Abstract
Epidural stimulation to trigger locomotion is a promising treatment after spinal cord injury (SCI). Continuous stimulation during locomotion is the conventional method. To improve recovery, we tested an innovative robot-driven epidural stimulation method, combined with a trunk-based neurorobotic system. The system was tested in rat, and the results were compared with the results of the neurorobotic therapy combined with the conventional epidural stimulation method. The rats had better recovery after treatment with the robot-driven epidural stimulation than conventional stimulation in our neurorobotic rehabilitation system.
I. Introduction
Triggering locomotion and balancing the body simultaneously is a major issue for rehabilitation after severe spinal cord injury (SCI) in rat models. Our goal was to improve autonomous locomotion after SCI in the rat model. To achieve this goal, we designed a robot-based rehabilitation system to promote better body weight support and for facilitating locomotion generated after complete spinal cord transection, and tested this in the animal model.
After complete spinalization of adult rats at the thoracic level, hind limb locomotion can be driven by central pattern generators (CPGs) in the isolated spinal cord circuitry. However, special measures are needed to activate the CPGs. Recent studies show that electric spinal epidural stimulation can drive CPGs and trigger hind limb locomotion [1-4]. Parts of the rats’ trunk above the lesion remain under supraspinal control after complete transection at thoracic levels. In neonatal spinalized rats, these circuits can be driven with cortical aid to support well-integrated weight-bearing whole-body locomotion. Thus, the recovery process and rehabilitation should be designed not only to train the spinal circuitry; there should also be training of integration with the supraspinal systems. Since the pelvis is the main mechanical junction between the trunk and the hind limbs, we designed a pelvic orthoses to connect a rat to a robot. This trunk-based neurorobotic system assists the rehabilitation of weight support and the interaction and integration between supraspinal and spinal systems. Long term adaptation occurred and the motor function of the rats improved significantly [5].
Continuous epidural stimulation has been tested as a practicable method to trigger locomotion [2-4, 6-10]. In order to improve the recovery, we modified our neurorobotic system to drive electric spinal epidural stimulation so as to help restore spinalized rats’ hindlimb stepping motor function. We found that the rats with the robot-controlled stimulation achieved a higher degree of hindlimb weight support than others, had less lateral deviation, and achieved higher function scores earlier than those in the continuous stimulation or control groups. This robot-driven stimulation combined with trunk-based neurorobotics is a possible solution to help trigger locomotion, promote better stepping and train balancing of the body simultaneously.
II. Methods
A. Overview
19 intact rats were used in the experiment, spinalized at vertebral level T9/T10, and implanted with an epidural stimulation electrode under the arch of vertebral level L2. Rats were separated into 3 groups: 6 rats in the control group (no stimulation), 6 rats in the conventional stimulation group, and 7 rats in the robot-driven epidural stimulation group. All of the rats were trained on a treadmill with the PHANTOM® neurorobotic system after complete spinal transection. The robot arm detected the position of the rat’s pelvis and provided feedback to the system PC to decide the strength and direction of the applied force and, where appropriate, the timing of stimulation. The interaction forces of the rats with the robot and the pelvic position were collected by the robot system software. The vertical force data were used to assess recovery of active hindlimb weight support, and the lateral position deviation were used to assess the walking stability. All processes were video recorded. An adapted motor score (hindlimb adapted BBB, or ‘AOB’ for spinalized animals) was used to assess kinematic recovery [11]. This score evaluated hindlimb joint motion, range of motion, rhythmicity, alternation, apparent weight support and plantar placement on a numerical scale. A joint marker tracking software, MaxTRAQ®, was also used to assist analyzing kinematic data.
B. Surgery
All surgical procedures were under aseptic conditions, and in compliance with IACUC recommendations. Rats were anesthetized by a ketamine cocktail (KXA) [ketamine hydrochloride (50 mg/Kg), xylazine (5 mg/Kg), acepromazine (0.75 mg/Kg)] (1 ml/kg), and maintained at a deep level of anesthesia by supplemental doses of KXA (0.38 ml/kg) per hour.
Transection and epidural stimulation electrode implantation
A partial laminectomy was performed at T9~T11, and the spinal cord was completely transected by iridectomy scissors and fine vacuum extraction. Gel foam was inserted into the gap created by the transection. Another partial laminectomy at the end of L1 allowed a stimulation electrode to be placed under the L2 arch. A ground electrode was sutured on the back muscle of the rat. A pelvic orthosis was implanted for robot attachment as described in [5] and epidural wiring routed to connectors mounted on it.
C. Neurorobotic System and Epidural Stimulation
We used a PHANTOM® Premium 1.0 device developed by SensAble Technologies, Inc. to assist weight support of the rats, as in Fig. 1(a). The robot arm can apply an elastic force field to the rat to assist weight support during training. The elastic field equation used was:
| (1) |
whereas F = force applied by the robot arm; k = stiffness of the elastic field; ℓ0 = the desired center of the elastic field; ℓ = the current point of the pelvic junction. After setting the desired stiffness and the desired center point of the elastic field, the pelvic junction is constrained around the center of the elastic field, so that the rat can be weight-supported by the robot arm. As rats regain self weight support and approach the robot field equilibrium, the robot assistance will be naturally diminished, i.e., as ℓ = ℓ0, robot force F = k·0 = 0.
Figure 1.
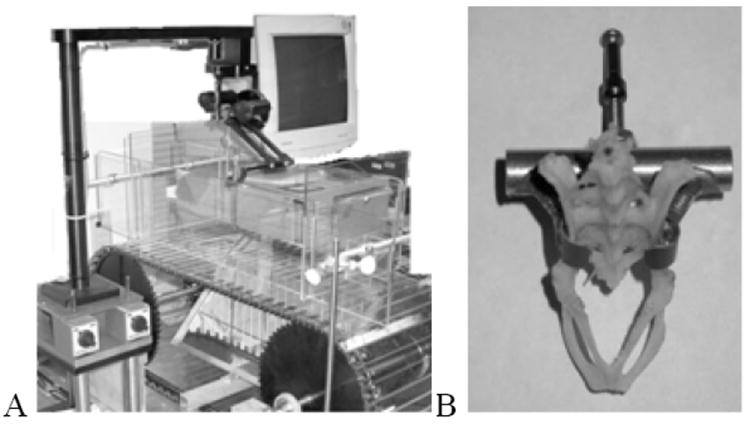
Robot training and stimulation driving framework. PHANTOM® Premium 1.0 device integrated with our treadmill. The robot arm connects to a control center, which is under the treadmill, and connects to a card interface to the ISA port of the PC. We can use a specific operating system for PHANTOM®, which is also developed by SensAble Technologies, to set the parameters of the robot arm. We can also collect feedback data from the robot arm by this OS. (b) The pelvic orthoses we used to connect the robot and the rat’s pelvis, demonstrated on a museum beetle cleaned pelvis.
For conventional epidural stimulation, parameter settings utilized those from previous work by other groups [2, 3, 6]. Frequency was 40Hz, (inter pulse period (IPP) set to be 25 ms), pulse duration was set to be 200 μs, and amplitude was set to be 3V [see 2,3,4,8,9]. This stimulation series then operated through the whole training process, for 15 minutes.
For robot-driven epidural stimulation, we used the same minimum inter-pulse period, pulse duration, and amplitude. The only difference was that the robot arm was used to detect the rat’s pelvic position, and the robot software thereby decided the timing of stimulation. The algorithm used was:
Robot arm encodes record the height of the rat’s pelvis and this is sent back to the program.
If the height < the center point of the elastic field, and interpulse period < 25ms, send a biphasic electric pulse to the spinal cord. (This limits stimulus frequency to < 40Hz)
Go to 1.
The program monitored the rat’s pelvis height every 1 ms. If the pelvis was lower than the elastic center and IPP>25ms, a 200 μs 3V pulse was delivered. At the early stage of rehabilitation, the pelvis of the rat was often under the elastic center, because of the absence of self weight support. In this case, the stimulation was nearly a continuous series at 40 Hz, nearly identical to the conventional stimulation group. After the rat more fully recovered however, the stimulation was diminished naturally in parallel with the force.
D. Training and Data Collection
Animals were trained on the treadmill for 15 minutes in each trial, 5 days a week, for 5 – 7 weeks. Treadmill speed was set as 12 cm/s. All processes were video recorded and force data were recorded by the neurorobotic system. Data analysis including: (1) Functional scoring: AOB scoring was used to assess qualitative kinetic recovery in the rats [5], (2) Self weight support examination: Z-force data from PHANTOM® robot were used as a measure of functional restoration of self weight support, (3) Walking balance examination: Y-position data from PHANTOM® robot were used as a measure of the lateral stability and precision of the rats’ walking, and (4) Kinematic data analysis: an image tracking program, MaxTRAQ® (Innovision Systems, Inc.), was used to track kinematic changes throughout training (not described or presented here).
III. Results
A. AOB Scores
AOB scoring was used to assess the qualitative functional recovery of the rats after treatment. Fig. 2(a) shows average AOB scores of the control group, while 2(b) shows average AOB score of the conventional stimulation group, and 2(c) shows average AOB score of the robot-driven stimulation group. Both conventional and robot-driven epidural stimulation show significant improvements over controls. Further, robot driven AOB in week 6 and 7 was significantly greater than either conventional or control (p<0.05, U test).
Figure 2.
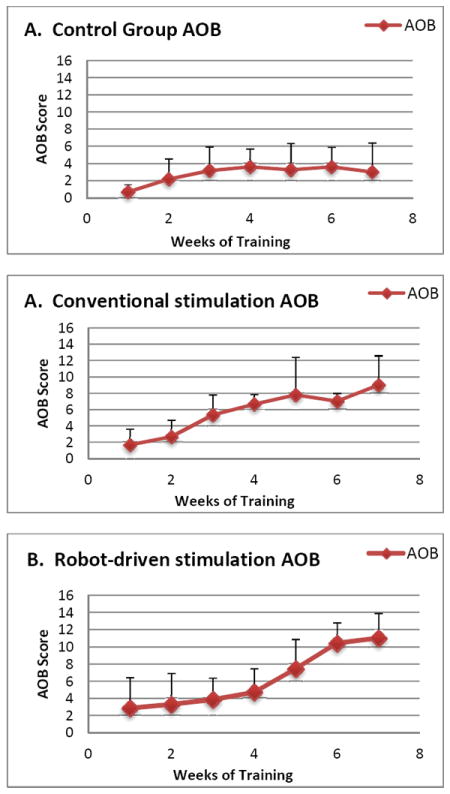
Qualitative function based AOB scores: A. Control with no stimulation but robot support. B. Conventional continuous 40Hz epidural stimulation combined with robot support. C. Robot driven epidural stimulation. In weeks 6 and 7 all AOB scores are significantly different (Mann-Whitney U, p<0.05) and robot driven is significantly greater than conventional epidural stimulation.
B. Data from the Neurorobotic System
The PHANTOM® robot system detected position, and velocity and delivered force in 3-dimensions. Delivered Z-direction force of the robot was inversely proportional to self body weight support, so we could thus use it to assess the recovery of self body weight support. From Y-direction position data, which is the lateral pelvis position, we could see the scale of yaw and roll movements, and the deviation from the field center. These indicated the difference in walking stability and precision of the rats. For the control and conventional stimulation group, we found no significant trends in Z-force, so we show the average value of Z-direction force instead of a trend line. Fig. 3(a) shows the average Z-direction force of the control group, Fig. 3(b) shows average Z-direction force of the conventional stimulation group, and Fig. 3(c) shows average Z-direction force trend of the robot-driven stimulation group. The average Y-direction position and standard deviation of the control group, conventional stimulation group, and robot-driven stimulation group all also differed. Statistical tests (Kruskal-Wallis test, p = 0.004≪0.05) showed that the Y-position data of these three groups were significantly different, with the robot-driven epidural stimulation group ultimately showing the smallest standard deviation, and the smallest systematic bias away from the field center, one that was also not significantly different from 0 after training (U test p>0.05). Thus the robot driven stimulation group used the minimal lateral assist from the robot and showed the best yaw and roll control.
Figure 3.
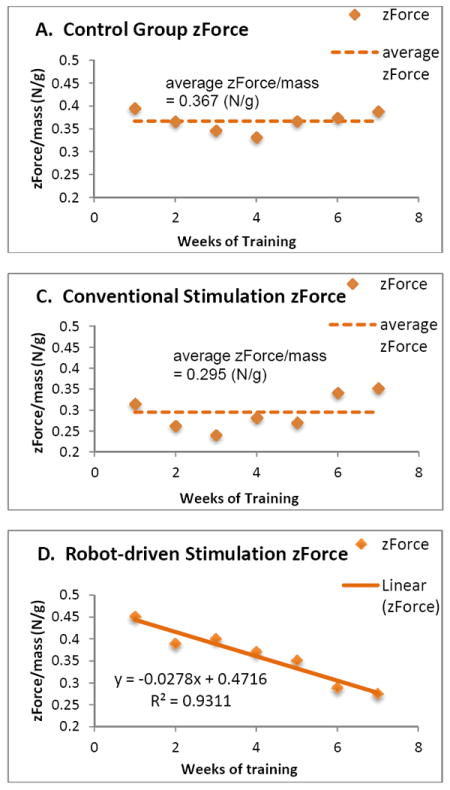
Z-force weight-support contribution of robot: mean Z-force. A. Z-direction force of the control group. B. Z-direction force of the conventional stimulation group. C. Z-direction force of the robot-driven stimulation group. Significant trend downward in robot contribution is seen only in C. Mean levels in A and B differ significantly. The final mean level of Z is lowest in C, using robot driven epidural spinal stimulation.
IV. Discussion
A. Robot- driven Epidural Stimulation
To our knowledge this study is the first to explore robot driven epidural stimulation as a treatment paradigm for SCI. Conceivably, the robot-driven stimulation is also a form of artificial feedback or the intermittency allows dis-habituation. The framework is readily transferred to an orthosis-driven stimulation approach for subsequent off-robot function.
B. Functional Recovery
From the average functional AOB scores of each group, we found that rats in the control group slightly recovered after training on the treadmill for 7 weeks, but the recovery level only reached 4. In AOB scoring this means very occasional right-left alternation of the hind limbs can be observed, and the amplitude is weak. The conventional stimulation group could recover to around 9 in their AOB score, which means consistent right-left alternation of the hind limbs could be observed, and the amplitude was large. The robot-driven stimulation group could recover to 11 in AOB score, which means not only right-left alternation, but also some planter stepping was observed. The average starting AOB score of the robot-driven stimulation group was also the highest among the three. Based on the information above, the robot-driven stimulation had the best functional recovery scores. The rats were trained daily (5 days per week) in a massed training paradigm.
C. Robot Data Analysis
The Z-direction force contribution of the robot can be used to assess the recovery of self-body weight support. This measure differed among all treatments but only showed major trend in robot driven stimulation. The rats were similarly prepared, yet the z-force in robot-driven epidural stimulation began higher. The initial AOB scores were similar. We believe these robot force differences indicate the robot driven stimulation likely allowed more initial drops of the pelvis. Statistical tests of the z-force showed significant differences among the treatments. Based on the z-force data, self weight-support level after treatment with the neurorobotic system was not significantly altered over time in either the control group, or in the conventional stimulation group. It was improved by conventional stimulation significantly over control, but was only significantly increased (with a clear and statistically significant trend of downward robot contribution) in the robot- driven stimulation group. The robot-driven stimulation was less effective initially, but likely provided superior improvement long term. We conclude that rats in the robot-driven stimulation group are likely to ultimately have better recovery of self body weight support.
From the average lateral position of the rats, we saw that rats often showed a mean bias and tended to lean on a specific side as they walked on the treadmill, with most showing a slight leftward bias. We estimated the mean lateral deviations as the rats walked on the treadmill. Both mean bias and lateral variation showed the differences in walking stability and precision in each group. Rats in the control group showed large variances of the left-right movements of the pelvis. The rats in the conventional stimulation group showed reduced bias, but their deviations were increased. The standard deviation of the lateral position of this group was the largest among the three, presumably indicating greater roll, and less precision. The rats in the robot-driven stimulation group showed least bias and variance, they stayed close to the field center, and the standard deviations were small, indicating more stability and precision in their lateral motion. In conclusion, the robot-driven stimulation rats had better walking stability and support, as expected from [12,13].
V. Conclusion
Rats recovered better with a combination of our trunk based neurorobotic system, and epidural stimulation, when compared to controls. However, when treated by our novel robot-driven epidural stimulation rats achieved a still higher and significantly improved level of recovery, compared to the conventional stimulation treated rats.
Acknowledgments
This work was supported in part by the National Institute of Health (NIH) NS44564, NIH NS54894, the Craig Neilsen Foundation, and Pa Dept Health, Tobacco settlement award.
Contributor Information
F. H. Hsieh, Email: fuhan@Drexel.edu, School of Biomedical Engineering, Science & Health Systems, Drexel University, Philadelphia, PA 19104 USA.
S. F. Giszter, Email: Simon.Giszter@DrexelMed.edu, Department of Neurobiology and Anatomy, Drexel University College of Medicine, Philadelphia, PA 19129 USA.
References
- 1.Jilge B, et al. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res. 2004 Feb;154:308–26. doi: 10.1007/s00221-003-1666-3. [DOI] [PubMed] [Google Scholar]
- 2.Ichiyama RM, et al. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005 Aug 5;383:339–44. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 3.Gerasimenko YP, et al. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods. 2006 Oct 30;157:253–63. doi: 10.1016/j.jneumeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Ichiyama RM, et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008 Jul 16;28:7370–5. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udoekwere UI, et al. Robot application of elastic fields to the pelvis of the spinal transected rat: a tool for detailed assessment and rehabilitation. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3684–7. doi: 10.1109/IEMBS.2006.259633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavrov I, et al. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol. 2006 Oct;96:1699–710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- 7.Edgerton VR, et al. Training locomotor networks. Brain Res Rev. 2008 Jan;57:241–54. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavrov I, et al. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J Neurosci. 2008 Jul 30;28:7774–80. doi: 10.1523/JNEUROSCI.1069-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavrov I, et al. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J Neurosci. 2008 Jun 4;28:6022–9. doi: 10.1523/JNEUROSCI.0080-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtine G, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009 Oct;12:1333–42. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antri M, et al. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002 Aug;16:467–76. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- 12.Giszter SF, et al. How spinalized rats can walk: biomechanics, cortex, and hindlimb muscle Scaling implications for rehabilitation. Ann N Y Acad Sci. 2010 Jun;1198:279–93. doi: 10.1111/j.1749-6632.2010.05534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giszter SF, et al. Trunk sensorimotor cortex is essential for autonomous weight-supported locomotion in adult rats spinalized as P1/P2 neonates. J Neurophysiol. 2008 Aug;100(2):839–51. doi: 10.1152/jn.00866.2007. Epub 2008 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]


