Abstract
Objective
To identify predictors and outcomes associated with a birth weight of 4000 g or more in Lusaka, Zambia.
Methods
Data from women who delivered between February 2006 and August 2011 were obtained from electronic perinatal records at 25 public sector facilities in Lusaka. Macrosomia was defined as a birth weight of 4000 g or more and normal birth weight as 2500–3999 g. Maternal and newborn characteristics were analyzed for association with macrosomia.
Results
There were 4717 macrosomic and 187 117 normal birth weight newborns. The strongest predictors of macrosomia were high BMI (adjusted odds ratio [AOR], 2.88; 95% confidence interval [CI], 1.95–4.24), prior macrosomic newborn (AOR, 7.60; 95% CI, 6.81–8.49), and history of diabetes (AOR, 3.09; 95% CI, 1.36–6.98). Macrosomic newborns were at increased risk for cesarean delivery (AOR, 1.63; 95% CI, 1.35–1.96), fresh stillbirth (AOR, 2.24; 95% CI, 1.56–3.21), Apgar score of under 7 at 5 minutes (AOR, 2.03; 95% CI, 1.33–3.11), and neonatal intensive care admission (AOR, 2.07; 95% CI, 1.32–3.23).
Conclusion
Screening for macrosomia should be considered for high-risk patients in Sub-Saharan Africa. Institutional delivery at facilities with operating rooms and neonatal intensive care services should be encouraged.
Keywords: Cesarean delivery, Macrosomia, Predictors, Stillbirth, Zambia
1. Introduction
Fetal macrosomia is associated with an increased risk of morbidity and mortality to both the pregnant woman and her newborn. Maternal complications include higher rates of cesarean delivery [1,2] and third and fourth degree perineal lacerations during vaginal delivery [2–4]. Potential newborn complications include perinatal mortality, shoulder dystocia, and birth asphyxia [1,5]. Where access to cesarean delivery is limited, macrosomia increases the probability of maternal death from obstructed labor, sepsis, and/or postpartum hemorrhage [5,6].
In high-income countries, the prevalence of fetal macrosomia is a public health issue of growing concern. Mean newborn birth weight has steadily risen over the past 4 decades in Europe, North America, and Australia, as has the relative number of macrosomic newborns [4,7–11]. Increased maternal body mass index (BMI) and decreased cigarette smoking have been proposed as reasons behind this trend [12,13]. By contrast, fewer data are available for lower-income countries, particularly in Sub-Saharan Africa where the prevalence of overweight and obese adults is also on the rise [14]. A better understanding of the clinical epidemiology of fetal macrosomia would greatly inform obstetric care in such resource-constrained settings.
Lusaka, Zambia, is a city that has a population of approximately 2 million and has an extensive public-sector network for prenatal and postnatal services across 25 facilities. Most pregnant women deliver at 13 of these sites, which include the tertiary referral hospital University Teaching Hospital. The Zambian national guidelines regarding pregnancy, childbirth, postpartum, and newborn care are based on recommendations by the WHO that promote focused prenatal care comprising 4 routine visits and facility delivery by a skilled birth attendant [15]. Although higher risk pregnancies are referred to specialist doctors stationed at hospitals, nearly all maternal care in the country’s capital is provided by midwives. The aim of the present study was to identify predictors and adverse outcomes associated with fetal macrosomia in public-sector health centers in Lusaka, Zambia.
2. Materials and methods
Retrospective data from women who established prenatal care and delivered within the public sector in Lusaka, Zambia, between February 1, 2006, and August 31, 2011, were analyzed. Data were obtained from the Zambia Electronic Perinatal Record System (ZEPRS), a networked patient-level electronic medical record system that captures information regarding prenatal care, labor and delivery, and postnatal care [16] and that is used in all 24 primary care sites and University Teaching Hospital in Lusaka. Use of these routinely collected clinical data was approved by ethical review committees at the University of Zambia; the University of Alabama at Birmingham, USA, and the University of North Carolina at Chapel Hill, USA. Owing to the retrospective nature of the study, informed consent was not obtained.
Mother–newborn pairs were categorized according to the newborn’s birth weight. Although multiple definitions have been proposed, a newborn birth weight of 4000 g or more was classified as fetal macrosomia [4] and these newborns were not graded further [17] in the present study. The comparison group comprised only newborns with a birth weight between 2500 g and 3999 g. Newborns under 2500 g at birth were not included in the analysis. Because estimates of gestational age can be inaccurate in the study setting, newborns with a lower birth weight probably comprise a heterogeneous group of preterm deliveries and growth-restricted newborns. Multiple gestations were excluded for similar reasons.
Maternal demographic and clinical information that are routinely captured at the first prenatal visit were examined. Historical data, such as prior delivery of a macrosomic newborn, prior stillbirth, previous newborn death (at <1 year), and personal or family history of diabetes mellitus, were ascertained by self-report. BMI (calculated as weight in kilograms divided by the square of height in meters) was determined for those women whose weight and height were documented at the first prenatal visit. Other potential risk factors were evaluated for fetal macrosomia including maternal age at first prenatal visit, highest level of maternal education attained, parity, maternal weight of 90 kg or more at first prenatal visit, chronic hypertension, and sex of newborn.
Several outcome measures were considered. Hypertensive disorders of pregnancy were defined by any one of the following: a systolic blood pressure of 140 mm Hg or higher after 20 weeks of gestation, a diastolic blood pressure 90 mm Hg or higher after 20 weeks of gestation, or documented diagnosis of pre-eclampsia or eclampsia in the medical record. Maternal mortality included prepartum, intrapartum, and postpartum deaths that were recorded in ZEPRS.
Stillbirths were diagnosed and classified by health providers attending the delivery. A macerated stillbirth was defined as a neonate born dead with degenerative skin changes, as recorded by the delivering clinician, and was presumed to have occurred 12 hours or more before delivery. A fresh stillbirth lacked such skin changes and probably occurred within 12 hours of delivery. Descriptions of neonatal death were limited to the early period of 7 days or fewer after birth; ascertainment of neonatal deaths was generally poor after this 1-week threshold, especially when newborns were readmitted after initial hospital discharge.
Other outcomes, all of which were extracted from individual medical records, were mode of delivery, cephalopelvic disproportion or dystocia as an indication for cesarean delivery, perineal laceration, postpartum hemorrhage, any other maternal complication (including a range of diagnoses from septicemia to renal failure), an Apgar score at 5 minutes of less than 7, and admission to the neonatal intensive care unit (NICU). The outcomes of stillbirth, cesarean delivery, and NICU admission were also compared according to 4 birth weight categories: 2500–2999 g, 3000–3999 g, 4000–4499 g, and 4500 g or more.
All analyses were conducted via SAS version 9.1 (SAS Institute, Cary, NC, USA). Maternal and newborn predictors and pregnancy outcomes were analyzed for association with macrosomia via Pearson χ2 test, Wilcoxon rank-sum test, and logistic regression to convey the crude odds ratio (COR) with corresponding 95% confidence interval (CI). For continuous variables, medians and interquartile ranges (IQR) were determined; for categorical variables, percentages were calculated.
An adjusted odds ratio (AOR) was calculated by using a generalized linear model with a binomial family of distributions and logit link function accounting for repeated measures of multiple pregnancies. Maternal and newborn characteristics that were significant at a P value of less than 0.05 were included in the multivariable regression. When 2 variables showed high correlation with each another, only 1 was selected for inclusion in the model.
3. Results
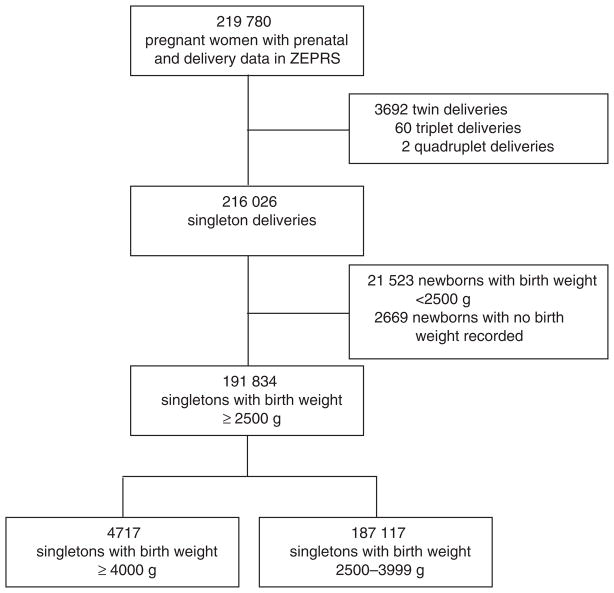
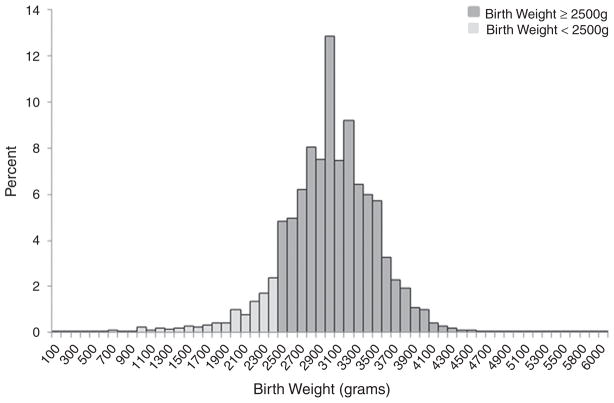
During the study period, 219 780 documented newborn deliveries with at least 1 prenatal visit were recorded in ZEPRS (Fig. 1). Of these, 216 026 (98.3%) newborns were singletons. The birth weight distribution of all 213 357 singletons with a documented birth weight ranged from 100 g to 6000 g with a mean birth weight of 3005 ± 501.6 g (Fig. 2). For the analysis, 21 523 (10.0%) newborns weighing less than 2500 g and 2669 (1.2%) newborns with missing birth weight data were excluded.
Fig. 1.
Study flow diagram for the inclusion of data on macrosomic neonates born in Lusaka, Zambia, between February 2006 and August 2011.
Fig. 2.
Distribution of birth weight for singletons born in Lusaka, Zambia, between February 2006 and August 2011 (n = 216 026). The birth weight distribution is divided by 100-g intervals and reflects all singleton newborns with birth weight documented in ZEPRS. The lighter-colored bars represent newborns with a birth weight of less than 2500 g who were excluded from the analysis.
For the 191 834 women included in the analysis cohort, the median maternal age at the first prenatal visit was 25 years (IQR, 21–29 years), the median parity was 1 (IQR, 0–3 years), and the median baseline BMI was 23.5 (IQR, 21.5–26.2). Among the 191 834 singletons included in the analysis, 4717 (2.5%) weighed more than 4000 g and were thus categorized as macrosomic.
Compared with women delivering a newborn weighing 2500–3999 g, women who gave birth to macrosomic newborns were older and more educated, and had higher parity (P < 0.01) (Table 1). They also had a higher baseline BMI (P < 0.01). Other risk factors that were significant included maternal weight of more than 90 kg at first prenatal visit, prior newborn weighing 4000 g or more, prior stillbirth, previous death of infant younger than 1 year, personal or family history of diabetes mellitus, chronic hypertension, and male sex of newborn. Several risk factors remained significant in the multivariable analysis (Table 2). The strongest predictors of macrosomia were baseline BMI of 30 or more, prior newborn weighing 4000 g or more, and personal or family history of diabetes mellitus.
Table 1.
Maternal and newborn characteristics among normal birth weight and macrosomic pregnancies in Lusaka, Zambia (n = 191 834).a
| Characteristic | Birth weight 2500–3999 g (n = 187 117) | Birth weight ≥4000 g (n = 4717) | P value |
|---|---|---|---|
| Maternal age, y | 25 (21–29) | 28 (24–32) | <0.01d |
| <20 | 33 675 (18.0) | 268 (5.7) | <0.01e |
| 20–34 | 139 431 (74.5) | 3847 (81.6) | |
| ≥35 | 14 011 (7.5) | 602 (12.8) | |
| Maternal education | |||
| None or primary | 73 101 (45.3) | 1719 (40.9) | <0.01e |
| Secondary and higher | 88 178 (54.7) | 2483 (59.1) | |
| Parityb | 1 (0–3) | 2 (1–3) | <0.01d |
| 0 | 51 095 (29.3) | 547 (12.7) | <0.01e |
| 1–4 | 114 136 (65.6) | 3,362 (78.4) | |
| ≥5 | 8879 (5.1) | 382 (8.9) | |
| Baseline BMIc | 23.5 (21.5–26.1) | 26.2 (23.4–29.8) | <0.01d |
| <18.5 | 3289 (2.8) | 41 (1.4) | <0.01e |
| 18.5–24.9 | 75 043 (63.2) | 1135 (38.4) | |
| 25.0–29.9 | 30 464 (25.7) | 1057 (35.7) | |
| ≥30.0 | 9934 (8.4) | 724 (24.5) | |
| Weight >90 kg at first prenatal visit | 2968 (1.9) | 369 (9.3) | <0.01e |
| Prior newborn weighing ≥4000 g | 5596 (5.3) | 998 (30.8) | <0.01e |
| Prior stillbirth | 2860 (1.5) | 104 (2.2) | <0.01e |
| Previous infant death | 15 494 (8.3) | 474 (10.0) | <0.01e |
| Personal or family history of diabetes mellitus | 135 (0.1) | 22 (0.5) | <0.01e |
| Chronic hypertension | 3923 (2.1) | 170 (3.6) | <0.01e |
| Sex of newborn | |||
| Female | 92 405 (49.4) | 1643 (34.8) | <0.01e |
| Male | 94 712 (50.6) | 3074 (65.2) | |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
Values are given as median (interquartile range) or number (percentage) unless stated otherwise.
Data were available for 174 110 women in the first group and 4291 women in the second group.
Data were available for 118 730 women in the first group and 2957 women in the second group.
By Wilcoxon rank-sum test.
By Pearson χ2 test.
Table 2.
Crude and adjusted odds ratios for predictors of macrosomic newborns in Lusaka, Zambia (n = 4717).
| Characteristic | Crude OR (95% CI) | Adjusted OR (95% CI)a |
|---|---|---|
| Maternal age, y | ||
| <20 | 1.0 | 1.0 |
| 20–34 | 3.45 (3.05–3.91) | 1.92 (1.29–3.02) |
| ≥35 | 5.36 (4.64–6.21) | 1.90 (1.35–2.66) |
| Maternal education | ||
| None or primary | 1.0 | 1.0 |
| Secondary and higher | 1.20 (1.12–1.27) | 1.29 (1.17–1.42) |
| Parity | ||
| 0 | 1.0 | 1.0 |
| 1–4 | 2.75 (2.51–3.01) | 1.63 (1.19–2.22) |
| ≥5 | 4.01 (3.51–4.58) | 1.86 (1.29–2.67) |
| Baseline BMI | ||
| <18.5 | 1.0 | 1.0 |
| 18.5–24.9 | 1.20 (0.88–1.64) | 0.92 (0.63–1.34) |
| 25.0–29.9 | 2.75 (2.01–3.76) | 1.72 (1.17–2.52) |
| ≥30.0 | 5.79 (4.22–7.94) | 2.88 (1.95–4.24) |
| Weight >90 kg at first prenatal visitb | 5.35 (4.77–5.99) | |
| Prior newborn weighing ≥4000 g | 7.97 (7.37–8.63) | 7.60 (6.81–8.49) |
| Prior stillbirth | 1.46 (1.20–1.78) | 1.03 (0.74–1.42) |
| Previous infant death | 1.24 (1.12–1.36) | 1.02 (0.87–1.18) |
| Personal or family history of diabetes mellitus | 6.07 (3.83–9.61) | 3.09 (1.36–6.98) |
| Chronic hypertension | 1.73 (1.48–2.03) | 1.12 (0.87–1.43) |
| Sex of newborn | ||
| Female | 1.0 | 1.0 |
| Male | 1.82 (1.71–1.94) | 1.95 (1.76–2.15) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); CI, confidence interval; OR, odds ratio.
ORs were adjusted for age, education, parity, BMI, prior newborn weighing ≥4000 g, prior stillbirth, previous infant death, personal or family history of diabetes, chronic hypertension, and sex of newborn.
The generalized linear model accounting for covariance was not applicable.
Pregnancy outcomes were evaluated for macrosomic newborns (Table 3). Compared with newborns weighing 2500–3999 g, macrosomic newborns were at increased risk for cesarean delivery, and cephalopelvic disproportion or dystocia was a more likely indication for cesarean delivery in fetal macrosomia. Women with macrosomic newborns were more likely than women with newborns weighing 2500–3999 g to develop hypertensive disorders of pregnancy. Macrosomic newborns were at increased risk for fresh stillbirth, low Apgar score (<7) at 5 minutes, and NICU admission compared with newborns weighing 2500–3999 g at birth. No significant differences were observed between the groups for other complications, including perineal laceration, postpartum hemorrhage, maternal mortality, and early neonatal death.
Table 3.
Pregnancy outcomes for normal birth weight and macrosomic newborns in Lusaka, Zambia (n = 191 834).a
| Pregnancy outcome | Birth weight 2500–3999 g (n = 187 117) | Birth weight ≥4000 g (n = 4717) | Crude OR (95% CI) | Adjusted OR (95% CI)b |
|---|---|---|---|---|
| Mode of delivery | ||||
| Spontaneous or assisted vaginal delivery | 178 834 (96.5) | 4244 (90.7) | 1.0 | 1.0 |
| Cesarean delivery | 6472 (3.5) | 435 (9.3) | 2.78 (2.51–3.07) | 1.63 (1.35–1.96) |
| Cephalopelvic disproportion or dystocia as indication for Cesarean delivery | 1536 (23.8) | 148 (34.0) | 1.66 (1.35–2.04) | 2.88 (1.96–4.24) |
| Hypertensive disorders of pregnancy including eclampsia | 10 418 (5.6) | 467 (9.9) | 1.85 (1.68–2.04) | 1.42 (1.23–1.65) |
| Perineal laceration | 70 (0.0) | 4 (0.1) | 2.27 (0.83–6.21) | 1.97 (0.42–9.19) |
| Postpartum hemorrhage | 441 (0.3) | 16 (0.4) | 1.47 (0.89–2.42) | 1.07 (0.46–2.47) |
| Maternal death | 228 (0.1) | 8 (0.2) | 1.39 (0.69–2.82) | 1.13 (0.34–3.67) |
| Any other maternal complication | 568 (0.3) | 14 (0.3) | 0.98 (0.58–1.66) | 0.97 (0.44–2.12) |
| Fresh stillbirth | 1115 (0.6) | 65 (1.4) | 2.33 (1.81–3.00) | 2.24 (1.56–3.21) |
| Macerated stillbirth | 787 (0.4) | 39 (0.8) | 1.97 (1.41–2.74) | 1.38 (0.80–2.37) |
| 5-min Apgar score <7 | 1345 (0.8) | 49 (1.2) | 1.42 (1.07–1.90) | 2.03 (1.33–3.11) |
| NICU admission | 1031 (0.6) | 60 (1.3) | 2.33 (1.79–3.02) | 2.07 (1.32–3.23) |
| Early neonatal death | 740 (0.4) | 19 (0.4) | 1.02 (0.65–1.61) | 1.23 (0.62–2.42) |
Abbreviations: CI, confidence interval; NICU, neonatal intensive care unit; OR, odds ratio.
Values are given as number (percentage) unless stated otherwise.
ORs were adjusted for age, body mass index, prior newborn weighing ≥4000 g, prior stillbirth, previous infant death, personal or family history of diabetes, and chronic hypertension.
The frequency of stillbirth, cesarean delivery, and NICU admission were stratified by birth weight (Fig. 3). Among the 4 categories, newborns weighing 3000–3999 g seemed to have the lowest risk for stillbirth and NICU admission, whereas those weighing 4500 g or more seemed to have the highest risk. The proportion of cesarean deliveries increased as the birth weight increased: the highest incidence of cesarean delivery (20.2%) occurred for newborns weighing 4500 g or more.
Fig. 3.
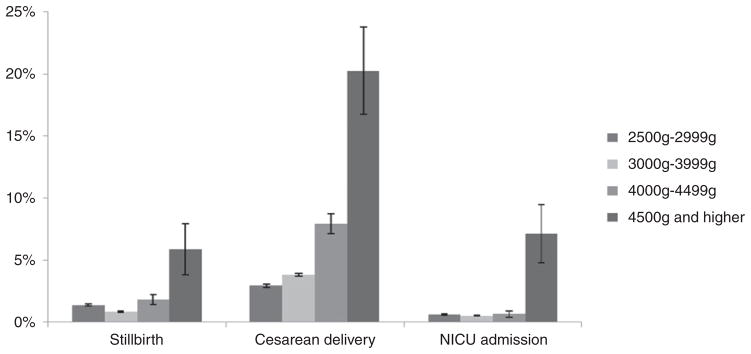
Frequencies and percentages of stillbirth, cesarean delivery, and NICU admission according to birth weight band. Data were obtained from ZEPRS for neonates born in Lusaka, Zambia, between February 2006 and August 2011 (n = 191 834).
4. Discussion
In the present population-based study of approximately 200 000 deliveries in Lusaka, Zambia, there was a 2.5% prevalence of fetal macrosomia among singletons weighing 2500 g or more. Macrosomia was associated with increased risk for cesarean delivery and perinatal complications such as fresh stillbirth, low Apgar score, and NICU admission. The findings show clear associations between specific maternal characteristics and fetal macrosomia, suggesting potential targets for public health interventions.
The strength of the present analysis is its population-based approach. All public sector facilities—where most women seek prenatal care in Lusaka—were included rather than a single clinic or referral hospital [1,5,18,19]. However, the study has several limitations. First, similar to other studies that limited their comparison group by weight instead of gestational age and weight [1,18], newborns weighing less than 2500 g were excluded. Because small-for-gestational-age term neonates, who may have increased morbidity and mortality, were not considered, the comparison group might have been enriched with healthier newborns. Second, only facility-based deliveries and their outcomes were reported. Macrosomic newborns who are delivered at home probably have worse outcomes, given the lack of neonatal monitoring and timely operative delivery [20]. Third, results from the present urban-based cohort may not be applicable to rural sites. The capacity for cesarean delivery and NICU referrals, for example, is likely to have improved perinatal outcomes. Studies have also shown that women living in urban settings have higher average weights than those in rural areas, a finding that could influence the baseline distribution of birth weight [21]. Fourth, the present analysis used programmatic data captured in routine clinical settings and probably underreports the frequency of complications in general. Last, screening and diagnostic testing for co-morbidities is limited in the study setting. Untreated gestational diabetes, for example, might also be an important predictor of fetal macrosomia; however, it was not possible to establish such links owing to a paucity of routine screening to determine the prevalence of this disease.
Macrosomia in the study cohort was at the lower range of reported prevalence in Africa (2.5%–14.7%) [5,18,22]; however, most African studies on fetal macrosomia originate from settings in West Africa that may be different in terms of urbanization and resources. In addition, unlike many hospital-based studies, the relatively low prevalence of macrosomia may reflect the general prenatal population who deliver at the Lusaka network of primary care centers. Despite the low prevalence of macrosomia, the predictors for high birth weight were consistent with those of other studies carried out both in the region and in high-resource countries [1,5,19,22].
The study showed that maternal and neonatal complications were increased for newborns weighing 4000 g or more. Macrosomic newborns were at higher risk for cesarean delivery compared with normal birth weight newborns: the incidence of cesarean delivery was higher than 20% when the birth weight was 4500 g or more. Similar increases in cesarean delivery have been previously reported [1,18], although not all studies are consistent [5]. In line with other reports [1,5,18], perinatal mortality and NICU admission increased for macrosomic newborns. Clinical interventions around the time of delivery might reduce the rate of perinatal mortality in cases of fetal macrosomia.
In the present cohort, women with risk factors for gestational diabetes were at increased risk of delivering a macrosomic newborn. Because the timely diagnosis and treatment of gestational diabetes can reduce the risk of fetal macrosomia and other perinatal complications [23], targeted screening should be considered in settings where routine testing is not feasible. While the International Association of the Diabetes and Pregnancy Study Groups allows flexibility for local implementation of such screening [24], some form of prenatal testing for gestational diabetes mellitus [1,5,25] should be prioritized, particularly as obesity is increasing in many African and Asian countries. Future research should include feasibility studies and cost–benefit analyses of screening, whether routine or targeted, for gestational diabetes in resource-limited settings. Interventions such as nutrition counseling and oral hypoglycemic agents should also be examined for effectiveness and cost–benefit in Sub-Saharan Africa.
The present results show that fetal macrosomia is associated both with risk factors that are easy to screen and with poor pregnancy outcomes that may benefit from early referral. More rigorous screening of predictors to identify and potentially prevent fetal macrosomia should be adopted. Although maternal diabetes is the most common cause of fetal macrosomia, modifiable risk factors include pre-pregnancy maternal weight, maternal weight gain during pregnancy, and delivery after 40 weeks of gestation. Public health campaigns and group health education should focus more on nutrition in reproductive age women. Whenever possible, delivery should occur at facilities with cesarean delivery capacity and/or neonatal intensive care. Targeted public health interventions aimed at reducing fetal macrosomia in resource-limited settings might help to meet Millennium Development Goal 5 by closing each gap that contributes to maternal and perinatal morbidity and mortality.
Acknowledgments
The Zambia Electronic Perinatal Record System was designed, developed, and implemented via support from the Bill and Melinda Gates Foundation. Additional trainee and investigator support was provided by the US National Institutes of Health (R24 TW007988, R24 TW008877, D43 CA153784). Funding institutions had no involvement in study design, data collection, data analysis, or manuscript writing.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Adesina OA, Olayemi O. Fetal macrosomia at the University College Hospital, Ibadan: a 3-year review. J Obstet Gynaecol. 2003;23(1):30–3. doi: 10.1080/0144361021000043182. [DOI] [PubMed] [Google Scholar]
- 2.Stotland NE, Caughey AB, Breed EM, Escobar GJ. Risk factors and obstetric complications associated with macrosomia. Int J Gynecol Obstet. 2004;87(3):220–6. doi: 10.1016/j.ijgo.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86(1):14–7. doi: 10.1016/0029-7844(95)00099-D. [DOI] [PubMed] [Google Scholar]
- 4.Lahmann PH, Wills RA, Coory M. Trends in birth size and macrosomia in Queensland, Australia, from 1988 to 2005. Paediatr Perinat Epidemiol. 2009;23(6):533–41. doi: 10.1111/j.1365-3016.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 5.Kamanu CI, Onwere S, Chigbu B, Aluka C, Okoro O, Obasi M. Fetal macrosomia in African women: a study of 249 cases. Arch Gynecol Obstet. 2009;279(6):857–61. doi: 10.1007/s00404-008-0780-7. [DOI] [PubMed] [Google Scholar]
- 6.Melah GS, El-Nafaty AU, Massa AA, Audu BM. Obstructed labour: a public health problem in Gombe, Gombe State, Nigeria. J Obstet Gynaecol. 2003;23(4):369–73. doi: 10.1080/01443610310001119510. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann RL, Richter R, Bergmann KE, Plagemann A, Brauer M, Dudenhausen JW. Secular trends in neonatal macrosomia in Berlin: influences of potential determinants. Paediatr Perinat Epidemiol. 2003;17(3):244–9. doi: 10.1046/j.1365-3016.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 8.Wen SW, Kramer MS, Platt R, Demissie K, Joseph KS, Liu S, et al. Secular trends of fetal growth in Canada, 1981 to 1997. Paediatr Perinat Epidemiol. 2003;17(4):347–54. doi: 10.1046/j.1365-3016.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- 9.Odlind V, Haglund B, Pakkanen M, Otterblad Olausson P. Deliveries, mothers and newborn infants in Sweden, 1973–2000. Trends in obstetrics as reported to the Swedish Medical Birth Register. Acta Obstet Gynecol Scand. 2003;82(6):516–28. [PubMed] [Google Scholar]
- 10.Alberman E. Are our babies becoming bigger? J R Soc Med. 1991;84(5):257–60. doi: 10.1177/014107689108400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79(6):440–9. [PubMed] [Google Scholar]
- 12.Kramer MS, Morin I, Yang H, Platt RW, Usher R, McNamara H, et al. Why are babies getting bigger? Temporal trends in fetal growth and its determinants. J Pediatr. 2002;141(4):538–42. doi: 10.1067/mpd.2002.128029. [DOI] [PubMed] [Google Scholar]
- 13.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104(4):720–6. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- 14.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 15.Mtonga V, Njobvu C, Mkumba G, Tembo E, Amafumba R, Sambo P, et al. Pregnancy, Childbirth, Postpartum and Newborn Care Guidelines. Lusaka: Ministry of Health; 2004. [Google Scholar]
- 16.Chi BH, Vwalika B, Killam WP, Wamalume C, Giganti MJ, Mbewe R, et al. Implementation of the Zambia electronic perinatal record system for comprehensive prenatal and delivery care. Int J Gynecol Obstet. 2011;113(2):131–6. doi: 10.1016/j.ijgo.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372–8. doi: 10.1067/mob.2003.302. [DOI] [PubMed] [Google Scholar]
- 18.Ojule JD, Fiebai PO, Okongwu C. Perinatal outcome of macrosomic births in Port Harcourt. Niger J Med. 2010;19(4):436–40. doi: 10.4314/njm.v19i4.61971. [DOI] [PubMed] [Google Scholar]
- 19.Onyiriuka AN. High birth weight babies: incidence and foetal outcome in a mission hospital in Benin City, Nigeria. Niger J Clin Pract. 2006;9(2):114–9. [PubMed] [Google Scholar]
- 20.Tuladhar H, Dali SM, Pradhanang V. Complications of home delivery: a retrospective analysis. JNMA J Nepal Med Assoc. 2005;44(159):87–91. [PubMed] [Google Scholar]
- 21.van der Sande MA, Ceesay SM, Milligan PJ, Nyan OA, Banya WA, Prentice A, et al. Obesity and undernutrition and cardiovascular risk factors in rural and urban Gambian communities. Am J Public Health. 2001;91(10):1641–4. doi: 10.2105/ajph.91.10.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Fekih C, Mourali M, Ouerdiane N, Oueslati S, Hadj Hassine A, Chaabene M, et al. Maternal and fetal outcomes of large fetus delivery: a comparative study. Tunis Med. 2011;89(6):553–6. [PubMed] [Google Scholar]
- 23.Ugboma HA, Aburoma H, Ukaigwe P. Gestational Diabetes: Risk Factors, Perinatal Complications and Screening Importance in Niger Delta Region of Nigeria: A Public Health Dilemma. Int J Trop Dis Health. 2012;2(1):42–54. [Google Scholar]
- 24.McIntyre HD, Oats JJ, Zeck W, Seshiah V, Hod M. Matching diagnosis and management of diabetes in pregnancy to local priorities and resources: an international approach. Int J Gynecol Obstet. 2011;115(Suppl 1):S26–9. doi: 10.1016/S0020-7292(11)60008-8. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal MM, Weigl B, Hod M. Gestational diabetes screening: the low-cost algorithm. Int J Gynecol Obstet. 2011;115(Suppl 1):S30–3. doi: 10.1016/S0020-7292(11)60009-X. [DOI] [PubMed] [Google Scholar]