour objects in the piriform cortex. Convergent findings from human and rodent models suggest that distributed piriform ensemble patterns of olfactory qualities and categories are crucial for maintaining the perceptual constancy of ecologically inconstant stimuli.
Understanding how sensory objects map onto the brain is a fundamental question that has preoccupied philosophers, psychologists and scientists at least since the time of the ancient Greeks1. To a large extent this question remains unsolved, although recent studies have considerably advanced our knowledge of object perception and coding, particularly with regard to visual objects2–10.
This Review is about olfactory objects and about how the brain is optimized to recognize, categorize and discriminate them. Given the complexity and variability of odours that are naturally encountered in the environment, the ability of the olfactory system to generate stable percepts from unstable inputs is essential for species that rely on the sense of smell for survival. Indeed, without mechanisms to internalize perceptual representations of olfactory objects, most animals would find themselves lost, hungry and on the shortlist of the plats du jour.
The concept of an olfactory object challenges conventional notions of what an object can or cannot be. It is generally presumed that a ‘thing’ has to have visual features to be deemed an object. This widespread assumption may have its origin in the visuocentric nature of human sensory experience11, and it has long dominated neuroscientific models of object processing. Thus, objects are commonly considered to be seen and not heard (or smelled): they are solid, opaque, tinged with colour and anchored in the environment, with edges delimiting their position in space. Described in these terms, the very idea of an olfactory object seems fallacious: odours are typically unseen, gaseous, invisible, amorphous and physically disconnected from their source.
However, a more charitable perspective suggests that these criteria for ‘objects’ are unnecessarily restrictive. For example, auditory sources (objects that produce sounds, such as a lion) and auditory events (sounds that emanate from objects, such as a roar) satisfy the criteria for auditory objects, as they present object information to the senses11,12. Applying the same logic, olfactory sources (objects that produce odours, such as a lion) and olfactory events (odours that emanate from objects, such as a musky lion smell) can be thought of as olfactory objects13,14. In fact, one of the earliest cited uses of the noun ‘object’ was framed in olfactory terms:
“That the earth … should give to the nose objecte so swete or minister scent so strong”15.
Basic research on the sense of smell has traditionally focused on the initial processing stages between olfactory receptor neurons and the olfactory bulb (BOX 1). Systematic research on information processing beyond the bulb has only recently begun to gain traction. The emergence of new methods and the increasing regard for the central olfactory system has shed new light on how odour representations are encoded in higher-order olfactory regions. A key challenge for olfactory neuroscience research is to understand which of these odour representations best conform to odour object perception.
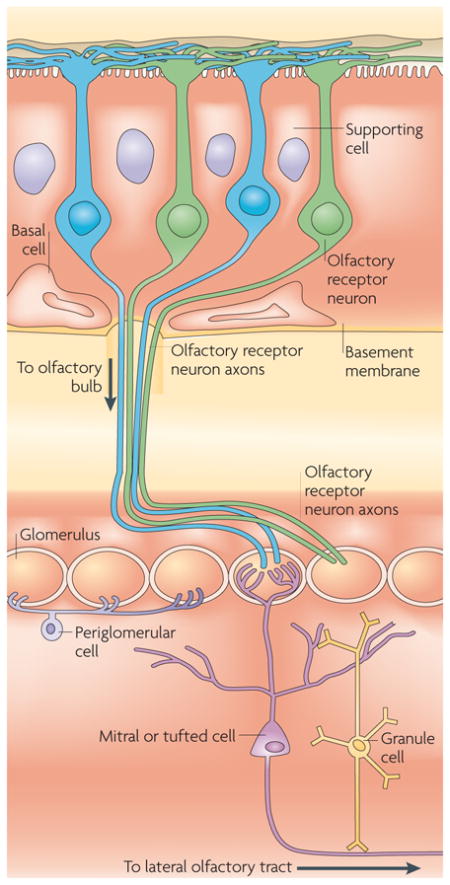
Box 1. Upstream stages of olfactory processing.
An odorant that arrives at the nose makes contact with the sensory endings of olfactory receptor neurons (ORNs) in the nasal epithelium (see the figure). Each ORN expresses just 1 receptor subtype out of approximately 1,000 possible receptors in rodents (380 in humans). A particular odorant may have a high affinity for a particular receptor subtype, and vice versa, but there is widespread promiscuity. A single odorant can bind to multiple receptor subtypes and a single receptor can bind to multiple, different odorants172,173. Receptor subtypes are randomly distributed through the epithelium without any distinguishable topographical organization. However, ORNs that express the same receptor converge on only two glomeruli in the rodent olfactory bulb, where they synapse with the dendritic terminals of second-order neurons that are known as mitral and tufted cells. The olfactory bulb also contains several classes of GABA (γ-aminobutyric acid)-ergic interneurons, including granule cells, which receive centrifugal input from higher-order regions and exert inhibitory effects on mitral and tufted cells21,174,175. This provides a feedback mechanism by which cortical brain areas modulate the afferent olfactory message at an early level of olfactory processing.
Numerous experimental approaches have been used to demonstrate that structurally related odorants evoke similar spatial patterns of glomerular activity16,72, although recent studies suggest that this chemotopic organization is less apparent on a finer spatial scale73. These organizational features have led to the idea that the perceptual identity of an odorant is represented as a combinatorial array of activity in the olfactory bulb176, and complementary behavioural evidence has demonstrated that rats have greater difficulty in discriminating odorants that have greater pattern overlap in the olfactory bulb177,178. Interestingly, a recent histological study indicates that the convergence ratio of receptor subtypes to glomeruli may be much lower in humans179. This is suggestive of a more distributed form of information processing that may deviate from rodent models of olfactory bulb function.
This article will draw from recent studies in humans and rodents to highlight the principles that underlie central mechanisms of odour object perception, particularly in the piriform cortex. Despite being commonly referred to as ‘primary olfactory cortex’, the piriform cortex is crucial for the integration of odour inputs with higher-order cortical information. Emphasis will be placed on olfactory studies that provide concomitant behavioural and neural measurements, in cases in which these are available, given that such approaches offer the most direct way to relate odour percepts to their cortical representations. A detailed overview of the neural computations that take place between olfactory receptor neurons and olfactory cortex is beyond the scope of this article, but this topic is discussed in other recent reviews16–26.
Anatomy of an odour
A pertinent question regarding the neural basis of olfactory object perception is: what is the natural form of odours that are encountered in the environment? Monomolecular odorants are useful in chemosensory research, but real-world aromas are complex mixtures of dozens, or even hundreds, of different molecules. The olfactory system seamlessly weaves these elements into perceptual wholes and therefore configural odour perception rather than elemental odour perception takes place27. In humans this bias towards configural (or synthetic) perception is so strong that even trained wine experts cannot reliably distinguish more than three components in an odour blend28,29. This has distinct computational advantages in that it minimizes the amount of information that the brain needs to encode about a given smell, and prevents perceptual confusion between odours that contain some of the same components.
Another key feature of real-world scents is their inconstancy. The presentation of an olfactory object to the nose may vary according to wind direction, air temperature and humidity, resulting in fluctuations in the perceived intensity and quality of the odour. The chemical composition that arrives at the nose from an odour source varies through time. Urine, that telltale canine calling card freshly sprinkled on the morning grass, may undergo dramatic olfactory alterations as the midday sun bakes off the most volatile constituents. Ecological factors such as these introduce further complexity to naturally-occurring odours, posing challenges for an olfactory system that aims to maintain constancy of object perception.
In addition to these so-called ‘anatomical’ features, odour perception is complicated by the fact that most odorous objects are perceived against a background of other odours. Sometimes there is a need to discriminate between two odours with considerable molecular similarity, or to categorize together two odours with little molecular similarity. How the olfactory brain handles such challenges will be discussed in the following sections.
The olfactory system
The environmental complexity of natural odours has shaped the evolutionary development of the olfactory system. Odour perception relies on the synthesis, consolidation and retrieval of behaviourally salient olfactory objects, and the anatomical organization of higher-order brain regions, in particular the piriform cortex, is ideally suited for these tasks.
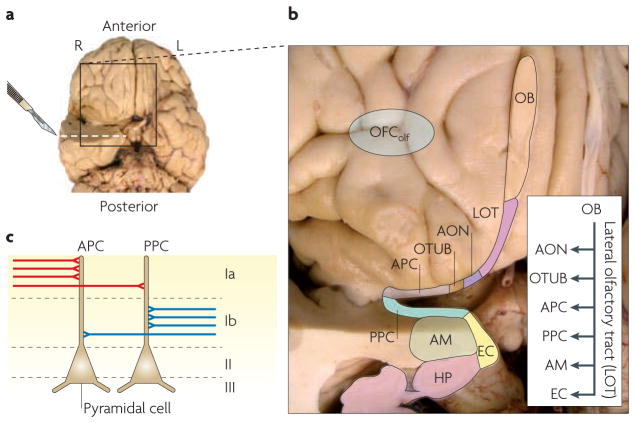
The cascade of events that culminates in odour object perception begins in the olfactory epithelium, where odorants bind to the receptors of olfactory sensory neurons, the axons of which project to olfactory bulb glomeruli and form synapses with the dendrites of mitral and tufted cells (BOX 1). Axonal projections from these cells are conveyed via the lateral olfactory tract (LOT) and terminate on several areas in the basal frontal and medial temporal lobes, including the anterior olfactory nucleus, olfactory tubercle, piriform cortex, amygdala and rostral entorhinal cortex (FIG. 1). These areas are sometimes collectively referred to as the ‘primary olfactory cortex’.
Figure 1. Anatomy of the human olfactory brain.
a | A ventral view of the human brain in which the right anterior temporal lobe has been resected in the coronal plane to expose the limbic olfactory areas. The area that is outlined by a box in part a is magnified in part b following a second resection along the axial plane of the right temporal lobe (shown by the dashed line and scalpel). b | Afferent output from the olfactory bulb (OB) passes through the lateral olfactory tract (LOT) and projects monosynaptically to numerous regions, including the anterior olfactory nucleus (AON), olfactory tubercle (OTUB), anterior piriform cortex (APC), posterior piriform cortex (PPC), amygdala (AM) and entorhinal cortex (EC). Downstream relays include the hippocampus (HP) and the putative olfactory projection site in the human orbitofrontal cortex (OFColf) that has been identified on the basis of a neuroimaging meta-analysis180. As noted in the inset, information is not transferred serially through this circuit. Monosynaptic projections from the lateral olfactory tract reach numerous downstream regions in parallel and these regions are then reciprocally interconnected (not shown). c | Schematic representation of the cellular organization of the piriform cortex. Pyramidal neurons are located in cell body layers II and III, and their apical dendrites project to molecular layer I. Layer I is subdivided into a superficial layer (Ia) that contains the sensory afferents from the olfactory bulb (shown in red) and a deeper layer (Ib) that contains the associative inputs from other areas of the primary olfactory cortex and higher-order areas (shown in blue)33. The majority of layer Ia afferents terminate in the APC, whereas the majority of layer Ib associative inputs terminate in posterior piriform cortex (PPC). Photographs in parts a and b prepared with the help of E. H. Bigio, Northwestern University Feinberg School of Medicine, USA.
Downstream relays from the primary olfactory cortex to the orbitofrontal cortex (OFC), agranular insula, hypothalamus, lateral and basolateral amygdala, perirhinal cortex, hippocampus and striatum link odour inputs to systems that are associated with affective learning and memory30–36 (FIG. 1). This extended olfactory network, which encompasses a large portion of limbic and paralimbic cortices37, reflects the importance of the sense of smell for mediating physiological and behavioural responses to emotionally arousing events in many animals38. Many of these connections are bidirectional, as are those between the olfactory bulb and primary olfactory structures. Indeed, the high density of fibres that carry information from the piriform cortex back to the olfactory bulb30,39,40 highlights the importance of the piriform cortex in shaping bulbar output.
Piriform cortex: a sensory-associative cortex
The piriform cortex, so named for its pear-shaped anatomy (Supplementary information S1), is a three-layer paleocortex (FIG. 1c) that lies at the medial junction of the frontal and temporal lobes. It is the largest recipient of bulbar projections. The piriform cortex is reciprocally and extensively connected with several high-order areas of the cerebral cortex, including the prefrontal, amygdaloid, perirhinal and entorhinal cortices31,34,41,42. Some piriform neurons project to more than one of these areas, with long-range axonal arbors spanning much of the brain34. Moreover, neighbouring piriform cells can have highly dissimilar projection targets34. This widely distributed pattern of connectivity — with direct links to brain networks that regulate cognition, emotion, memory, and behaviour — is highly reminiscent of a sensory association cortex, where representations of individual components are assembled into holistic objects.
The demonstration that the human piriform cortex is activated during higher-order tasks that are related to learning, memory, and motivational and cognitive states43–52 supports the idea that its function goes beyond merely relaying odour information. In patients with medically refractory epilepsy, unilateral temporal lobectomy has been associated with reduced discrimination, identification, matching and memory of odours53–58. Interestingly, in the well-known patient H.M., who underwent bilateral temporal resection, odour detection thresholds were preserved but judgements of perceptual similarity between pairs of odours were impaired59. This finding suggests that the temporal lobes are crucial for accessing information about odour object quality.
On the basis of anatomical, physiological and functional differences30,39,48,60–67, the piriform cortex can be divided into the anterior piriform cortex (APC) and the posterior piriform cortex (PPC). Afferent input from the olfactory bulb does not show any distinctive spatial patterning across the piriform cortex34,41,68–71. In contrast to the coarse chemotopy in the olfactory bulb16,72,73, a modular spatial architecture has not been identified in the piriform cortex. Early olfactory stimulation studies using 2-deoxyglucose (2DG) methods failed to reveal an effect of odour quality on 2DG activity-related uptake patterns in the APC or the PPC68–70. A recent study identified discrete spatial bands of cells that express FOS, a marker for neuronal activity, in the APC after exposure to particular odours, but no clear relationship to concentration or quality could be determined71. An in vivo study combining optical imaging and single-unit recordings in the guinea pig APC demonstrated a spread of odour-evoked activation from rostral to caudal areas in response to increases in stimulus concentration74. These findings are compatible with an increasing gradient of pyramidal cell inhibition from rostral to caudal APC by local interneurons. This mechanism has received recent support from a study that used fluorescent imaging and glutamate uncaging to characterize inhibitory postsynaptic potentials in layer II and III piriform cells75.
More recent electrophysiological and topological data have improved our understanding of cortical representations of odours at the cellular and synaptic levels. Voltage-clamp recordings from rat piriform slices indicate that focal electrical stimulation of single axons from mitral and tufted cells in the LOT is sufficient to induce robust activity in individual piriform neurons76. On average, each axon makes five synaptic contacts onto each neuron76. As a multi-component odour would activate several glomeruli with divergent cortical projections, this implies that a particular percept is likely to be represented in a spatial ensemble manner across the piriform cortex. Electrophysiological work in vivo77 has helped to refine this idea: non-selective odour-evoked global inhibition across the population of piriform pyramidal neurons — probably mediated through local GABA (γ-aminobutyric acid)-ergic interneurons in a feedforward manner77,78 — dampens all but the strongest bulbar inputs, leading to a sparsening of cortical activity that favours selective activation of neurons that represent the most relevant odour77.
A multi-electrode study in the APC of rats has shown that neural representations of odours are transformed into distributed ensemble patterns across piriform cortical neurons79. Individual neurons responded to multiple odorants, whereas individual odorants evoked responses in multiple neurons. In addition, odour-evoked responses in a given neuron varied across olfactory stimuli in the magnitude of spike firing and in temporal characteristics (including spike latency, duration and respiratory entrainment)79, suggesting that odour representations in the APC are distributed both spatially and temporally.
A recent study addressed the question of odour coding in the piriform cortex by combining in vivo two-photon microscopy and calcium-sensitive dyes to record from a large population of pyramidal neurons at single-cell resolution80. Activation patterns were highly dispersed in response to a diverse panel of odorants and they overlapped across piriform ensembles. A given neuron responded to structurally dissimilar odorants, and neurons responding to a given odorant were spatially dispersed instead of being localized to specific clusters in a single imaging field. Moreover, pyramidal cells immediately adjacent to each other responded to structurally distinct odorants, suggesting a discontinuity in the receptive-field profile that differs not only from the topographical arrangement in the olfactory bulb but also from the canonical organization of primary sensory cortices in other modalities81–83. These findings further support the concept of the piriform cortex as a sensory-associative region that is optimized to incorporate cognitive and experiential factors into the assembly of odour object percepts.
Neocortical projections, thalamocortical relays?
A distinctive feature of the olfactory system is the absence of an obligatory thalamic relay between the sensory periphery and neocortical areas. Monosynaptic projections from the piriform cortex to the OFC31 ensure that odour information has access to the neocortex without passing through the thalamus first. This anatomical arrangement implies either that the olfactory system has no need for the functions that the thalamus carries out in other sensory modalities (for example, feature extraction, gain control, perceptual awareness and corticocortical communication) or that an alternative area, such as the olfactory bulb or the piriform cortex, fulfills this role84–88. In fact, an indirect transthalamic pathway does exist from the piriform cortex to the mediodorsal thalamus and from there to the OFC89,90. The same region of the OFC that receives direct piriform input also receives indirect input by way of the piriform–mediodorsal thalamus pathway90, allowing for an interaction between these two pathways. The role of the mediodorsal thalamus in human olfaction is poorly understood, but some putative functions elucidated by recent studies include attentional processing, reward and valence coding, and associative learning50,51,91–93.
Comparatively more is known about the function of the OFC in olfactory processing. In evolutionary terms, the OFC arrived late on the scene, first appearing about 175 million years ago in mammals94. The fact that non-mammalian vertebrates continue to negotiate the olfactory environment without an OFC suggests that this structure is not essential for odour-guided behaviour95. Instead, what the OFC provides is the ability to decouple odour stimulation from prepotent responses, conferring a behavioural flexibility that is difficult to achieve for ‘lower’ animals. In non-human primates, the tuning specificity of odour-evoked single-unit activity progressively narrows from the olfactory bulb to the OFC96. Furthermore, the involvement of the OFC in odour discrimination learning97,98, encoding of food-based reward value99 and multisensory integration100 underscores its role in higher-order olfactory processing. This reactivity to olfactory valence, as well as associative learning, reward value, odour quality discrimination and multisensory interactions have also been observed in many functional MRI studies of the human OFC43,45,46,49,51,63,101–110.
Interestingly, human patients with focal orbitofrontal lesions — typically those that involve head trauma, tumour or cerebrovascular disease — have notable difficulties with odour discrimination, identification and memory56,111–113, whereas odour detection is relatively spared. This suggests a causal link between higher-order olfactory computations and OFC function. A recent clinical report suggests that olfactory conscious awareness relies on an intact right OFC: a 33-year-old man developed anosmia following right orbitofrontal injury but demonstrated preserved odour-evoked autonomic responses to unpleasant smells, with concomitant activation of the piriform cortex (bilaterally) and the left OFC114. These findings imply a central role of the right OFC in transforming olfactory inputs into conscious percepts, and suggest that the left olfactory pathway is not sufficient to sustain conscious olfaction (see REF. 115 for further discussion of consciousness in olfaction).
Odour object perception
Studies in which olfactory behavioural states and brain activity are monitored simultaneously in the same animal have advanced our understanding of odour object percepts and their cortical signatures. By using a psychological framework to consider questions of object recognition and discrimination, this section will discuss how the properties of real-world odours have shaped the mechanisms of odour object perception in the central olfactory system.
Odorant feature synthesis
Most real-world odours are naturally encountered as complex blends of different odorous molecules. Therefore, a necessary step in the perception of an odour object is the synthesis of discrete odorant parts into a unified perceptual whole. The odour of cooked clam broth, for example, is composed of 49 different odorants, including a caramel-like furanone, a popcorn-like thiazole and a boiled potato-like 3-methyl-thiopropanal116. It is the combined presentation of these molecules to the nose that creates the singular percept of brewed clams.
An effective way to demonstrate synthetic olfactory processing would be to identify brain activity patterns that are selectively triggered by the whole odour rather than by the odorant parts. In one study117, anaesthetized rats were presented with a two-odorant binary mixture ‘A + B’ or one of its components in isolation ‘A’. After 50 s of habituation to the mixture, the rats exhibited reduced spike firing in the olfactory bulb when presented with either A or A + B, suggesting that this brain region did not discriminate between the mixture and its components. However, in the APC the reduction only occurred after A + B presentation, suggesting that A and A + B were recognized as distinct perceptual entities at this level. This indicates that perceptual experience with the binary mixture induced encoding of a novel olfactory object in the piriform cortex but not in the olfactory bulb.
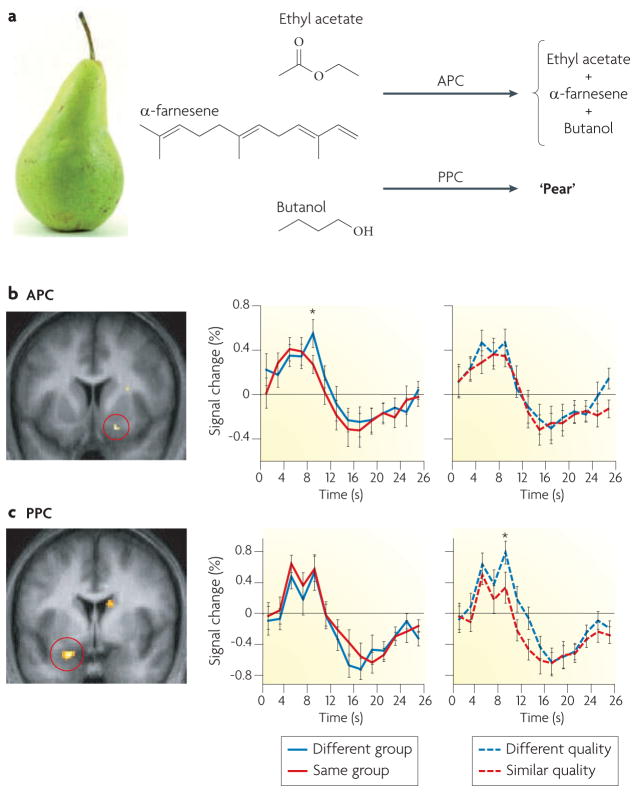
Additional studies have extended these findings. An fMRI cross-adaptation experiment in humans118 showed that odour coding is functionally dissociable in piriform subregions, such that representations of odorant functional group were identified in the APC and representations of odour perceptual quality were identified in the PPC (FIG. 2). In an intriguing example of cross-species functional homology, this dissociation was also observed in an electrophysiological study in rodents: neuronal ensembles of odour-evoked single-unit activity selectively responded to the chemical identity of odorants in the APC and to their perceptual features in the PPC119. The hierarchical transformation of information coding from APC to PPC closely agrees with their anatomical connectivity120,121: most afferent fibres from the olfactory bulb innervate the APC, which is therefore ideally suited to represent elemental features of an odour, whereas the PPC mainly receives associative projections, allowing elemental representations to be unified into perceptual wholes.
Figure 2. Odorant feature synthesis.
a | The d’Anjou pear produces 44 volatile components181, 3 of which are depicted here: ethyl acetate, α-farnesene and butanol. Elemental processing in the anterior piriform cortex (APC) results in coding of odorant chemical identity (top right), whereas synthetic processing in the posterior piriform cortex (PPC) results in coding of odour object quality (bottom right)118,119. b | Sequential presentation of odorants that contain the same functional group results in decreased, or cross-adapting, responses in the APC. The image (left) shows a statistical parametric map of functional MRI data111 from the APC after the sequential presentation, showing the cross-adapting effect. The time-course plots for signal change illustrate a significant main effect for repetition of functional group (left-hand graph) but not for repetition of perceptual quality (right-hand graph). c | In the PPC, cross-adaptation is elicited by the sequential presentation of odorants that contain similar perceptual qualities. The image (left) shows the statistical parametric map of fMRI data111 from the PPC after the sequential presentation. The time-course plots show no significant main effect for repetition of functional group (left-hand graph) but a significant effect for repetition of quality (right-hand graph). The red circles indicate the APC (b) and the PPC (c). * significant at P < 0.05. Parts b and c are modified, with permission, from REF. 118 © (2006) Cell Press.
Several studies have directly compared piriform cortical activity in response to single odorants with that in response to combinations of odorants to assess the mechanisms of information integration. Single-unit recordings in the rodent APC provide evidence for mixture addition (in which the net response exceeds the sum of the component responses) and mixture suppression (in which the net response is lower than the sum of the component responses)117,122,123, with the latter occurring more commonly. Recent in vivo imaging data from rodents indicate that similar rules apply across large populations of piriform neurons in the APC and the PPC: 20–40% of neurons showed a supra-additive effect to binary mixtures, but another 40–60% exhibited a sub-additive effect80. Thus, it appears that piriform representations of odour mixtures involve both synergistic and suppressive mechanisms, both of which can generate a unique ensemble code that is distinguishable from that generated by the parts of the odour mixture.
Odour-background segmentation
An object that is newly presented to the senses can be perceived only if irrelevant (background) stimulus information can be effectively filtered or tuned out. This property is known as figure–ground segmentation124, and its principles apply as much to odour object perception as to object perception in other sensory modes (FIG. 3a).
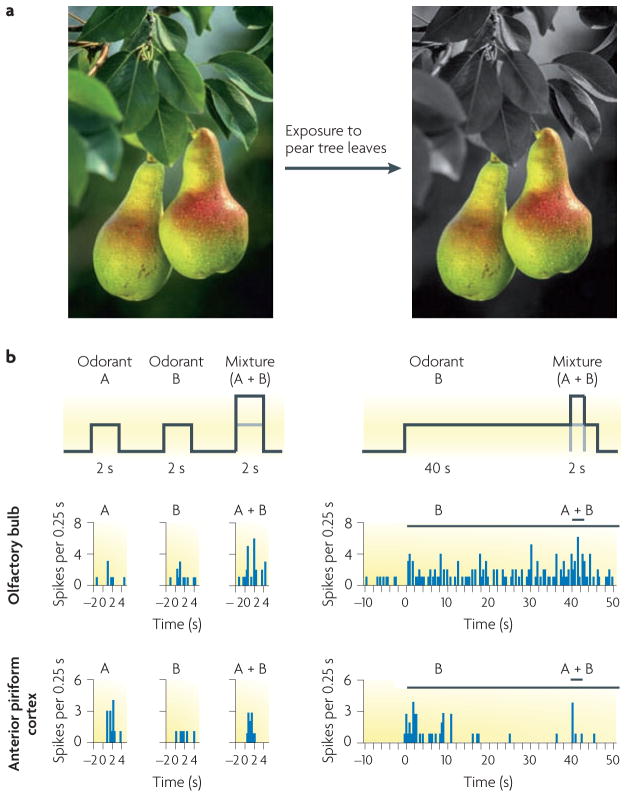
Figure 3. Odour-background segmentation.
a | When considering pears hanging from a tree as an odour object, the olfactory features that arise from the leaves of the pear tree constitute the background stimulus. Prolonged exposure to this background stimulus induces sensory-specific habituation in the piriform cortex, thereby bringing the smell of pears to the foreground of perception. b | In a study performed in rodents, odorants ‘A’ and ‘B’ were presented individually or as a mixture (‘A + B’) on separate trials that lasted for 2 s (top part, left side). In a subsequent habituation phase, B was continuously presented for 40 s, followed by a presentation of the mixture for 2 s (top part, right side). In this presentation of the mixture, B constituted the background against which A was presented. During habituation to B, single-unit activity persisted in the olfactory bulb, showing a constant profile resembling that elicited by the 2-s presentation (middle part, right side), but in the anterior piriform cortex, the activity declined progressively during habituation to B (bottom part, right side). When A was presented together with B immediately after habituation, firing rates in the olfactory bulb were similar to those evoked by individual presentations of A + B (middle part, left side). By contrast, anterior piriform cortex firing rates in response to presentation of the mixture (bottom part, right side) were more similar to those evoked by A alone (bottom part, left side), reflecting the effective segregation of odorant A from the background (odorant B). Part b is modified, with permission, from REF. 122 © (2006) The American Physiological Society.
In the olfactory system, sensory habituation is one method of segmenting a novel odour from older (background) odours. Recent electrophysiological data from rodents indicate that this process takes place in the piriform cortex122 (FIG. 3b). Single-unit responses in the APC and the olfactory bulb were first established during brief presentations (2 s) of 2 odorants and their binary mixture. Rats were subsequently habituated to one of the odorants (the ‘background odorant’) for 40 s and then presented with the other odorant (the ‘target odorant’) concurrently with the background odorant. Single-unit activity in the APC progressively declined during habituation, and presentation of the target odorant along with the background odorant elicited responses that differed from those obtained after a single-step presentation of the binary mixture. Thus, APC neurons responded as if only a single odorant was present. By comparison, neural activity in the olfactory bulb was sustained during habituation and although spike firing rates proportionally increased on addition of the target odorant, these responses were similar in magnitude to those evoked by the binary mixture. Together, these results suggest that filtering of odour background information takes place in the APC, enabling segmentation of new odour objects.
In a variant of the paradigm described above125, rats were trained on a Go/No-go task to respond to a single odorant ‘A’ and to withhold responses to a binary mixture ‘A + B’. In a subsequent manipulation, the other odorant ‘B’ was presented as a background stimulus for 3 minutes. Subsequently, the rats responded to the A + B mixture with a ‘go’ response as if only A had been perceived. In a computational model simulating activity- dependent response adaptation between the olfactory bulb and the APC125–127, it was shown that synaptic depression of projections from the olfactory bulb to APC pyramidal cells was sufficient to separate odour from background, a result mirroring that of the behavioural study. Interestingly, functional imaging data in humans show that piriform cortex exhibits robust olfactory habituation128 in an odorant-specific manner129, indicating that this area may carry out such computations. It remains to be tested whether the human piriform cortex can exhibit odour–ground segmentation.
Discrimination of an odour against its background is not necessarily restricted to the piriform cortex. In fact, stimulus sampling at the nose appears to be the first step in this process. High-frequency sniffing in awake rats during delivery of a tonic background odorant was associated with attenuated odour-evoked input activity to the olfactory bulb and enhanced response sensitivity to a test odorant that was presented subsequently130. Given the rapid kinetics of both olfactory receptor adaptation131,132 and intraglomerular feedback inhibition133,134, either of these mechanisms could plausibly account for the response decrease that was observed during high-frequency sniffs. Such effects were not observed during low- frequency sniffing130, highlighting the importance of the behavioural state in the modulation of odour response patterns even at the initial stages of olfactory information processing, although the contribution of higher-order centrifugal influences on sniff dynamics remains to be determined.
Object constancy and categorization
The sensory input from real-world objects can be unpredictable. Consider a dog, as perceived through different sensory channels. The visual object ‘dog’, could look sausage-shaped from one angle and round from another. The olfactory object ‘dog’, could smell like a wet dog on one day and a freshly shampooed dog on another day. But in all cases, they retain their objectness of dog, and can be identified as such. Thus, the efficiency of object recognition, regardless of the sensory domain, is optimized in a brain that can generalize across multiple versions of the same object and across multiple exemplars of the same object category (FIG. 4a). The ecological variability of odour objects places an additional burden on olfactory systems that are aiming to extract perceptual constancy from inconstant inputs.
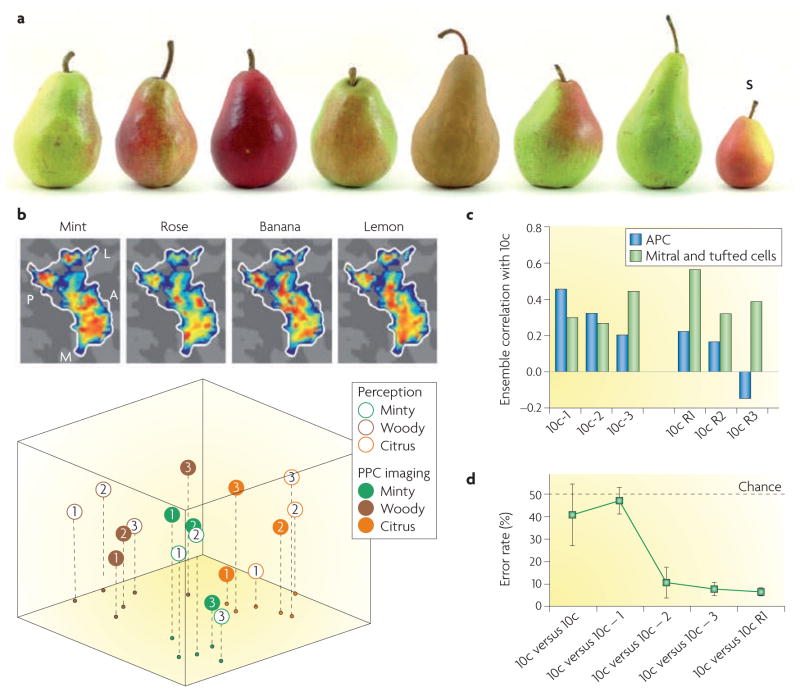
Figure 4. Constancy and categorization of objects.
a | Despite differences in odour, colour, size, shape and texture, all of the objects shown belong to the category ‘pears’. In the piriform cortex, distributed ensemble patterns of odour-evoked activity provide a mechanism for perceptual pattern completion139 of partial, fragmented or non-canonical stimulus inputs. This allows the distinction of one object from a group of objects of the same category — for example, the distinction of the small Seckel pear (S) from a group of pears. b | Data from a human subject, presented on a flattened cortical map of the left posterior piriform cortex (PPC), showing that odorants that differ in perceptual quality evoke distributed and overlapping but unique functional MRI activity patterns in this structure (top part). Considerable response overlap was observed at the level of individual voxels, suggesting that odorants that differ in perceived quality might activate the same voxel. However, at the multi-voxel level, qualitatively distinct odorants evoked unique ensemble patterns of activity. From a dataset of 3 minty, 3 woody, and 3 citrus odorants, 2 ‘distance’ matrices were generated: a 9-by-9 imaging matrix composed of the multi-voxel fMRI signal correlation in the PPC for every odorant pair and a 9-by-9 perceptual matrix composed of perceived differences (reported by the subject) in odour quality between every odorant pair. Multidimensional scaling projections of these distance matrices onto a common three-dimensional space (bottom part) demonstrated robust spatial correspondence between the projected PPC imaging map (shown by filled circles) and the projected perceptual map (shown by open circles)128. A, anterior; L, lateral; M, medial; P, posterior. c | In the rodent anterior piriform cortex (APC), virtual ensemble activity patterns for a 10-odorant mixture (10c) were highly correlated with similar mixtures from which 1 component was removed (10c – 1). These pattern correlations progressively decreased as more odorants were either removed (10c – 2, 10c – 3) or replaced (10c R1, 10c R2, 10c R3) in the mixture (shown by blue bars). This was not observed in the mitral and tufted cell ensembles (shown by green bars). d | A 2-choice odour discrimination test shows that rats make many more errors when trying to distinguish between 10c and 10c – 1 (pattern completion) than when trying to tell apart 10c from 10c – 2, 10c – 3, and 10c R1 (pattern separation). This finding is in agreement with the pattern correlations that are depicted in part c. Part b is modified, with permission, from REF. 139 © (2009) Macmillan Publishers Ltd. All rights reserved. Parts c and d are modified, with permission, from REF. 137 © (2008) Macmillan Publishers Ltd. All rights reserved.
One important aspect of object constancy is the extraction of perceptual sameness across different stimuli. This is known as object categorization. For example, the monomolecular odorants L-carvone and methyl salicylate are different stimuli, but they share a prominent ‘minty’ odour note and can therefore be categorized together. The classification of different stimuli into the same perceptual group helps to conserve cognitive resources, optimize behavioural responses and generalize past experiences to future stimulus encounters135,136.
Recent studies in humans indicate that categorical perception of odours is encoded in the PPC137. High-resolution functional imaging was combined with cortical flattening algorithms and multivariate fMRI analyses to show that odorants that differ in perceptual quality evoked response patterns in the PPC that were both spatially distributed and overlapping, with no evidence for discernible local clusters or patches of activity (FIG. 4b). However, qualitatively distinct odorants elicited unique multi-voxel patterns of PPC ensemble activity, to the extent that category membership for a given odorant could be determined from its voxel-wise pattern. Likewise, the more that odorants were perceived as being similar in odour quality, the more that their fMRI ensemble patterns overlapped in the PPC137. The absence of such effects in the APC, amygdala or OFC highlights the regional specificity of odour categorical perception. These results underscore the advantages of multivariate analysis138 for imaging investigations of olfactory coding, as conventional univariate fMRI analyses did not discriminate between perceptually distinct odorants137. The demonstration of widely dispersed and overlapping odour-evoked responses in the human piriform cortex agrees with the results in rodents, as previously discussed71,79,80, and suggests a common architecture for odour representations in the piriform cortex.
Another key aspect of object constancy is the extraction of perceptual sameness from different samplings or ‘views’ of the same stimulus. The presentation of a natural odour stimulus to the nose may vary depending on weather conditions, wind direction, angle of the nose and respiratory phase, to name just a few possibilities. The consequence is a partial or corrupted stimulus input that needs to be reconstructed to achieve a stable percept. One proposed mechanism to accomplish this is ‘pattern completion’, which has been shown to occur in the rodent piriform cortex (FIG. 4c,d). Rats had difficulty in discriminating between a mixture of 10 odorants and a related mixture from which 1 of the 10 odorants had been removed139, demonstrating a tendency to ‘fill in’ the missing information. Virtual ensembles of single-unit activity in the APC, but not in the olfactory bulb, also disregarded this minor stimulus variation, restoring the pattern representation of the full mixture and thereby ensuring perceptual stability. After the removal of additional components, the behavioural discrimination between full and impoverished mixtures greatly improved, with concomitant divergence of ensemble patterns in the APC. This is consistent with ‘pattern separation’ for odour representations that have more pronounced qualitative differences.
These studies, which span different paradigms and species, provide converging evidence for distributed ensemble coding in the piriform cortex as a mechanism for object categorization and consistency in the olfactory system. Insofar as information about an odorant’s perceptual identity can be estimated from piriform ensemble activity, the fact that these patterns have direct relevance for perception and behaviour fulfills an important criterion140 for what constitutes a genuine odour code. Finally, the distributed pattern representations of odour objects in the PPC resemble the way in which visual objects are encoded in the visual association cortex141–143, supporting the idea that the PPC is an associative brain region.
Odour object discrimination
The ability to group different objects into the same category must be tempered with the flexibility to discriminate among the individual objects. A stereotyped response to all members of a category is non-adaptive as it would lead to diversion and impulsivity that may be an animal’s undoing. A kitten charging towards all rodent-smelling objects may find itself at the beastly whims of an enormous rat, like poor Tom Kitten smeared with butter and rolled up in pastry dough in The Tale of Samuel Whiskers144. Thus, a brain that is optimized for processing information about objects must be able to preserve the identity of specific objects.
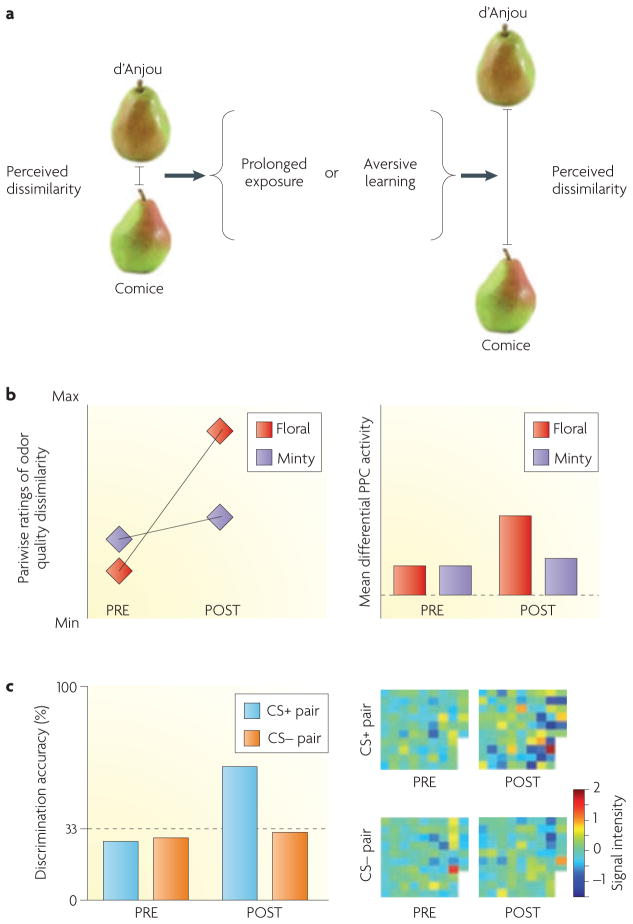
Discriminating among different exemplars of an object category is facilitated by perceptual learning and experience145,146. In the case of odour objects, one simple mechanism for object discrimination is olfactory habituation, which is a form of non-associative perceptual learning that arises from passive, prolonged exposure to a smell147,148. As described earlier, olfactory habituation in the rat piriform cortex has been shown to underlie the discrimination of binary odour mixtures from their components117 and of odour foreground from background122. In humans, continuous exposure (habituation) to 1 odorant for 3 min enhanced the ability to differentiate between categorically similar odorants129 (FIG. 5a,b). Hence, after prolonged experience with a floral-like odorant, subjects were better able to distinguish among different floral odorants, effectively becoming floral ‘experts’. These perceptual changes were paralleled by an odour-evoked enhancement of mean fMRI activity in the PPC and OFC (but not in the APC), implying that these regions are involved in refining odour quality information. Interestingly, across the group of subjects, the magnitude of the learning-induced fMRI signal change in the OFC correlated with the ability to discriminate the odours, suggesting that the OFC may play an active role in mediating this phenomenon.
Figure 5. Odour object discrimination.
a | Perceptual learning. Two varieties of pears, the d’Anjou and the Comice, have similar smells (left part). The ability to discriminate between the two can increase after prolonged exposure to their odours or after aversive learning (middle and right parts). This is an example of learning-induced perceptual plasticity in humans. b | In a functional MRI study of olfactory perceptual learning in humans129, prolonged exposure to one odorant enhanced perceptual differentiation among qualitatively related odorants. After continuous delivery of a floral odorant for 3.5 min, the ratings of odour quality dissimilarity given by subjects increased between floral odorants (left part, shown in red) but not between minty odorants (left part, shown in purple), demonstrating the category-specificity of these learning effects. In the same subjects, experience-dependent changes were observed in the right posterior piriform cortex (PPC) (right part), with greater differences in mean fMRI activity in response to floral odours pre-exposure (PRE) compared with post-exposure (POST), showing how perceptual experience can modulate neural representations of odour quality. c | In an olfactory fMRI study of aversive learning, subjects smelled two odour enantiomers, or mirror-image molecules, before and after an aversive conditioning session in which one of the two enantiomers (the conditioned stimulus, CS) was repeatedly paired with a mild electric shock (the unconditioned stimulus)154. After conditioning, there was an improvement in perceptual discrimination between the previously indistinguishable enantiomers (CS+) (left part, shown by blue bars) but this was not the case for a control pair of odour enantiomers (CS−) (left part, shown by orange bars). Aversive learning was also associated with a reorganization of fMRI ensemble activity patterns in the PPC specifically for the enantiomer pair that was used during conditioning. The grids depict odorant pairwise activation differences at each PPC voxel, with bolder colors indicating greater differences in activation per voxel (right part). Voxels are arranged in columns from top left to bottom right of each grid, in ascending order of signal intensity for the conditioned stimulus before conditioning. Part b is modified, with permission, from REF. 129 © (2006) Cell Press. Part c is modified, with permission, from REF. 154 © (2008) American Association for the Advancement of Science.
Associative learning is another potent mechanism for sharpening odour object discrimination. In rodents there is a long tradition of using aversive conditioning paradigms to test the effects of negative reinforcement on behavioural modulation and odour coding in the olfactory bulb149–153. Such a paradigm was used to determine whether learned fear association between an odorant and a mild footshock enhances the discrimination of odour objects in humans154. After odour–shock pairing, subjects gained the ability to discriminate between odorants that had been perceived as identical before conditioning (FIG. 5c). Multivariate analysis of fMRI data obtained from the same subjects echoed these perceptual improvements: ensemble patterns of PPC activity that were evoked by the two odorants were de-correlated after conditioning154. These findings suggest a potential mechanism by which closely related odour objects can become perceptually individuated, and by which perceptual acuity for behaviourally salient odorants can be enhanced.
Olfactory attentional selection
In the real world, odour objects are rarely encountered in isolation. When simultaneously confronted with several odour objects the olfactory system needs to focus processing resources towards objects that are behaviourally relevant (FIG. 6a). Selective attention also helps to modulate whether an object is grouped with, or differentiated from, other members of a perceptual category.
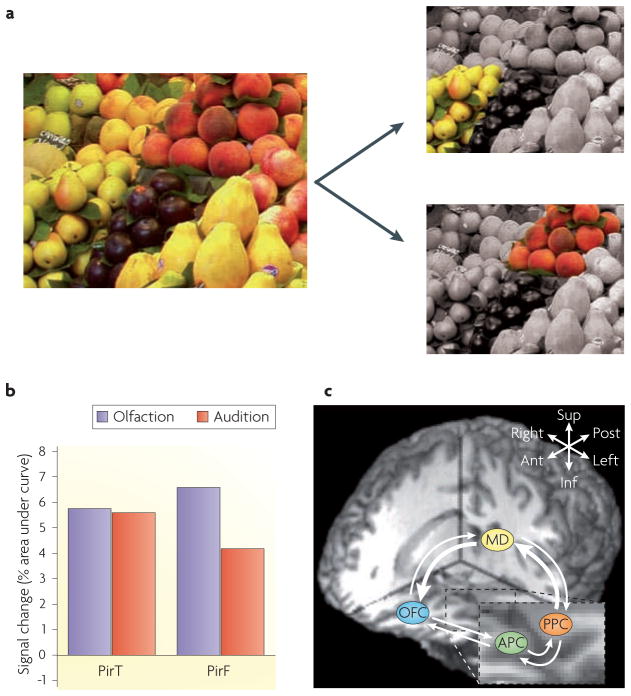
Figure 6. Olfactory attentional selection.
a | The olfactory system may be confronted with many different odorous objects simultaneously, such as an array of different fruit smells at a market (left part). Attentional mechanisms provide a dynamic way of selecting among these competing alternatives, bringing one odour object to the perceptual foreground (right part) in accordance with physiological needs and motivational states. b | In a functional MRI study in humans48, participants were presented with a bimodal olfactory–auditory stimulus and asked to attend to the odour or to the tone. Odorant-evoked activity in the temporal piriform cortex (PirT; roughly approximating to the posterior piriform cortex [PPC]) was not affected by the attentional state, whereas activity in the frontal piriform cortex (PirF; roughly approximating the anterior piriform cortex [APC]) was attention-dependent and more pronounced when the subjects were paying attention to odour instead of tone. c | Odour attention enhances fMRI network coherence along the olfactory transthalamic pathway. The coupling between the PPC and mediodorsal thalamus (MD), and between the mediodorsal thalamus and orbitofrontal cortex (OFC) was strengthened as a result of directing attention to a smell50. This effect was specific to forward connections (that is, connections from the MD to the OFC, rather than from the OFC to the MD), and to the indirect trans-thalamic pathway (compared to the direct pathway between the APC and the OFC). Thick arrows indicate forward pathway connections that were strengthened during odour versus tone attention. Thin arrows indicate other forward and backward connections that were not affected by attentional manipulation. The key (top right of the image) indicates anterior (Ant), posterior (Post), superior (Sup)-inferior (Inf), right and left axes of the brain image. Part b is modified from REF. 48 © (2005) Macmillan Publishers Ltd. All rights reserved. Part c modified, with permission, from REF. 50 © (2008) Society for Neuroscience.
Several recent studies have hinted at some of the brain regions and mechanisms that help to direct olfactory attention to perceptually salient smells. Human fMRI studies have implicated the piriform cortex and the OFC in attention-dependent modulation of odour information processing48,50,155. In subjects who simultaneously smelled an odour and heard a tone48, selective attention to the olfactory (as opposed to the auditory) stimulus elicited differential fMRI activity in frontal, but not temporal, areas of the piriform cortex (FIG. 6b). This shows that anatomically discrete piriform regions are differentially sensitive to the attentional state of the subject. In a variation of this paradigm50, attending to the olfactory stimulus was found to enhance fMRI effective connectivity156 between the PPC and the mediodorsal thalamus, and between the mediodorsal thalamus and the OFC (FIG. 6c), suggesting that the olfactory trans-thalamic pathway is involved in the conscious analysis of smell. Strengthening of the indirect trans-thalamic pathway may also enhance information exchange between the olfactory system and the non-olfactory cortical centers that are connected to the mediodorsal thalamus, providing further opportunities for enriching odour discrimination.
In broad terms, sleep can be considered a global type of attentional shift, in which neural access to sensory information in the environment progressively declines from wakefulness to deep sleep. Single-unit recordings from anaesthetized rats showed that odour-evoked spike firing in the APC was preserved during fast-wave (light) sleep, but blunted during slow-wave (deep) sleep157. In contrast, spike activity in the olfactory bulb was preserved throughout both sleep stages, suggesting that odour information is likely to be filtered at the level of the piriform cortex, although it is also possible that the attenuation in the response in the piriform cortex might be mediated by the OFC or the mediodorsal thalamus. Cortical access to olfactory inputs during light sleep may provide a mechanism by which odours can modulate vigilance, arousal and even cognition — although, paradoxically, a recent study found that odour presented during slow-wave sleep helped consolidate memory for object locations158.
Conclusions and future directions
One of the main aims of this Review has been to describe the concept of an odour object, with a specific focus on the key neurobiological steps that are involved in transforming odorant parts into perceptual wholes. Although the visual and olfactory systems have evolved under different ecological pressures, many of the basic principles that underlie visual object perception are also valid for olfactory objects. This underscores the idea that the function of sensory systems is optimized to detect and encode behaviourally relevant events (objects) that are encountered in the real world. A purely hedonic definition of odour objects has also been proposed. According to this alternative definition, the complexity of odour space reduces to a single continuum of pleasantness, along which each odour has a unique pleasantness score. It is this hedonic score that constitutes the odour object159. Insofar as synthesis, segregation, categorization, discrimination and selection are equally pertinent for guiding behavioural distinctions among smells of different valence, this alternative hypothesis is not incompatible with the definition adopted in this Review.
The studies described here make a strong case for the piriform cortex as an important substrate of odour object perception. The data suggest that the APC and the PPC have different roles in this process. Odorant identity, the composite sum of an odorant’s molecular and chemical constituents, is encoded in the APC, where signal fidelity of the original stimulus can be preserved. Odour quality is encoded in the PPC, a sensory-associative area where object representations are defined and updated through learning and experience. Thus, the APC retains a more static snapshot of the olfactory environment, which in the PPC is transformed into a dynamic percept that varies according to an individual’s past history, present circumstances and future expectations. Finally, the proposed role of the OFC in guiding experience-dependent perceptual plasticity129 is in agreement with the idea that this region provides a top-down signal that helps to resolve odour object representations in the piriform cortex, particularly under conditions of high stimulus uncertainty.
Interestingly, the architecture and connectivity of the piriform cortex bear close resemblance to the network structure of content-addressable memory models160, which excel at memory retrieval despite errors in the input pattern. Given the ecological variance or ‘noise’ of odour object inputs, a content-addressable memory scheme would be a highly effective mechanism for achieving olfactory pattern completion, stimulus generalization and categorical perception. Theoretical and computational studies121,161,162 have shown that the piriform cortex could serve as a content-addressable memory system, and the demonstration of pattern-based odour representations in the piriform cortex139,137,80 provides direct evidence for such a role. This Review has emphasized the links between odour object perception and spatial ensemble coding. However, whether odour object information is embedded in the form of temporal or spatiotemporal codes163 remains to be answered.
The contribution of higher-order cortical regions to odour object coding certainly begs closer scrutiny. Besides a few notable exceptions66,67,97,98,164, contemporary olfactory research in animal models has focused almost exclusively on the neurobiology of olfactory receptor neurons and on the olfactory bulb. This is gradually changing, with some research teams turning their attention towards the new frontier of the piriform cortex. Nevertheless, future work will need to move deeper still, into the amygdala, entorhinal cortex and the OFC, ideally using multi-site recordings to develop more comprehensive models that take into account both ‘bottom- up’ and ‘top-down’ contributions to odour processing at the anatomical, physiological and systems levels. Crucial to this development will be a shift toward using awake, freely sniffing animals165 as anaesthesia is likely to blunt the reciprocal communication between higher-order cortical centres and the piriform cortex165.
As emphasized throughout this Review, the presentation of odour stimuli in their natural context provides a unique way to investigate olfactory brain function, but the experimental simplicity of using synthetic monomolecular odorants has dominated olfactory research so far. However, changes are afoot. Recent studies in rodents166,167 and moths168,169 have begun to explore the impact of complex natural odours and their fract ionated odorant components on behaviour and neural activity in the olfactory system. This provides the opportunity to study odour object coding and perception simultaneously at the level of individual molecules, behaviour and neurobiology, a research direction that should gather momentum.
I conclude with a nod to olfactory research in humans. Due to their ability to provide direct verbal reports and ratings of their perceptual experiences, human subjects offer distinct advantages as research subjects over non-verbal animals, whose percepts can only be inferred indirectly170. In addition, state-of-the-art neuroimaging methods in conjunction with multivariate analytical approaches now make it possible to collect the type of ensemble pattern data in humans that was formerly only possible using animal models. Future improvements on the existing technology will further enhance the scientific value of fMRI in the study of human olfaction. First, the development of new imaging protocols with narrowly defined acquisition windows that are delimited to olfactory regions of interest, should make it possible to surpass the current voxel resolution of 1–2 mm without reducing signal-to-noise ratios. Second, the use of complementary techniques, such as diffusion-tensor imaging and transcranial magnetic stimulation is likely to widen the range of questions that can be investigated and answered. Unquestionably, the olfactory bulb is still the Holy Grail for fMRI studies of the human olfactory system. However, owing to its small size and its location at the interface between the brain and sinus, it has not been possible to investigate the activation patterns of the olfactory bulb in fMRI studies thus far. This has prevented researchers from gaining a good understanding of olfactory processing in humans. Ultimately, for imaging techniques to realize their full potential in the study of human olfaction, it will be essential to obtain simultaneous measurements of odour-evoked activity from the olfactory bulb and the cortex. Recent technical advances using novel imaging sequences that are less susceptible to signal artefact in the area of the olfactory bulb171 hold the promise of bringing the field closer to this goal.
Supplementary Material
Acknowledgments
The author would like to thank the members of the Gottfried Laboratory for helpful comments and suggestions. The author is supported by grants from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, USA.
Glossary
- Odours
Perceived smells that emanate from an odorant or mixture of odorants
- Odorant
A chemical stimulus that is capable of evoking a smell. In terrestrial animals, most odorants are organic molecules of low molecular weight that can gain airborne access to the olfactory system
- Configural odour perception
The perception of an odour mixture that differs from the perception of the mixture elements
- that is
the mixture configuration is perceived holistically rather than as the sum of its parts
- Elemental odour perception
The perception of an odour mixture that is the same as the perception of the summed mixture elements
- Chemotopy
The idea that information about odorant chemical composition is projected onto topographically ordered spatial maps in the olfactory brain
- 2-deoxyglucose methods
A functional brain mapping technique that uses radioactive 2-[14C]deoxyglucose to measure local metabolic patterns of activity-dependent glucose uptake in the central nervous system
- Odour quality
The perceptual character of a smell, such as mintiness, that emanates from an odorous object, as opposed to other perceptual features of a smell, such as intensity or pleasantness
- Focal electrical stimulation
An electrophysiological technique that limits the extent of electrical stimulation to single axon fibres. This permits quantification of synaptic transmission at the level of individual synapses
- Respiratory entrainment
The time at which odorant-evoked neural activity, usually in the form of single-unit spike firing, is most prominent during a particular phase of the respiratory cycle
- Prepotent responses
Behavioural responses with the greatest (‘most potent’) tendencies of being evoked by given sensory stimuli — often innate, reflexive responses
- Tuning specificity
The idea that the response activity (or ‘tuning’) of a given neuron is specific for a particular range of stimulus inputs. This tuning may be highly specific and ‘narrow’ (or non-specific and ‘broad’)
- Olfactory valence
The appetitive or aversive nature of an olfactory stimulus
- Anosmia
Complete loss of the sense of smell, typically caused by trauma, infection or nasal–sinus disease, but often arising without an identifiable cause
- fMRI cross-adaptation
A paradigm based on the concept that sequential presentation of stimuli that share a particular feature, such as olfactory quality, causes response decline (or adaptation) in neural populations that are sensitive to that feature
- Figure–ground segmentation
The ability to discriminate, or segment, foreground details from background distracters. It is also referred to as figure–ground separation or segregation and is a necessary aspect of object perception
- Go/No-go task
In this classic discrimination task, animal or human subjects are required to make a response (‘go’) when presented with a particular stimulus cue and to withhold a response (‘no-go’) when presented with a different one
- Intraglomerular feedback inhibition
An important mechanism of synaptic inhibition in the olfactory bulb glomerulus, in which GABA (γ-aminobutyric acid)-ergic interneurons send direct inhibitory projections back to the same odour-activated mitral or tufted cells, forming a disynaptic feedback arc
- Cortical flattening algorithm
A computational method for unfolding a three-dimensional image of the brain into a flattened two-dimensional cortical sheet, making it easier to visualise topographical patterns of functional activity. These algorithms have been widely applied in retinotopic mapping of the primary visual cortex
- Multivariate fMRI analysis
A method of functional MRI data analysis designed to preserve activity-dependent signal change at the level of individual voxels and that allows the characterization of multi-voxel, pattern-based information within a brain region of interest
- Voxel-wise pattern
An ensemble pattern of functional MRI activity distributed spatially across a set of voxels
- Univariate fMRI analysis
A method of functional MRI data analysis in which data are spatially averaged and smoothed across trials, voxels and subjects, and that yields a mean estimate of peak fMRI activity for a given region of interest
- Pattern completion
A concept that is pertinent to content-addressable memory and in which an object-specific pattern representation can be fully reconstituted, or ‘completed’, from an incomplete stimulus input, helping to achieve perceptual constancy
- Virtual ensembles of single-unit activity
An analytical method that pools single-unit responses from different cells and different subjects into a ‘virtual’ ensemble of a spatially distributed activity
- Aversive conditioning
A type of associative learning paradigm in which a previously innocuous stimulus acquires behavioural salience after being repetitively paired with an aversive event such as an electric shock
- fMRI effective connectivity
A technique that is used to compute the causal links, or ‘effects’, that one brain region exerts on another, based on functional MRI data sets
- Signal fidelity
In the context of neural information processing and transformation, this term refers to how closely an output signal or representation corresponds to the input
- Content-addressable memory
A computationally robust form of associative memory that effectively functions as a reference table that allows the retrieval of specific memories in response to a particular smell
- Object coding
The neurobiological processes by which perceptually relevant information about an object is encoded or represented in the brain
- Diffusion-tensor imaging
An MRI technique that provides a three-dimensional image of water diffusion in the brain. As water diffuses more readily along the axis of myelinated nerve-fibre tracts, this method can be used to obtain a non-invasive estimate of anatomical connectivity between brain areas
- Transcranial magnetic stimulation
A method that involves applying local magnetic stimulation at the scalp to induce electrical excitation of the underlying cortical areas and their projections. The technique can be used to study cortical excitability and reorganization, as well as to disrupt or enhance activity in specific brain regions
Footnotes
Competing interests statement
The author declares no competing financial interests.
References
- 1.Aristotle. On Sense and the Sensible. The Internet Classics Archive. 350 BC [online], http://classics.mit.edu/Aristotle/sense.html.
- 2.Gross CG. Representation of visual stimuli in inferior temporal cortex. Phil Trans R Soc Lond B. 1992;335:3–10. doi: 10.1098/rstb.1992.0001. [DOI] [PubMed] [Google Scholar]
- 3.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis NK, Sheinberg DL. Visual object recognition. Annu Rev Neurosci. 1996;19:577–621. doi: 10.1146/annurev.ne.19.030196.003045. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K. Inferotemporal cortex and object vision. Annu Rev Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- 6.Wallis G, Rolls ET. Invariant face and object recognition in the visual system. Prog Neurobiol. 1997;51:167–194. doi: 10.1016/s0301-0082(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 7.Treisman AM, Kanwisher NG. Perceiving visually presented objects: recognition, awareness, and modularity. Curr Opin Neurobiol. 1998;8:218–226. doi: 10.1016/s0959-4388(98)80143-8. [DOI] [PubMed] [Google Scholar]
- 8.Riesenhuber M, Poggio T. Neural mechanisms of object recognition. Curr Opin Neurobiol. 2002;12:162–168. doi: 10.1016/s0959-4388(02)00304-5. [DOI] [PubMed] [Google Scholar]
- 9.Palmeri TJ, Gauthier I. Visual object understanding. Nature Rev Neurosci. 2004;5:291–303. doi: 10.1038/nrn1364. [DOI] [PubMed] [Google Scholar]
- 10.Freedman DJ, Miller EK. Neural mechanisms of visual categorization: insights from neurophysiology. Neurosci Biobehav Rev. 2008;32:311–329. doi: 10.1016/j.neubiorev.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Kubovy M, Van Valkenburg D. Auditory and visual objects. Cognition. 2001;80:97–126. doi: 10.1016/s0010-0277(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths TD, Warren JD. What is an auditory object? Nature Rev Neurosci. 2004;5:887–892. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- 13.Dubois D. Categories as acts of meaning: the case of categories in olfaction and audition. Cogn Sci Quart. 2000;1:35–68. [Google Scholar]
- 14.Stevenson RJ, Wilson DA. Odour perception: an object-recognition approach. Perception. 2007;36:1821–1833. doi: 10.1068/p5563. Drawing from psychological studies in humans and electrophysiological studies in rodents, this review provides a thoughtful account of odour object perception. [DOI] [PubMed] [Google Scholar]
- 15.Maplet J. A greene forest or a naturall historie, wherein may be seene first the most sufferaigne vertues in all the whole kinde of stones and metals: next of plantes, as of herbes, trees, and shrubs, lastly of brute beastes, foules, fishes, creeping wormes, and serpents, and that alphabetically: so that a table shall not neede. H Denham; London: 1567. [Google Scholar]
- 16.Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson RI. Neural and behavioral mechanisms of olfactory perception. Curr Opin Neurobiol. 2008;18:408–412. doi: 10.1016/j.conb.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay LM, et al. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linster C, Cleland TA. Glomerular microcircuits in the olfactory bulb. Neural Netw. 2009;22:1169–1173. doi: 10.1016/j.neunet.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Restrepo D, Doucette W, Whitesell JD, McTavish TS, Salcedo E. From the top down: flexible reading of a fragmented odor map. Trends Neurosci. 2009;32:525–531. doi: 10.1016/j.tins.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strowbridge BW. Role of cortical feedback in regulating inhibitory microcircuits. Ann NY Acad Sci. 2009;1170:270–274. doi: 10.1111/j.1749-6632.2009.04018.x. [DOI] [PubMed] [Google Scholar]
- 22.Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban NN, Arevian AC. Computing with dendrodendritic synapses in the olfactory bulb. Ann NY Acad Sci. 2009;1170:264–269. doi: 10.1111/j.1749-6632.2009.03899.x. [DOI] [PubMed] [Google Scholar]
- 24.Zou DJ, Chesler A, Firestein S. How the olfactory bulb got its glomeruli: a just so story? Nature Rev Neurosci. 2009;10:611–618. doi: 10.1038/nrn2666. [DOI] [PubMed] [Google Scholar]
- 25.Cleland TA. Early transformations in odor representation. Trends Neurosci. 2010;33:130–139. doi: 10.1016/j.tins.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isaacson JS. Odor representations in mammalian cortical circuits. Curr Opin Neurobiol. 2010 Mar 5; doi: 10.1016/j.conb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay LM, Crk T, Thorngate J. A redefinition of odor mixture quality. Behav Neurosci. 2005;119:726–733. doi: 10.1037/0735-7044.119.3.726. [DOI] [PubMed] [Google Scholar]
- 28.Laing DG, Francis GW. The capacity of humans to identify odors in mixtures. Physiol Behav. 1989;46:809–814. doi: 10.1016/0031-9384(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 29.Livermore A, Laing DG. Influence of training and experience on the perception of multicomponent odor mixtures. J Exp Psychol Hum Percept Perform. 1996;22:267–277. doi: 10.1037//0096-1523.22.2.267. [DOI] [PubMed] [Google Scholar]
- 30.de Olmos J, Hardy H, Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978;181:213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- 31.Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. A tour de force study combining anatomical tracers and electrophysiological recordings to delineate olfactory connectivity in primate olfactory cortex. [DOI] [PubMed] [Google Scholar]
- 32.Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30:123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Haberly LB. In: The Synaptic Organization of the Brain. Shepherd GM, editor. Oxford Univ. Press; New York: 1998. pp. 377–416. [Google Scholar]
- 34.Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleland TA, Linster C, Doty RL. In: Handbook of Olfaction and Gustation. Doty RL, editor. Marcel Dekker; New York: 2003. pp. 165–180. [Google Scholar]
- 36.Wilson DA, Sullivan RM, Doty RL. In: Handbook of Olfaction and Gustation. Doty RL, editor. Marcel Dekker; New York: 2003. pp. 181–201. [Google Scholar]
- 37.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson RJ. An initial evaluation of the functions of human olfaction. Chem Senses. 2010;35:3–20. doi: 10.1093/chemse/bjp083. [DOI] [PubMed] [Google Scholar]
- 39.Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- 40.Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci. 1998;112:541–553. doi: 10.1037//0735-7044.112.3.541. [DOI] [PubMed] [Google Scholar]
- 41.Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 42.Chen S, Murakami K, Oda S, Kishi K. Quantitative analysis of axon collaterals of single cells in layer III of the piriform cortex of the guinea pig. J Comp Neurol. 2003;465:455–465. doi: 10.1002/cne.10844. [DOI] [PubMed] [Google Scholar]
- 43.O’Doherty J, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- 44.Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- 45.Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 47.Gottfried JA, Smith AP, Rugg MD, Dolan RJ. Remembrance of odors past: human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42:687–695. doi: 10.1016/s0896-6273(04)00270-3. [DOI] [PubMed] [Google Scholar]
- 48.Zelano C, et al. Attentional modulation in human primary olfactory cortex. Nature Neurosci. 2005;8:114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
- 49.Cerf-Ducastel B, Murphy C. Neural substrates of cross-modal olfactory recognition memory: an fMRI study. Neuroimage. 2006;31:386–396. doi: 10.1016/j.neuroimage.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Plailly J, Howard JD, Gitelman DR, Gottfried JA. Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci. 2008;28:5257–5267. doi: 10.1523/JNEUROSCI.5607-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zelano C, Montag J, Khan R, Sobel N. A specialized odor memory buffer in primary olfactory cortex. PLoS ONE. 2009;4:e4965. doi: 10.1371/journal.pone.0004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henkin RI, Comiter H, Fedio P, O’Doherty D. Defects in taste and smell recognition following temporal lobectomy. Trans Am Neurol Assoc. 1977;102:146–150. [PubMed] [Google Scholar]
- 54.Abraham A, Mathai KV. The effect of right temporal lobe lesions on matching of smells. Neuropsychologia. 1983;21:277–281. doi: 10.1016/0028-3932(83)90045-3. [DOI] [PubMed] [Google Scholar]
- 55.Eskenazi B, Cain WS, Novelly RA, Friend KB. Olfactory functioning in temporal lobectomy patients. Neuropsychologia. 1983;21:365–374. doi: 10.1016/0028-3932(83)90023-4. [DOI] [PubMed] [Google Scholar]
- 56.Jones-Gotman M, Zatorre RJ. Olfactory identification deficits in patients with focal cerebral excision. Neuropsychologia. 1988;26:387–400. doi: 10.1016/0028-3932(88)90093-0. [DOI] [PubMed] [Google Scholar]
- 57.Martinez BA, et al. Olfactory functioning before and after temporal lobe resection for intractable seizures. Neuropsychology. 1993;7:351–363. [Google Scholar]
- 58.West SE, Doty RL. Influence of epilepsy and temporal lobe resection on olfactory function. Epilepsia. 1995;36:531–542. doi: 10.1111/j.1528-1157.1995.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 59.Eichenbaum H, Shedlack KJ, Eckmann KW. Thalamocortical mechanisms in odor-guided behavior. I Effects of lesions of the mediodorsal thalamic nucleus and frontal cortex on olfactory discrimination in the rat. Brain Behav Evol. 1980;17:255–275. doi: 10.1159/000121803. [DOI] [PubMed] [Google Scholar]
- 60.Litaudon P, Mouly AM, Sullivan R, Gervais R, Cattarelli M. Learning-induced changes in rat piriform cortex activity mapped using multisite recording with voltage sensitive dye. Eur J Neurosci. 1997;9:1593–1602. doi: 10.1111/j.1460-9568.1997.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 61.Chabaud P, et al. Exposure to behaviourally relevant odour reveals differential characteristics in rat central olfactory pathways as studied through oscillatory activities. Chem Senses. 2000;25:561–573. doi: 10.1093/chemse/25.5.561. [DOI] [PubMed] [Google Scholar]
- 62.Mouly AM, Fort A, Ben-Boutayab N, Gervais R. Olfactory learning induces differential long-lasting changes in rat central olfactory pathways. Neuroscience. 2001;102:11–21. doi: 10.1016/s0306-4522(00)00476-0. [DOI] [PubMed] [Google Scholar]
- 63.Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. Eur J Neurosci. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- 65.Martin C, Gervais R, Chabaud P, Messaoudi B, Ravel N. Learning-induced modulation of oscillatory activities in the mammalian olfactory system: the role of the centrifugal fibres. J Physiol (Paris) 2004;98:467–478. doi: 10.1016/j.jphysparis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Calu DJ, Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in posterior piriform cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:1342–1349. doi: 10.1093/cercor/bhl045. This article and those cited in references 67 and 97 represent the small handful of rodent studies that have provided methodical comparisons between odor-related single-unit responses in the piriform cortex and the orbitofrontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:643–652. doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharp FR, Kauer JS, Shepherd GM. Laminar analysis of 2-deoxyglucose uptake in olfactory bulb and olfactory cortex of rabbit and rat. J Neurophysiol. 1977;40:800–813. doi: 10.1152/jn.1977.40.4.800. [DOI] [PubMed] [Google Scholar]
- 69.Astic L, Cattarelli M. Metabolic mapping of functional activity in the rat olfactory system after a bilateral transection of the lateral olfactory tract. Brain Res. 1982;245:17–25. doi: 10.1016/0006-8993(82)90335-3. [DOI] [PubMed] [Google Scholar]
- 70.Cattarelli M, Astic L, Kauer JS. Metabolic mapping of 2-deoxyglucose uptake in the rat piriform cortex using computerized image processing. Brain Res. 1988;442:180–184. doi: 10.1016/0006-8993(88)91449-7. [DOI] [PubMed] [Google Scholar]
- 71.Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- 72.Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- 73.Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nature Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. A comprehensive imaging investigation of odorant-evoked sensory tuning in rodent olfactory bulb that challenges the longstanding dogma that there is a tight systematic relationship between glomerular position and odorant chemical features. [DOI] [PubMed] [Google Scholar]
- 74.Sugai T, Miyazawa T, Fukuda M, Yoshimura H, Onoda N. Odor-concentration coding in the guinea-pig piriform cortex. Neuroscience. 2005;130:769–781. doi: 10.1016/j.neuroscience.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 75.Luna VM, Pettit DL. Asymmetric rostro-caudal inhibition in the primary olfactory cortex. Nature Neurosci. 2010;13:533–535. doi: 10.1038/nn.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 77.Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luna VM, Schoppa NE. GABAergic circuits control input-spike coupling in the piriform cortex. J Neurosci. 2008;28:8851–8859. doi: 10.1523/JNEUROSCI.2385-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. Results from this in vivo optical imaging study indicate that odorant-related response patterns are spatially distributed and overlapping across neuronal ensembles in the mouse piriform cortex. These findings complement well the human data in reference 137. [DOI] [PubMed] [Google Scholar]
- 81.Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- 82.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 84.Kay LM, Sherman SM. An argument for an olfactory thalamus. Trends Neurosci. 2007;30:47–53. doi: 10.1016/j.tins.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 85.Shepherd GM. Perspectives on olfactory processing, conscious perception, and orbitofrontal cortex. Ann NY Acad Sci. 2007;1121:87–101. doi: 10.1196/annals.1401.032. [DOI] [PubMed] [Google Scholar]
- 86.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Phil Trans R Soc Lond B. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones EG. The Thalamus. Cambridge Univ. Press; Cambridge, UK: 2006. [Google Scholar]
- 88.Kastner S, Schneider KA, Wunderlich K. Beyond a relay nucleus: neuroimaging views on the human LGN. Prog Brain Res. 2006;155:125–143. doi: 10.1016/S0079-6123(06)55008-3. [DOI] [PubMed] [Google Scholar]
- 89.Yarita H, Iino M, Tanabe T, Kogure S, Takagi SF. A transthalamic olfactory pathway to orbitofrontal cortex in the monkey. J Neurophysiol. 1980;43:69–85. doi: 10.1152/jn.1980.43.1.69. [DOI] [PubMed] [Google Scholar]
- 90.Price JL, Slotnick BM. Dual olfactory representation in the rat thalamus: an anatomical and electrophysiological study. J Comp Neurol. 1983;215:63–77. doi: 10.1002/cne.902150106. [DOI] [PubMed] [Google Scholar]
- 91.Zelano C, Montag J, Johnson B, Khan R, Sobel N. Dissociated representations of irritation and valence in human primary olfactory cortex. J Neurophysiol. 2007;97:1969–1976. doi: 10.1152/jn.01122.2006. [DOI] [PubMed] [Google Scholar]
- 92.Sela L, et al. Spared and impaired olfactory abilities after thalamic lesions. J Neurosci. 2009;29:12059–12069. doi: 10.1523/JNEUROSCI.2114-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tham WW, Stevenson RJ, Miller LA. The functional role of the medio dorsal thalamic nucleus in olfaction. Brain Res Rev. 2009;62:109–126. doi: 10.1016/j.brainresrev.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Jerison HJ. In: Development of the Prefrontal Cortex: Evolution, Neurobiology, and Behavior. Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Paul, H. Brookes; Baltimore, Maryland: 1997. pp. 27–47. [Google Scholar]
- 95.Gottfried JA. What can an orbitofrontal cortex-endowed animal do with smells? Ann NY Acad Sci. 2007;1121:102–120. doi: 10.1196/annals.1401.018. [DOI] [PubMed] [Google Scholar]
- 96.Tanabe T, Iino M, Takagi SF. Discrimination of odors in olfactory bulb, pyriform-amygdaloid areas, and orbitofrontal cortex of the monkey. J Neurophysiol. 1975;38:1284–1296. doi: 10.1152/jn.1975.38.5.1284. [DOI] [PubMed] [Google Scholar]
- 97.Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- 98.Critchley HD, Rolls ET. Olfactory neuronal responses in the primate orbitofrontal cortex: analysis in an olfactory discrimination task. J Neurophysiol. 1996;75:1659–1672. doi: 10.1152/jn.1996.75.4.1659. [DOI] [PubMed] [Google Scholar]
- 99.Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- 100.Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC. Flavor processing: more than the sum of its parts. Neuroreport. 1997;8:3913–3917. doi: 10.1097/00001756-199712220-00014. [DOI] [PubMed] [Google Scholar]
- 102.Royet JP, et al. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- 103.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 104.Anderson AK, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 105.De Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- 106.Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–386. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 107.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 108.Small DM, et al. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 2004;92:1892–1903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- 109.Osterbauer RA, et al. Color of scents: chromatic stimuli modulate odor responses in the human brain. J Neurophysiol. 2005;93:3434–3441. doi: 10.1152/jn.00555.2004. [DOI] [PubMed] [Google Scholar]
- 110.Boyle JA, Frasnelli J, Gerber J, Heinke M, Hummel T. Cross-modal integration of intranasal stimuli: a functional magnetic resonance imaging study. Neuroscience. 2007;149:223–231. doi: 10.1016/j.neuroscience.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 111.Potter H, Butters N. An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia. 1980;18:621–628. doi: 10.1016/0028-3932(80)90101-3. [DOI] [PubMed] [Google Scholar]
- 112.Zatorre RJ, Jones-Gotman M. Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain. 1991;114:71–84. [PubMed] [Google Scholar]
- 113.Jones-Gotman M, Zatorre RJ. Odor recognition memory in humans: role of right temporal and orbitofrontal regions. Brain Cogn. 1993;22:182–198. doi: 10.1006/brcg.1993.1033. [DOI] [PubMed] [Google Scholar]
- 114.Li W, et al. Right orbitofrontal cortex mediates conscious olfactory perception. Psychol Sci. doi: 10.1177/0956797610382121. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stevenson RJ. Phenomenal and access consciousness in olfaction. Conscious Cogn. 2009;18:1004–1017. doi: 10.1016/j.concog.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 116.Sekiwa Y, Kubota K, Kobayashi A. Characteristic flavor components in the brew of cooked clam (Meretrix lusoria) and the effect of storage on flavor formation. J Agric Food Chem. 1997;45:826–830. [Google Scholar]
- 117.Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. References 117, 147 and 148 comprise an important set of studies establishing that odour configural processing occurs in rodent anterior piriform cortex as a consequence of olfactory habituation; a non-associative form of perceptual learning. Insights from this study paved the way for a similar but human-based study that is described in reference 129. [DOI] [PubMed] [Google Scholar]
- 118.Gottfried JA, Winston JS, Dolan RJ. Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron. 2006;49:467–479. doi: 10.1016/j.neuron.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 119.Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci USA. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwob JE, Price JL. The cortical projection of the olfactory bulb: development in fetal and neonatal rats correlated with quantitative variations in adult rats. Brain Res. 1978;151:369–374. doi: 10.1016/0006-8993(78)90891-0. [DOI] [PubMed] [Google Scholar]
- 121.Haberly LB. Neuronal circuitry in olfactory cortex: anatomy and functional implications. Chem Senses. 1985;10:219–238. An authoritative review of olfactory cortical anatomy and function. It was among the first to propose that the piriform cortex might serve as a content-addressable memory system for efficient perceptual reconstruction of odour inputs. [Google Scholar]
- 122.Kadohisa M, Wilson DA. Olfactory cortical adaptation facilitates detection of odors against background. J Neurophysiol. 2006;95:1888–1896. doi: 10.1152/jn.00812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoshida I, Mori K. Odorant category profile selectivity of olfactory cortex neurons. J Neurosci. 2007;27:9105–9114. doi: 10.1523/JNEUROSCI.2720-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lamme VA. The neurophysiology of figure-ground segregation in primary visual cortex. J Neurosci. 1995;15:1605–1615. doi: 10.1523/JNEUROSCI.15-02-01605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Linster C, Henry L, Kadohisa M, Wilson DA. Synaptic adaptation and odor-background segmentation. Neurobiol Learn Mem. 2007;87:352–360. doi: 10.1016/j.nlm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 126.Best AR, Thompson JV, Fletcher ML, Wilson DA. Cortical metabotropic glutamate receptors contribute to habituation of a simple odor-evoked behavior. J Neurosci. 2005;25:2513–2517. doi: 10.1523/JNEUROSCI.5298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yadon CA, Wilson DA. The role of metabotropic glutamate receptors and cortical adaptation in habituation of odor-guided behavior. Learn Mem. 2005;12:601–605. doi: 10.1101/lm.41405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sobel N, et al. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 2000;83:537–551. doi: 10.1152/jn.2000.83.1.537. This pioneering olfactory fMRI experiment demonstrated that continuous odour stimulation elicits profound response habituation in the human piriform cortex, providing an important foundation for the design of subsequent fMRI studies. [DOI] [PubMed] [Google Scholar]
- 129.Li W, Luxenberg E, Parrish T, Gottfried JA. Learning to smell the roses: experience-dependent neural plasticity in human piriform and orbitofrontal cortices. Neuron. 2006;52:1097–1108. doi: 10.1016/j.neuron.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nature Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- 131.Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. J Neurosci. 1998;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duchamp-Viret P, Duchamp A, Chaput MA. Peripheral odor coding in the rat and frog: quality and intensity specification. J Neurosci. 2000;20:2383–2390. doi: 10.1523/JNEUROSCI.20-06-02383.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McGann JP, et al. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 134.Wachowiak M, et al. Inhibition [corrected] of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol. 2005;94:2700–2712. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosch EH. In: Cognition and Categorization. Rosch EH, Lloyd B, editors. Erlbaum Associates; Hillsdale, New Jersey: 1978. pp. 27–48. [Google Scholar]
- 136.Miller EK, Nieder A, Freedman DJ, Wallis JD. Neural correlates of categories and concepts. Curr Opin Neurobiol. 2003;13:198–203. doi: 10.1016/s0959-4388(03)00037-0. [DOI] [PubMed] [Google Scholar]
- 137.Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nature Neurosci. 2009;12:932–938. doi: 10.1038/nn.2324. In this study, high-resolution olfactory fMRI was combined with multivariate pattern analyses to show that odour object categories are encoded as spatially distributed ensemble representations in the human posterior piriform cortex. These findings complement well the mouse data in reference 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38:649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nature Neurosci. 2008;11:1378–1380. doi: 10.1038/nn.2217. This article presents compelling evidence for the role of rodent piriform cortex, but not olfactory bulb, in the perceptual pattern completion of degraded odour inputs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- 141.Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 142.Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage. 2003;19:261–270. doi: 10.1016/s1053-8119(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 143.Haushofer J, Livingstone MS, Kanwisher N. Multivariate patterns in object-selective cortex dissociate perceptual and physical shape similarity. PLoS Biol. 2008;6:e187. doi: 10.1371/journal.pbio.0060187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Potter B. The Tale of Samuel Whiskers, or The Roly-Poly Pudding. Frederick Warne & Co; London: 1908. [Google Scholar]
- 145.Gibson EJ. An Odyssey in Learning and Perception. MIT Press; Cambridge, Massachusetts: 1991. [Google Scholar]
- 146.Goldstone RL. Perceptual learning. Annu Rev Psychol. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- 147.Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- 148.Wilson DA. Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol. 2000;84:3036–3042. doi: 10.1152/jn.2000.84.6.3036. [DOI] [PubMed] [Google Scholar]
- 149.Pager J, Royet JP. Some effects of conditioned aversion on food intake and olfactory bulb electrical responses in the rat. J Comp Physiol Psychol. 1976;90:67–77. doi: 10.1037/h0077254. [DOI] [PubMed] [Google Scholar]
- 150.Freeman WJ, Schneider W. Changes in spatial patterns of rabbit olfactory EEG with conditioning to odors. Psychophysiology. 1982;19:44–56. doi: 10.1111/j.1469-8986.1982.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 151.Coopersmith R, Lee S, Leon M. Olfactory bulb responses after odor aversion learning by young rats. Brain Res. 1986;389:271–277. doi: 10.1016/0165-3806(86)90195-1. [DOI] [PubMed] [Google Scholar]
- 152.Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- 153.Sullivan RM, Wilson DA. Dissociation of behavioral and neural correlates of early associative learning. Dev Psychobiol. 1995;28:213–219. doi: 10.1002/dev.420280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. Following rodent models of aversive learning (references 149–153), this human imaging study demonstrated that previously indistinguishable odorants become discriminable after odour–footshock conditioning. This was observed both at the level of odour perception and at the level of functional MRI ensemble pattern activity in the human posterior piriform cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sabri M, Radnovich AJ, Li TQ, Kareken DA. Neural correlates of olfactory change detection. Neuroimage. 2005;25:969–974. doi: 10.1016/j.neuroimage.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 156.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 157.Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 158.Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 159.Yeshurun Y, Sobel N. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol. 2010;61:219–241. C211–215. doi: 10.1146/annurev.psych.60.110707.163639. [DOI] [PubMed] [Google Scholar]
- 160.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hasselmo ME, Wilson MA, Anderson BP, Bower JM. Associative memory function in piriform (olfactory) cortex: computational modeling and neuropharmacology. Cold Spring Harb Symp Quant Biol. 1990;55:599–610. doi: 10.1101/sqb.1990.055.01.057. [DOI] [PubMed] [Google Scholar]
- 162.Wilson M, Bower JM. Cortical oscillations and temporal interactions in a computer simulation of piriform cortex. J Neurophysiol. 1992;67:981–995. doi: 10.1152/jn.1992.67.4.981. [DOI] [PubMed] [Google Scholar]
- 163.Kay LM, Stopfer M. Information processing in the olfactory systems of insects and vertebrates. Semin Cell Dev Biol. 2006;17:433–442. doi: 10.1016/j.semcdb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 164.Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci. 2008;28:6664–6669. doi: 10.1523/JNEUROSCI.0178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rinberg D, Gelperin A. Olfactory neuronal dynamics in behaving animals. Semin Cell Dev Biol. 2006;17:454–461. doi: 10.1016/j.semcdb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 166.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 167.Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 168.Riffell JA, Lei H, Christensen TA, Hildebrand JG. Characterization and coding of behaviorally significant odor mixtures. Curr Biol. 2009;19:335–340. doi: 10.1016/j.cub.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Riffell JA, Lei H, Hildebrand JG. Inaugural Article: Neural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc Natl Acad Sci USA. 2009;106:19219–19226. doi: 10.1073/pnas.0910592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zelano C, Sobel N. Humans as an animal model for systems-level organization of olfaction. Neuron. 2005;48:431–454. doi: 10.1016/j.neuron.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 171.Parrish T, Chen YF, Li W, Howard J, Gottfried JA. High resolution 3D functional images of the human olfactory bulb using Passband SSFP at 3T. ISMRM website. 2008 [online], http://www.ismrm.org/08/EP4.htm#fMRI:AcquisitionMethods.
- 172.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 173.Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev. 2004;62:S184–188. doi: 10.1111/j.1753-4887.2004.tb00097.x. discussion S224–141. [DOI] [PubMed] [Google Scholar]
- 174.Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 175.Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 176.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 177.Linster C, et al. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- 179.Maresh A, Rodriguez Gil D, Whitman MC, Greer CA. Principles of glomerular organization in the human olfactory bulb — implications for odor processing. PLoS ONE. 2008;3:e2640. doi: 10.1371/journal.pone.0002640. This important anatomical study suggests that the human olfactory bulb is organized in a fundamentally different way to the rodent analogue, raising questions about cross-species functional homologies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Smith GE. Notes upon the natural subdivision of the cerebral hemisphere. J Anat Physiol. 1901;35:431–454. [PMC free article] [PubMed] [Google Scholar]
- 181.Sander J. In: Archiv für Anatomie, Physiologie und wissenschaftliche Medicin. Reichert CB, Du Bois-Reymond E, editors. Verlag von Veit et Comp; Leipzig: 1866. pp. 750–756. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.