Abstract
Background. Noroviruses cause significant morbidity and mortality from acute gastroenteritis in all age groups worldwide.
Methods.We conducted 2 phase 1 double-blind, controlled studies of a virus-like particle (VLP) vaccine derived from norovirus GI.1 genotype adjuvanted with monophosphoryl lipid A (MPL) and the mucoadherent chitosan. Healthy subjects 18–49 years of age were randomized to 2 doses of intranasal Norwalk VLP vaccine or controls 21 days apart. Study 1 evaluated 5-, 15-, and 50-μg dosages of Norwalk antigen, and study 2 evaluated 50-and 100-μg dosages. Volunteers recorded symptoms for 7 days after dosing, and safety was followed up for 180 days. Blood samples were collected for serological profile, antibody secreting cells (ASCs), and analysis of ASC homing receptors.
Results. The most common symptoms were nasal stuffiness, discharge, and sneezing. No vaccine-related serious adverse events occurred. Norwalk VLP-specific immunoglobulin G and immunoglobulin A antibodies increased 4.8-and 9.1-fold, respectively, for the 100-μg dosage level. All subjects tested who received the 50-or 100-μg vaccine dose developed immunoglobulin A ASCs. These cells expressed molecules associated with homing to mucosal and peripheral lymphoid tissues.
Conclusions. The intranasal monovalent adjuvanted Norwalk VLP vaccine was well tolerated and highly immunogenic and is a candidate for additional study.
Trial Registration. ClinicalTrials.gov identifier: NCT00806962.
Noroviruses (NoV), a member of the family Calicivir- [1]. The Norwalk agent was the first virus identified in idae , cause acute epidemic gastroenteritis in humans of this family, and sequencing of its RNA classified it as all ages in both developed and developing countries a new genus designated “ norovirus.” Noroviruses are genetically variable and include 25 genotypes that infect humans. The prototype Norwalk virus is a member of genogroup I, genotype 1 (GI.1). Currently, genogroup II, genotype 4 (GII.4) viruses are responsible for most outbreaks. Although gastroenteritis is short-lived in healthy individuals, it has a significant socioeconomic toll on businesses, hospitals, schools, and other settings [2]. Noroviruses are highly infectious and spread rapidly by person-to-person contact with aerosol droplets, environmental surfaces, and ingestion of contaminated foods, especially in hospitals, nursing homes, university campuses, military barracks, and cruise ships [3–7]. Recent estimates indicate that >90% of nonbacterial gastroenteritis outbreaks in the United States are caused by noroviruses [8, 9]. Recently, the Centers for Disease Control and Prevention estimated that 23 million cases occur annually in the United States, and up to 200,000 deaths of children <5 years old occur each year in developing countries [10].
A major development in norovirus research came with the successful cloning, sequencing, and expression of noroviruses in insect cells using baculovirus recombinants with the major capsid protein (VP1) that spontaneously folds into virus-like particles (VLPs) [11, 12]. Lacking a viral genomic RNA, these noninfectious VLPs have a preserved antigen conformation and interact with cellular receptors, eliciting a strong host immune response [11, 12]. Norovirus VLPs are highly immunogenic when given to animals parenterally, orally, or intranasally without adjuvant [13, 14] and are stable following lyophilization and when exposed to acid (pH 2.5). In phase 1 studies, VLPs administered orally without adjuvant or in edible transgenic plants were safe but only modestly immunogenic as measured by serum antibody and specific antibody-secreting cells (ASCs) [5, 15, 16]. The purpose of these studies was to determine the safety and immunogenicity of adjuvanted Norwalk VLP vaccine administered intranasally for the first time to humans.
Methods
Vaccine
Norwalk VLPs, derived from norovirus GI.1 genotype, were prepared under good manufacturing practice at Protein Sciences, Inc. The vaccine consists of (1) Norwalk VLPs produced by a recombinant baculovirus expression system; (2) monophosphoryl lipid A (MPL) adjuvant (GlaxoSmithKline Pharmaceuticals Inc), a Toll-like receptor 4 (TLR-4) agonist, derived from detoxified Salmonella minnesota lipopolysaccharide; (3) chitosan (ChiSys; Archimedes Development Ltd), a linear polysaccharide produced by alkaline hydrolysis (deacetylation) of chitin from shrimp shells, a mucoadhesive to nasal mucus and epithelial cells prolonging antigen adherence [17]; and (4) sucrose and mannitol excipients as bulking agents and preservatives to stabilize VLP structure during lyophilization. The vaccine was formulated under good manufacturing practice as a dry powder by Archimedes Development, Ltd.
Vaccine was administered intranasally using Bespak UniDose DP delivery devices (Milton Keynes). A dose of vaccine consisted of 2 loaded Bespak devices discharging 10 mg of dry powder vaccine formulation into each nostril for a total dose of 20 mg.
Study Design
Two phase 1 clinical studies were performed. Study 1 was a step-wise, dosage escalation trial, with safety reviews before each dose escalation. Study 2 was a dose comparison study of the 2 highest dosages. Subjects were 18–49-year-old healthy H type 1 secretor adults, given that only individuals with blood H type 1 antigen are susceptible to Norwalk infection [18]. Study 2 also excluded subjects with blood types B or AB because those individuals were reported to be less susceptible to Norwalk infection [19]. Subjects were counseled to ensure comprehension of the study and the risks, benefits, and procedures involved. The respective Institutional Review Boards approved the studies, and all subjects provided informed signed consent.
Study 1 was a single-site (University of Maryland), randomized, double-blind study of 3 dosage levels of adjuvanted Nor-walk VLP vaccine (Norwalk VLP vaccine, MPL, and chitosan) compared with adjuvant control (MPL and chitosan). Twenty-eight sequentially randomized adults received 2 intranasal doses of (1) 5 μg of Norwalk VLP vaccine (n = 5) or adjuvant control (n = 2), (2) 15 μg of Norwalk VLP vaccine (n = 5) or adjuvant control (n = 2), or (3) 50 μg of Norwalk VLP vaccine (n = 10) or adjuvant control (n = 4). The 2 doses were separated by 21 days. The vaccine and control preparations were administered with Bespak intranasal delivery devices.
Study 2 was a multicenter, randomized, double-blind study at 4 sites. Sixty-one healthy adults were enrolled and randomized 2:2:1:1, respectively, to receive either 2 doses of 50 μg of Norwalk VLP vaccine (n = 20), 100 μg of Norwalk VLP vaccine (n = 20), adjuvant control (n = 10), or true placebo (n = 11) consisting of a puff of air (no dry powder). All doses were administered intranasally with the Bespak device. The 2 doses were separated by 21 days.
Safety Evaluation
Safety was assessed after each dose by review of a 7-day symptom record for interim medical histories and clinical laboratory tests. The following 7 local symptoms were sought: runny nose and/or nasal discharge, nasal pain and/or discomfort, nasal stuffiness and/or congestion, nasal itching, sneezing, blood-tinged nasal mucus, nasal bleeding, and the following 8 systemic symptoms: headache, fever, malaise, nausea, vomiting, abdominal cramps and/or pain, diarrhea, and loss of appetite. In addition to these solicited adverse events, unsolicited adverse events were collected through study day 56 (35 days after the second dose). Serious adverse events and new medically significant conditions were collected for 180 days. Study 2 was amended to assess fibrinogen levels before and 1 and 7 days IgA antibody secreting cells (ASCs) were measured in peripheral after each vaccination of the first 12 subjects (9 vaccinees and 3 control participants).
Immune Responses
Serum antibodies. Serum samples were collected on days 0, 7, 21, 28, 56, and 180 after intranasal administration. Norwalk VLP-specific immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin M (IgM) were measured by enzyme-linked immunosorbent assay (ELISA) [5, 20]. Geometricmean titers (GMTs), geometric mean of fold rises, and seroresponse rates (⩾4-fold rises) were determined.
Antibody secreting cells. Norwalk VLP-specific IgG and IgA antibody secreting cells (ASCs) were measured in peripheral blood mononuclear cells (PBMCs) on days 0, 7, 21, and 28 [5]. The response rate and mean number of ASCs per 10 6 PBMCs were assessed. A positive response was defined as a postvaccination ASC count ⩾8 spots per 106 PBMCs and at least 3 standard deviations above the mean prevaccination ount for all subjects.
Norovirus hemagglutination inhibition assay. Red blood (IgA), and immunoglobulin M (IgM) were measured by en-cells were purified from type O peripheral blood of healthy zyme-linked immunosorbent assay (ELISA) [5, 20]. Geometric adult volunteers and maintained at 4°C in a 10% stock in
Alsever buffer; subsequent dilutions were made in 0.01 mol/L phosphate-buffered saline without Ca2+ or Mg2+ at pH 7.2 (Invitrogen). Serially diluted Norwalk VLPs (50 mL) were incubated with an equal volume of a 0.5% human red blood cell suspension in 96-well V bottom plates for 90 min at 4°C. The amount of Norwalk VLP antigen corresponding to 4 hemagglutination units was determined and confirmed by back titration. Test serum samples were heat inactivated at 56°C for 30 min and treated (in a 1:5 ratio) with freshly prepared 25% Kaolin suspension (Sigma). To eliminate serum inhibitors, test samples were also preadsorbed with red blood cells. The hemagglutination inhibition (HAI) assay was performed as follows: pretreated serum samples (diluted 2-fold in phosphate-buffered saline, pH 5.5) were added to 96 well V-plates (Costar) and incubated with an equal volume of Norwalk VLP antigen (LigoCyte, lot 45–06002) containing 4 hemagglutination units, for 30 min at 4°C. A suspension of 0.5% red blood cells was added to each well; plates were incubated for an additional 90 min at 4°C, checking for agglutination every 30 min. Wells containing only phosphate-buffered saline or antigen without serum served as negative and positive controls, respectively. HAI titers were calculated as the inverse of the highest dilution that inhibited hemagglutination, with a compact negative red blood cell pattern (button of red blood cells).
Expression of homing molecules by Norwalk-specific ASCs. Fresh PBMCs were stained with monoclonal antibodies to CD19-PE-Cy7 (clone J3–119, Beckman Coulter), CD27-PECy5 (Clone 1A4CD27, Beckman Coulter), CD62L-PE (L-selectin, Clone Dreg-56, B-D Biosciences), and integrin α4β7 (Clone ACT-1) conjugated to Alexa 488 using an Alexa labeling kit (Molecular Probes). Cells were then simultaneously sorted into 4 populations: (N/MB) B naive (CD19+ CD27-), (P) B memory (BM, CD19+CD27+) expressing CD62L but not integrin α4/β7 (CD62L+ α4/β7-), (M) BM expressing α4/β7 but not CD62L (CD62L-α4/β7+), or (M/P) BM expressing both integrin α4/β7 and CD62L (CD62L+ α4/β7+). Four-way sorting was performed in a MoFlo flow cytometer/cell sorter system (Beckman-Coulter). Purities of the sorted populations were 84.1%-94%. The presence of Norwalk VLP-specific IgG and IgA ASCs in each population was measured as described above.
Results
Safety
The safety data for study 2 are presented in Table 1. The safety data for study 1 (data not shown) were similar to those of study 2. After dose 1 or dose 2 of the 100-μg dosage of vaccine, local nasal symptoms were reported by 19 of 20 subjects and 18 of 20 subjects, respectively (Table 1). Likewise in the MPL plus chitosan control group without Norwalk antigen, 10 of 10 subjects and 8 of 10 subjects reported local nasal symptoms after each dose, respectively. In the true placebo group, 8 (73%) of 11 subjects and 3 (27%) of 11 subjects who received a puff of air (no dry powder) reported local nasal symptoms after dose 1 or dose 2, respectively. Local nasal symptoms were primarily mild (grade 1)—nasal stuffiness, nasal discharge, or sneezing. Two (5%) of 40 vaccinees reported mild (grade 1) nasal bleeds on a single day: 1 subject after dose 1 (100-μg vaccinee) and 1 subject after dose 2 (50 -μg vaccinee). No severe (grade 3) local nasal or systemic events were reported in the 7 days after either dose of vaccine or control. None of the 61 subjects in study 2 reported fever in the 7 days after either nasal administration. Headache and malaise were the most common systemic symptoms observed and were not statistically different between groups.
Table 1.
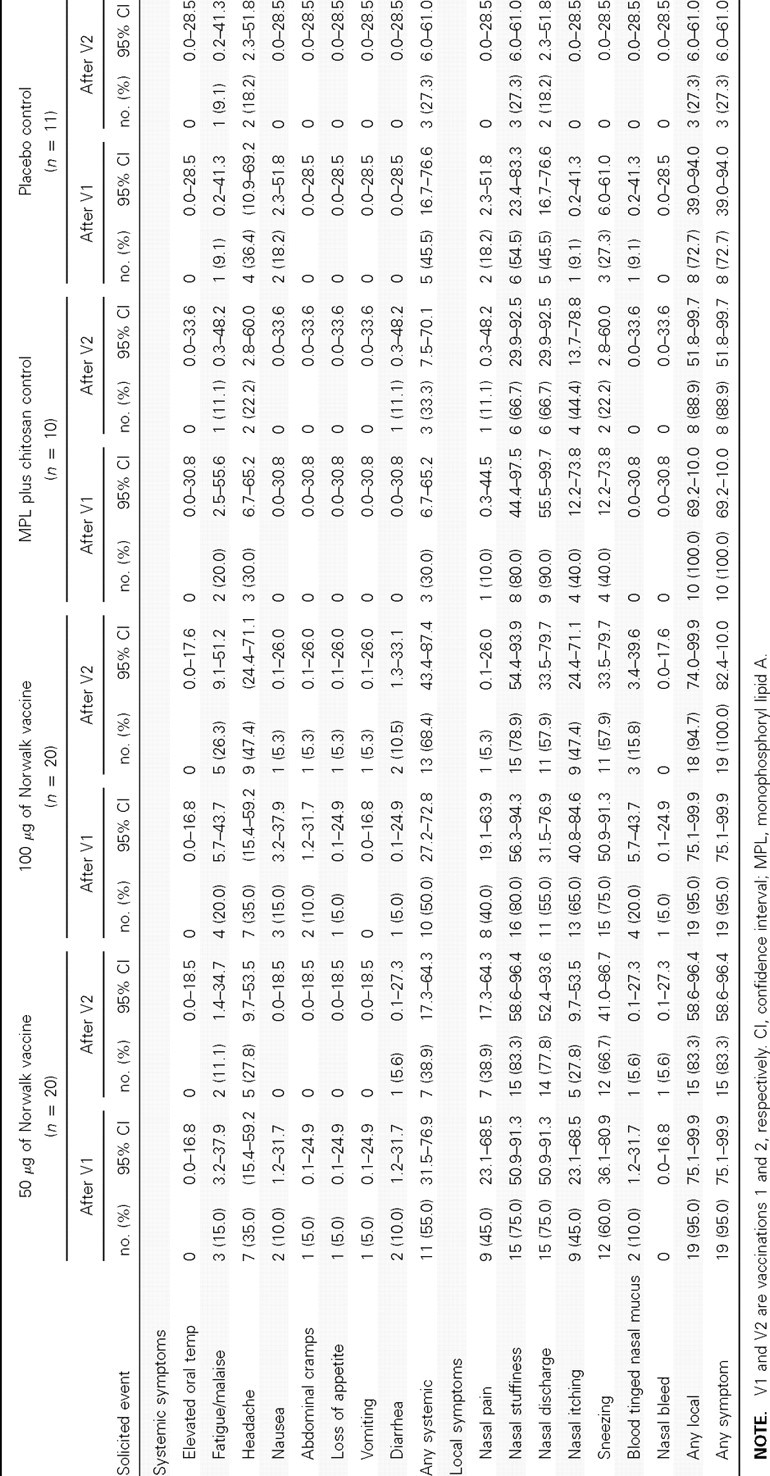
Frequency of Solicited Adverse Events Reported during the First 7 Days after Administration of Vaccine or Placebo in Study 2, by Group
A mild (grade 1) elevation in fibrinogen was observed in 1 of 9 vaccinees and in none of the 3 control subjects 1 day after dose 1 that returned to normal by day 7. No elevation in fibrinogen was observed at 1 day or 7 days after dose 2. Clinical laboratory abnormalities were infrequent and observed with similar frequency across study groups. Severe (grade 3) hematologic abnormalities were not observed. Two severe (grade 3) chemistry abnormalities were observed; an elevated aspartate aminotransferase in a recipient of the 50-μg dosage of vaccine and a decreased glucose in a placebo recipient. One serious adverse event not related to the vaccine was reported in the 180-day safety period, a hospitalization for appendectomy 111 days after the second dose of vaccine. No new onset, medically significant medical conditions were reported in the 180-day safety period.
Immune Responses
ASCs. Norwalk VLP-specific IgG and IgA circulating ASCs were detected at day 7, waned at day 21 (immediately prior to dose 2), and reappeared at day 28, 7 days after dose 2 (Table 2). In study 1, 7 (39%) of 18 subjects who were evaluated and received any vaccine dosage developed rises in specific IgA ASCs at day 7, and 10 (53%) of 19 subjects had ASC responses at day 28. In study 2, all 10 subjects evaluated (100%) who received 50 or 100 mg of vaccine developed IgA ASCs at days 7 and 28.
Table 2.
Response Rates of Norwalk Virus-Like Particle-Specific Immunoglobulin A (IgA) Antibody Secreting Cells (ASCs) and ASC Geometric Mean (GM), by Group
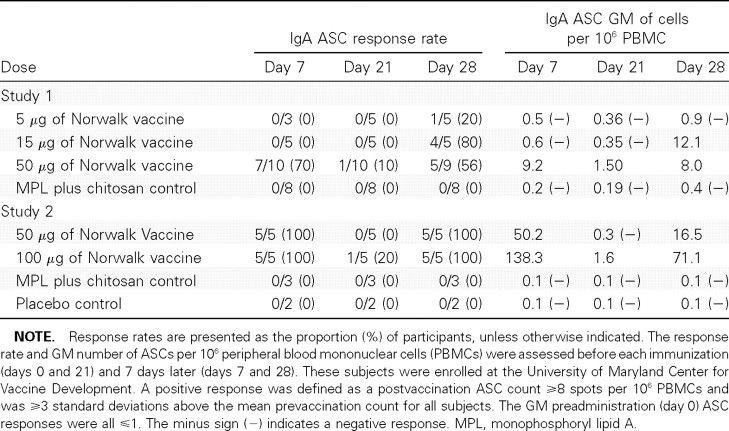
Serum antibodies. Norwalk VLP-specific IgG and IgA seroresponse rates and geometric mean of fold rises are presented in Table 3, and the kinetics of antibody production (geometric mean titers before and after vaccination) are presented in Figure 1. In study 1, the seroresponse rates showed a dose-dependent response with increased titers as the dosage of vaccine antigen increased; a logistic regression with dose as a continuous variable results in a χ2 Pvalue <.01 for IgG seroresponse rates and a χ2 Pvalue <.02 for IgA seroresponse rates. In study 2, 12 (63%) of 19 subjects in the 100-mg group seroresponded for IgG antibodies, and 15 (79%) of 19 subjects seroresponded for IgA antibodies at day 56. The 100-mg group developed higher titers than did the 50-μg group, but the differences were not statistically significant. Both vaccine groups developed higher serum IgG and IgA responses than those of the 2 control groups; a logistic regression with dose as a continuous variable results in a χ2 Pvalue <.001 for IgG and IgA seroresponse rates. No subjects developed ⩾4-fold rises in serum IgM antibody (data not shown).
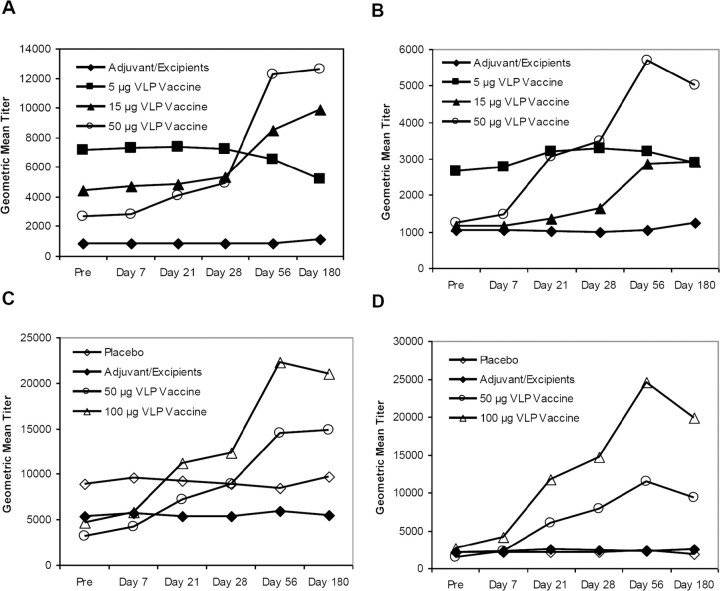
Vaccine-induced antibodies were also examined in their capacity to inhibit hemagglutination of O-type human red blood cells by Norwalk VLP. HAI titers (geometric mean titers, geometric mean of fold rises, and ⩾4-fold rises) are presented in Table 4, and the geometric mean titers are presented in Figure 2. Among subjects who received the 100-μg dosage of vaccine, the geometric mean HAI antibody titers peaked after the second dose, with a geometric mean of fold rises of 9.1 (95% confidence interval [CI], 4.0–20.7); seroresponse occurred in 73.7% of these subjects. The IgG and HAI data had a 72% agreement in 4-fold rise responses for day 56 (95% CI, 58%-83%). The IgA and HAI data had a 75% agreement in 4-fold rise responses for day 56 (95% CI, 62%-86%).
Homing potential of Norwalk-specific ASCs. To investigate the expression of homing molecules known to direct their migration to mucosal and peripheral lymphoid tissues, PBMCs from 5 subjects were stained, sorted simultaneously into 4 defined subsets, and assessed for their ability to secrete Norwalk IgG and IgA as described above (Table 5). The majority of IgA ASCs were observed in 2 main subsets: CD19+CD27 + CD62L +, integrin α4/β7+, that is, expressing both peripheral lymphoid tissue and mucosal homing molecules (∼700–10,700 ASCs per 10 6sorted cells); and CD19+CD27+ CD62L -integrin α4/β7+, that is, expressing exclusively mucosal homing molecules (∼2500–6700 ASCs per 10 6sorted cells). The latter was observed in 3 of 4 vaccinees.
Table 5.
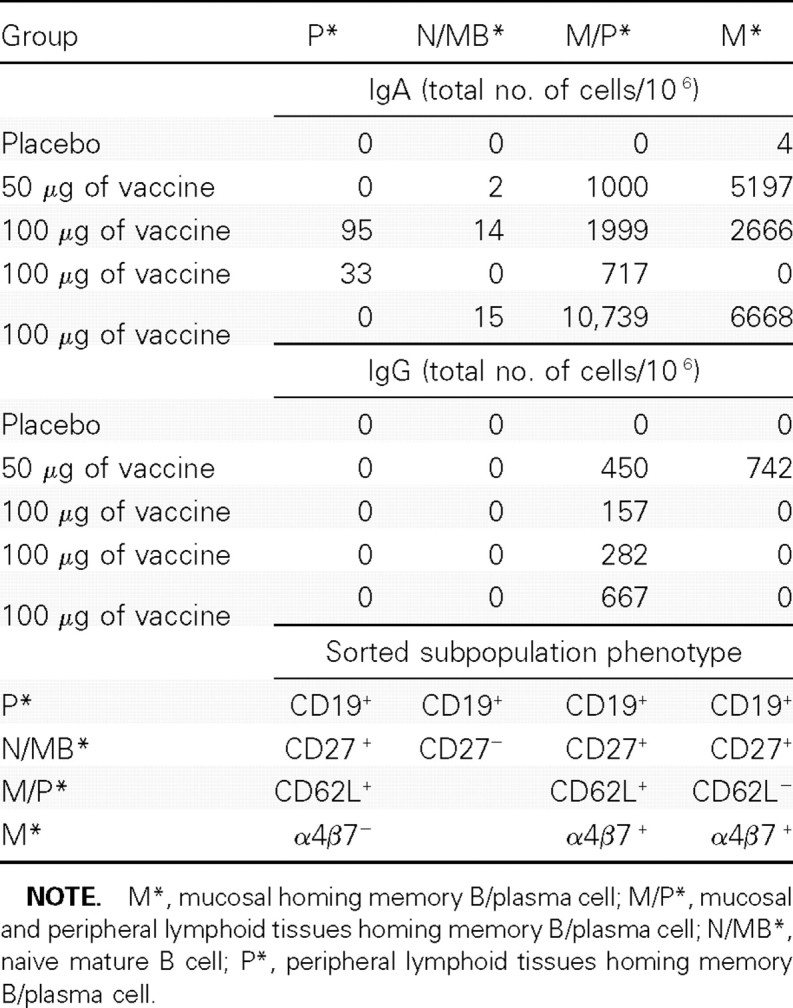
Norwalk Virus-Like Particle-Specific Immunoglobulin G (IgA) and Immunoglobulin G (IgG) Cell Surface Receptor Homing Molecules in Study 2
In contrast, the IgG ASCs, with the exception of 1 vaccinee, were of a single phenotype: CD19 +CD27 +CD62L +integrin α4/β7 +, and the frequencies (∼150–670 ASCs per106 sorted cells) were considerably lower than those observed for IgA ASCs. One vaccinee whose dosage was at the 50-μg level exhibited IgG ASC subsets bearing both mucosal and peripheral lymphoid tissues homing receptors.
No IgG or IgA ASCs exhibited a phenotype associated with either naive B cells (CD19+, CD27- or BM (CD19+,CD27+) cells expressing CD62L in the absence of integrin α4/β7, which would presumably home exclusively to peripherl lymphoid tissues.
Discussion
The intranasal monovalent adjuvanted Norwalk VLP vaccine was generally well tolerated and immunogenic. A second dose of vaccine provided increased serologic antibody responses, whereas the peak increase in ASC responses occurred after dose 1. HAI antibody (a functional measurement) increased only at the highest dosage tested, and the fold increase (9-fold) was similar to that of the serum IgA. Mucosal priming by the nasal mucosa was supported by the presence of high frequencies of IgA and IgG ASCs in peripheral blood. ASCs appear transiently in the circulation after naive B lymphocytes at an inductive site are exposed to a foreign antigen (eg, vaccine-primed B cells at the nasal associated lymphoid tissue return to the mucosa as immune effector cells). Norwalk IgA-specific ASC responses were observed 7 days after the first immunization in all 5 subjects tested that received the 100-μg vaccine dose, with a geometric mean of 138 cells per 10 6PBMCs. The ASC numbers reported in this study are higher than what has been previously observed after administration of oral nonadjuvanted Norwalk VLP vaccine or after ingestion of nonadjuvanted Norwalk VLP antigen in edible transgenic plants [5, 16]. These ASC counts are also generally higher than those induced by live oral vaccines or by wild-type challenges with enteric organisms [5, 21–24]. The correlates of protective immunity against Norwalk virus illness are not known; however, these mucosally primed ASCs in combination with the serum IgG and IgA antibodies may contribute to protection. Graham et al [25] reported a 35-fold mean increase in serum antibody titers in a population of 41 Norwalk virus-infected subjects after virus challenge. Gray et al [20] evaluated a subset of these serum samples for IgG and IgA by ELISA and observed Norwalk IgG peak titers of ∼15,900 from days 15 to 90 and IgA peak titers of ∼12,600 from days 24 and 90. A direct comparison of IgG and IgA between challenged and vaccinated subjects has not been performed but should be included in future studies.
We report for the first time to our knowledge the presence of circulating Norwalk-specific IgA and IgG ASCs after intranasal vaccination with adjuvanted VLPs. Hence, it was important to study the homing characteristics of these effector cells. It is widely accepted that CD62L is a key molecule implicated in the initial phase of migration through high endothelial venules in lymphoid tissues, including lymph nodes and Peyer patches, by binding to the peripheral lymph node addressins (PNad) and the mucosal addressin cell adhesion molecule (MAdCAM-1) present in the high endothelial venule vascular endothelium, which results in tethering and rolling [27–29]. In contrast, at mucosal effector sites, although a number of adhesion molecules are involved, vascular adhesion specificity is mediated by integrin α4/β7 interacting with the mucosal addressin cell adhesion molecule [27–29]. Thus, cells expressing integrin α4/β7 but not CD62L are destined to home to the gut mucosa, whereas cells expressing CD62L but not integrin a4/ b7 are destined to home to peripheral and mesenteric lymph nodes. As an example, a previous study that evaluated B cell surface markers in response to acute rotavirus infections showed that cells homing to the gut mucosa were CD27 +integrin α4/β7 +CD62L +/-[26]. In contrast, although it is likely that cells coexpressing integrin α4/β7 and CD62L can home to peripheral lymphoid tissues [27], the homing potential and activity of these cells remains controversial.
In the present study we observed that intranasal immunization elicited circulating VLP-specific IgA and IgG ASCs with different homing potentials. IgA-specific ASCs exhibited homing receptors likely to endow them with the ability to home to both the gut mucosa (CD19+CD27+ integrin α4/β7 +CD62L -) and peripheral lymphoid tissues (CD19 +CD27 +integrin α4/β7 +CD62L +), whereas IgG-ASCs expressed homing receptors that support homing to peripheral lymphoid tissues (CD19 +CD27 +integrin α4/β7 +CD62L +). The fact that intranasal immunization was able to elicit ASCs with such a diverse homing profile, including the gut mucosa, is noteworthy and demonstrates that this route has the capacity to induce potent systemic and mucosal immune responses, including effector cell activity at a distant site of infection (ie, the gastrointestinal tract in the case of Norwalk virus). However, intranasal immunization was not effective in inducing significant levels of specific IgG or IgA ASCs with the potential to home exclusively to peripheral lymphoid tissues [27].
This adjuvanted Norwalk VLP vaccine is one of only a few vaccine candidates directed against an enteric pathogen that has been delivered to humans by the intranasal route. An intranasal vaccine against Shigella flexneri (Invaplex 50) was generally well tolerated, but the immune responses were suboptimal; subjects who underwent subsequent challenge with S. flexneri were not protected [30]. The heat-labile enterotoxin of Escherichia coli and its enzymatically inactive mutants have been given as intranasal adjuvants in human studies; however, the toxin has been associated with facial nerve palsy [31], and intranasal administration of this antigen and/or adjuvant has been abandoned.
VLPs have been a successful strategy for development of parenteral vaccines, such as those against hepatitis B and human papillomavirus [32, 33]. Our data support that mucosal and systemic immune responses after adjuvanted Norwalk VLPs delivered by the intranasal route support this approach as an important vaccine development strategy.
Nasal vaccination has several advantages, including ease of administration, stimulation of mucosal dendritic cells to initiate immune responses locally and systemically, and ease of storage at room temperature. Disadvantages include the requirement of a specialized delivery device, the rapid clearance of antigens from the nose by mucociliary action, and that the mucosal immune response may be limited [34]. The intranasal adjuvanted Norwalk VLP vaccine was formulated with both MPL and chitosan to address these disadvantages [17, 35, 36]. MPL is a potent adjuvant for adaptive immunity signaling through TLR-4, inducing a blended Th1-and Th2-type immune response. MPL was recently approved by the FDA as a new adjuvant in the United States as a component of GlaxoSmithKline's human papillomavirus vaccine, CERVARIX [37, 38]. Chitosan, a cationic polysaccharide derived from chitin, has been used for nasal delivery because of its biocompatibility, biodegradability, and bioadhesiveness [17, 39, 40]. Chitosan has also been shown in animals to have immunostimulatory activity [41].
How effective the observed immune responses might be in preventing Norwalk infection and illness remains unknown. The immune responses to the prototype vaccine described here may not be cross-reactive against other genotypes, and a future norovirus VLP vaccine should include VLPs that represent the most epidemiologically important genotypes [9]. Preparations of wild-type Norwalk viruses used in challenges in humans established their pathogenicity [25, 42]. Norwalk shedding and clinical symptoms after Norwalk challenge in humans using virus of known genetic makeup and purity has recently been reported elsewhere [43]. Because this model of infection in human volunteers is established, the efficacy of adjuvanted Nor-walk VLP vaccine administered by the intranasal route should be assessed in the human challenge model.
Table 3.
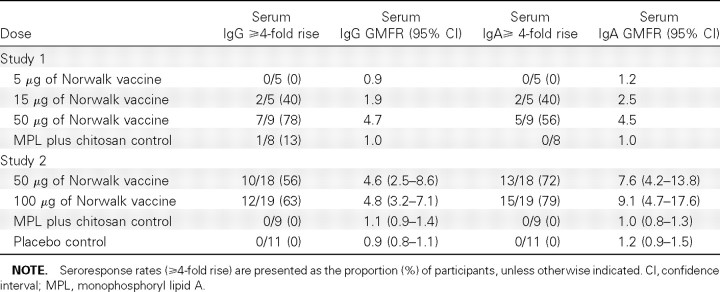
Norwalk Virus-Like Particle-Specific Immunoglobulin G (IgG) and Immunoglobulin A (IgA) Antibody Seroresponse Rates (percent of subjects with ⩾4-fold rise) and Geometric Mean of Fold Rise (GMFR), by Group by Study at Day 56 (35 days after Vaccination 2) Compared with Prevaccination
Table 4.
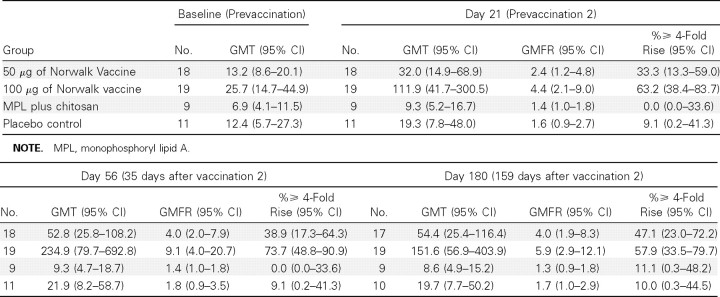
Norwalk Virus-Like Particle-Specific Hemagglutination Inhibition Antibody Geometric Mean Titers (GMTs), Geometric Mean of Fold Rises (GMFR), and Seroresponse Rates by Group in Study 2
Figure 1.
Norwalk virus-like particle (VLP)-specific immunoglobulin G (IgG) and immunoglobulin A (IgA) geometric mean antibody titers, by group in studies 1 and 2. In study 1, 28 adult subjects were randomized sequentially by group to receive 2 doses of (1) 5 μg of Norwalk VLP vaccine (5 subjects, squares) or adjuvant control (2 subjects, diamonds), (2) 15 μg of Norwalk VLP vaccine (5 subjects, triangles) or adjuvant control (2 subjects, diamonds), or (3) 50 μg of Norwalk VLP vaccine (10 subjects, circles) or adjuvant control (4 subjects, diamonds). A, Serum IgG geometric mean titers from study 1. B, Serum IgA geometric mean titers from study 1. In study 2, 61 healthy adult subjects were enrolled at 4 sites and randomized 2:2: 1:1, respectively, to receive either 2 doses of (1) 50 μg of Norwalk VLP vaccine (20 subjects, circles), (2) 100 μg of Norwalk VLP vaccine (20 subjects, triangles); (3) adjuvant control (10 subjects, filled diamonds), or (4) true placebo (11 subjects, open diamonds) consisting of a puff of air (no dry powder). C, Serum IgG geometric mean titers from study 2. D, Serum IgA geometric mean titers from study 2. All doses were delivered intranasally, and the 2-dose regimen was separated by 21 days.
Figure 2.
Norwalk virus-like particle (VLP)-specific hemagglutination inhibition antibody geometric mean titers, by group in study 2. Sixty-one healthy adult subjects were enrolled at 4 sites and randomized 2:2:1:1, respectively, to receive either 2 doses of (1) 50 μg of Norwalk VLP vaccine (20 subjects, circles), (2) 100 μg of Norwalk VLP vaccine (20 subjects, triangles), (3) adjuvant control (10 subjects, filled diamonds), or (4) true placebo (11 subjects, open diamonds) consisting of a puff of air (no dry powder). All doses were delivered intranasally, and the 2-dose regimen was separated by 21 days.
Acknowledgments
We are grateful for the recruitment and care of subjects provided by Mary Lou Mullen and JoAnna Becker at the University of Maryland, to Amy Cline at Cincinnati Children's Hospital, to Sharon Irby-Moore, Dr Edwin Anderson, and Dr Irene Graham at the Saint Louis University Center for Vaccine Development, and to Doreen Francis at the University of Rochester Medical Center. We thank Dr Wilbur Chen from the University of Maryland, Dr Nancy Browning from EMMES, and Dr Robert Belshe from Saint Louis University Medical Center for serving on the Safety Monitoring Committee. The authors acknowledge Julie Cordova and Robin Mertens for their operational expertise. James Freeman provided expertise in regulatory affairs and Brenda Dorsey in quality management. Special thanks to the Emmes Corporation, Rockville, Maryland, for data management and data analysis, and to DP Clinical, Rockville, Maryland, for data monitoring. We thank Dr Robert Goodwin of LigoCyte Pharmaceuticals, Inc, and Dr Min-Shi Lee of the National Health Research Institutes, Taiwan, who provided critical review of the manuscript. The ACT-1 monoclonal antibody to Integrin α4/β7 was kindly provided by Dr Mark Newman, PaxVax, Inc, San Diego, California.
Footnotes
Presented in part: National Foundation for Infectious Diseases Thirteenth Annual Conference on Vaccine Research, Bethesda, Maryland, 26–28 April 2010 (abstract S36); 5th International Conference on Vaccines for Enteric Diseases, Malaga, Spain, 9–11 September 2009; 3rd International Calicivirus Conference, Cancun, Mexico, 10–13 November 2007 (S5-3).
Potential conflicts of interest: S.S.E.-K. and M.F.P. have each been reimbursed for travel to one scientific meeting by LigoCyte Pharmaceuticals. P.M.M., R.S., C.R., and R.B. are employed by LigoCyte Pharmaceuticals.
Financial support: US Army Medical Research and Material Command (contract W8IXWH-05-C-0135), the University of Maryland General Clinical Research Center (grant M00 RR 16500), the General Clinical Research Center Program, the National Center for Research Resources, the National Institutes of Health, and LigoCyte Pharmaceuticals, Inc.
The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation. The investigators adhered to the policies regarding the protection of human subjects as prescribed by 45 CFR 36 and 32 CFR 219 (Protection of Human Subjects). Investigators adhered to Guidelines for Research Involving Recombinant DNA Molecules; Notice, 59 Federal Register 127 (1994). The nasal vaccine incorporates chitosan. This application of chitosan (ChiSys) has been licensed from Archimedes Development, Ltd. ChiSys is a trademark of Archimedes Development, Ltd, and is registered as a Community Trade Mark (CTM), as a US registered trademark, and in certain other jurisdictions.
References
- 1.Estes MK, Ball JM, Guerrero RA, et al. Norwalk virus vaccines: challenges and progress. J Infect Dis. 2000;181(Suppl 2):367–373. doi: 10.1086/315579. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Norovirus: technical fact sheet. http://www.cdc.gov/ncidod/dvrd/revb/gastro/norovirus-factsheet .htm. Accessed 22 October 2009. [Google Scholar]
- 3.Hyams KC, Malone JD, Kapikian AZ, et al. Norwalk virus infection among Desert Storm troops. J Infect Dis. 1993;167:986–987. doi: 10.1093/infdis/167.4.986. [DOI] [PubMed] [Google Scholar]
- 4.Sharp TW, Hyams KC, Watts D, et al. Epidemiology of Norwalk virus during an outbreak of acute gastroenteritis aboard a US aircraft carrier. JMed Virol. 1995;45:61–67. doi: 10.1002/jmv.1890450112. [DOI] [PubMed] [Google Scholar]
- 5.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 6.Johnston CP, Haoming Q, Ticehurst JR, et al. Outbreak management and implications of nosocomial Norovirus outbreak. Clin Infect Dis. 2007;45:534–540. doi: 10.1086/520666. [DOI] [PubMed] [Google Scholar]
- 7.Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long term travelers (US military and similar populations): a systemic review. Am J Trop Med Hyg. 2006;74(5):891–900. [PubMed] [Google Scholar]
- 8.Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI. Molecular epidemiology of “ Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998;178:1571–1578. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- 9.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel MM, Widdowson M-A, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of Noroviruses in Sporadic gastroenteritis. Emerg Infect Dis. 2008;14(8):1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkataram Prasad BV, Hardy ME, Estes MK. Structural studies of recombinant Norwalk capsids. J Infect Dis. 2000;181(Suppl 2):317–321. doi: 10.1086/315576. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ball JM, Hardy ME, Atmar RL, Conner ME, Estes MK. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998;72:1345–1353. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero RA, Ball JM, Krater SS, Pacheco SE, Clements JD, Estes MK. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J Virol. 2001;75:9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999;117:40–48. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 16.Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000;182:302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 17.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 18.indesmith L, Moe C, Marionneau S, et al. Human susceptibility and resistance to Norwalk infection. Nat Med. 2003;9(5):548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 19.Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection is associated with ABO histo-blood group type. J Infect Dis. 2002;185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 20.Gray JJ, unliffe C, Ball J, Graham DY, Desselberger U, Estes MK. Detection of immunoglobulin M (IgM), IgA, and IgG Norwalk virus-specific antibodies by indirect enzyme-linked immunosorbent assay with baculovirus-expressed Norwalk virus capsid antigen in adult volunteers challenged with Norwalk virus. J Clin Microbiol. 1994;32:3059–3063. doi: 10.1128/jcm.32.12.3059-3063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.otloff K, Sztein MB, Wassserman SS, Losonsky G, DeLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581–3590. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotloff KL, Noriega FR, Samandari T, et al. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA , is highly attenuated in humans. Infect Immun. 2000;68:1034–1039. doi: 10.1128/iai.68.3.1034-1039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenzie R, Bourgeois AL, Frech SA, Flyer DC, Bloom A, azempour K, Glenn GM. Transcutaneous immunization with the heat-labile toxin (LT) of entertoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine. 2007;25:3684–3691. doi: 10.1016/j.vaccine.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Kotloff KL, Simon JK, Pasetti MF, et al. Safety and immunogenicity of CVD 1208S, a live, oral D guaBADsenDset Shigella flexneri 2a vaccine grown on animal-free media. Hum Vaccin. 2007;3:268–275. doi: 10.4161/hv.4746. [DOI] [PubMed] [Google Scholar]
- 25.Graham DY, Jiang X, anaka T, Opekun AR, Madore HP, Estes MK. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 26.Jaimes MC, Rojas OL, Kunkel EJ, et al. Maturation and Trafficking Markers on Rotavirus-Specific B Cells during Acute Infection and Convalescence in Children. J Virol. 2004;78:10967–10976. doi: 10.1128/JVI.78.20.10967-10976.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandtzaeg P, Johansen F-E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 28.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 29.Shyjan AM, Bertagnolli M, Kenney CJ, Briskin MJ. Human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) demonstrates structural and functional similarities to the alpha 4 beta 7-integrin binding domains of murine MAdCAM-1, but extreme divergence of mucinlike sequences. J Immunol. 1996;156:2851–2877. [PubMed] [Google Scholar]
- 30.Harro C, Riddle MS, Kaminski RW, et al. Shigella flexneri 2a Invaplex 50 vaccine phase 2b challenge study. 5th International Conference on Vaccines against Enteric Diseases, 9–11 September 2009, Malaga, Spain [Google Scholar]
- 31.utsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 32.Boisgerault F, Moron G, Leclerc C. Virus-like particles: a new family of delivery systems. Expert Rev Vaccines. 2002;1:101–109. doi: 10.1586/14760584.1.1.101. [DOI] [PubMed] [Google Scholar]
- 33.Schiller JT, Lowy DR. Papillomavirus-like particle vaccines. J Natl Cancer Inst Monogr. 2001;28:50–54. doi: 10.1093/oxfordjournals.jncimonographs.a024258. [DOI] [PubMed] [Google Scholar]
- 34.Slutter B, Hagenaars N, Jiskoot W. Rational design of nasal vaccines. J Drug Target. 2008;16:1–17. doi: 10.1080/10611860701637966. [DOI] [PubMed] [Google Scholar]
- 35.Boland G, Beran J, Lievens M, et al. Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine. 2004;23:316–320. doi: 10.1016/j.vaccine.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 37.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Food and Drug Administration(FDA) CERVARIX approval letter. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186959.htm. 16 October 2009.
- 39.Huo Z, Sinha R, McNeela EA, et al. Induction of protective serum meningococcal bactericidal and diphtheria-neutralizing antibodies and mucosal immunoglobulin A in volunteers by nasal insufflations of the Neisseria meningitidis serogroup C polysaccharide-CRM197 conjugate vaccine mixed with chitosan. Infect Immun. 2005;73:8256–8265. doi: 10.1128/IAI.73.12.8256-8265.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Read RC, Naylor SC, Potter CW, et al. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23(35):4367–4374. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 41.Porporatto C, Bianco ID, Correa SG. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J Leukocyte Biol. 2005;78:62–69. doi: 10.1189/jlb.0904541. [DOI] [PubMed] [Google Scholar]
- 42.indesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol. 2005;79:2900–2909. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atmar RA, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14(10):1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]