Summary
Background
Puberty is characterized by increases in growth hormone (GH) and insulin-like growth factor-1 (IGF-1) and the pubertal growth spurt. Bone formation and resorption also increase, consistent with increased bone metabolism.
Objective
To determine the relationship between pubertal bone metabolism, GH and IGF-1. We hypothesized that bone turnover peaks at the time of greatest pubertal GH secretion.
Design and Subjects
Subjects included 86 girls and boys, 9–17 years-old (BMI 10th–90th percentiles). Because higher endogenous GH secretion is associated with a higher nadir following oral glucose, we used the GH nadir following a 2-h OGTT as indicative of GH status. Fasting serum IGF-1, aminoterminal propeptide of type 1 procollagen (P1NP) and carboxy-terminal collagen cross-links (CTX) were obtained. Subjects were grouped per expected timing of peak growth. Group 1: Tanner 1 girls and Tanner 1–2 boys (period preceding peak growth), Group 2: Tanner 2–3 girls and Tanner 3–4 boys (period of peak growth) and Group 3: Tanner 4–5 girls and Tanner 5 boys (period following peak growth).
Results
GH peaked at mid-puberty (Group 2) and IGF-1 in late puberty (Group 3). P1NP and CTX were highest in mid-puberty compared with early and late puberty (P = 0·0009 and 0·006 in girls and P = 0·005 and 0·04 in boys). GH, but not IGF-1, correlated with P1NP (r = 0·46 in both genders, P ≤ 0·008) and CTX (r = 0·37 and 0·38, P = 0·04 and 0·02 in girls and boys, respectively). Similarly, on regression modelling, GH (but not IGF-1) predicted both bone turnover markers in both genders.
Conclusion
GH is strongly associated with pubertal bone metabolism, independent of systemic IGF-1 in girls and boys.
Introduction
Puberty is characterized by marked hormonal changes and rapid growth. Early pubertal increases in testosterone and oestrogen secretion are followed by increases in growth hormone (GH) secretion and production of insulin-like growth factor-1 (IGF-1), factors important for skeletal growth and maturation.1–5 Following increases in levels of GH and IGF-1, rapid increases in height velocity and the pubertal growth spurt occur. During this growth phase, the skeleton undergoes marked linear growth at the epiphyses and increased bone mineral deposition. Puberty is characterized by bone modelling, which produces new bone formation at sites not undergoing resorption, and this occurs in conjunction with bone remodelling, a process characterized by new bone formation and resorption occurring at the same bone site. Both processes together lead to pubertal alterations in bone shape and in net increases in height and bone mass accrual.6 However, it is unknown whether GH or IGF-1 levels are stronger determinants of pubertal bone mass accrual.
Serum surrogate markers of collagen synthesis and collagen degradation products enable the assessment of bone turnover in vivo.7–9 Although it is generally assumed that increases in levels of surrogate markers of bone turnover (or bone metabolism) during puberty are consequent to changes in GH and IGF-1 secretion, recent studies have demonstrated relatively weak associations of IGF-1 levels and markers of bone turnover in puberty.3 Importantly, there are no reported studies at this time evaluating pubertal associations of GH with markers of bone turnover, particularly in relation to pubertal stages corresponding with peak growth. Because of the pulsatile nature of GH secretion, assessment of GH status entails either a stimulatory dynamic test of GH secretion (most useful in states of low endogenous GH secretion), frequent sampling to assess secretory characteristics, or assessment of the GH nadir following an oral glucose load (most useful in states of high endogenous GH secretion). Puberty is characterized by high levels of endogenous GH secretion, and we have previously demonstrated strong associations of the GH nadir following an oral glucose load in normal puberty with GH secretory characteristics.10,11
Girls and boys have similar levels of bone turnover markers at a given age before the onset of puberty.12 Increases in bone turnover markers coincide with increases in linear growth velocity.13–15 Because the timing of peak pubertal growth differs in boys versus girls, levels of bone turnover markers differ at any given age. Bone turnover markers in boys rise later, are greater and last longer compared with girls.14–16 GH levels and growth velocity increase during puberty in both boys and girls; however, in addition to differing in age of onset, these also differ in the phase of puberty during which the increase occurs. Girls typically have an earlier peak pubertal growth velocity than boys, occurring at Tanner stages 2–3 (mean age 11·5 years),17 and peak GH secretion occurs at Tanner stage 3.18 In contrast, peak pubertal growth occurs later in boys, during Tanner stages 3–4 (mean age 13·5 years), and highest levels of GH also occur at Tanner stage 4.17,19
Although many studies have demonstrated an association of increased bone turnover markers and peak pubertal growth, studies evaluating associations of GH, IGF-1 and bone turnover markers are limited, particularly in relation to gender. We hypothesized that bone modelling, as evidenced by increased levels bone turnover markers, increases during peak pubertal growth and is associated with increases in GH and IGF-1 secretion in both boys and girls.
Subjects and methods
Subject selection
Subjects were recruited between 9 and 17 years of age at various pubertal stages (Tanner stages) with a body mass index (BMI) between the 10th and 90th percentiles for age. A total of 107 subjects were initially recruited, 64 girls and 43 boys. Twenty-three girls and six boys were excluded because of incomplete data. Analyses were performed on 86 subjects; 45 boys and 41 girls as follows: Tanner stage 1 (n = 13) seven girls and six boys, Tanner stage 2 (n = 18) seven girls and eleven boys, Tanner stage 3 (n = 13) seven girls and six boys, Tanner stage 4 (n = 12) five girls and seven boys and Tanner stage 5 (n = 30) 15 girls and 15 boys. Ethnicity included 74% non-Hispanics, 24% Hispanics and 2% unknown. Self-reported racial make-up included 72% white subjects, 12% black subjects, 9% more than one race, 5% Asian and 2% unknown. Subjects were excluded if they had a medical condition or were taking medications within the preceding three months that affect the metabolism of glucose or GH (such as disorders of the thyroid or pituitary glands, Cushing’s syndrome, diabetes mellitus and renal failure) or were taking oestrogen. Subjects were recruited from paediatric practices and health care centres affiliated with Massachusetts General Hospital (MGH) paediatric practices using mass mailing and fliers. The institutional review board approved this study, and informed written consent and assent were obtained from all parents and subjects.
Experimental protocol
All subjects underwent a history and physical examination, including Tanner staging. Breast staging for girls and genital staging for boys were performed, and subjects were placed into three different groups based on stage of puberty, as previously reported.10 Tanner staging was performed by two paediatric endocrinologists who established agreement between their findings in 30 children before study initiation. This grouping was based on expected timing of peak height velocity, such that group 1 indicated the stage preceding peak growth (Tanner stage 1 in girls and stages 1–2 in boys), group 2 included subjects in Tanner stages associated with peak growth (Tanner stages 2–3 in girls and stages 3–4 in boys), and group 3 included subjects in Tanner stages following peak growth (Tanner stages 4–5 in girls and stage 5 in boys).10,14 Eligible subjects were studied at a single outpatient visit to the MGH Clinical Research Center. Post-menarchal girls were evaluated during the follicular phase (days 1–10) of their menstrual cycle.
Subjects came fasting for the study visit between 8:00 and 10:00 AM. A fasting blood sample was obtained for levels of IGF-1, aminoterminal propeptide of type 1 procollagen (P1NP) (a marker of bone formation), carboxy-terminal collagen crosslinks (CTX) (a marker of bone resorption) and 25(OH) vitamin D. An oral glucose tolerance test was performed (2·35 g/kg, with a maximum dose 100 g). Blood samples were drawn fasting at baseline and 30, 60, 90 and 120 min after oral glucose administration for GH levels. Pregnancy was ruled out in all girls. Data regarding GH levels, but not IGF-1 levels or levels of bone turnover markers, have been previously published.10
Biochemical assays
GH was measured with IRMA (Immulite 2000 Analyzer; Diagnostic Products Corp., Los Angeles, CA) (detection limit 0·01 ng/ml, intra-assay coefficient of variation (CV) 5·7%, inter-assay CV 8·2%). In addition to determining the nadir of GH over the two hours following oral glucose administration, we used Cluster analysis to determine area under the curve 12 for GH. IGF-1 was measured by the ISYS Analyzer (Immunodiagnostic Systems Inc., Scottsdale, AZ; detection limit 4·4 ng/ml, intra-assay CV 2·2% and inter-assay CV of 5·1%). PINP was measured using a RIA (Orion Diagnostica, Espoo, Finland), with a detection limit of 1·0 ng/ml, intra-assay CV of 2·9% and inter-assay CV of 4·6%. CTX was measured by the ISYS Analyzer (Immunodiagnostic Systems Inc., Scottsdale, AZ; detection limit 0·023 ng/ml, intra-assay CV 3·2% and inter-assay CV 6·2%). 25(OH) vitamin D was assessed using the ISYS Autoanalyzer (Immunodiagnostic Systems Inc., Scottsdale, AZ; limit of detection 1·5 ng/ml, intra-assay CV 4·4–8·3% and inter-assay CV 6·2–12·5%). Samples were stored at −80 °C until analysis and run in duplicate.
Statistical methods
Data are described as means ± SD. Version 5 of JMP (SAS Institute, Inc., Cary, NC) was used for data analysis. Natural log transformations were performed for data not normally distributed. Two-group comparisons of means were evaluated with the Student’s t-test. We used ANOVA, followed by the Tukey–Kramer test to adjust for multiple comparisons, for three-group comparisons. Levels of markers of bone turnover were compared across groups to determine whether the highest levels of these markers corresponded to pubertal stages known to be associated with peak pubertal growth. Nontransformed values for the mean and range are reported for values when logarithmic conversions were used, for ease of interpretation. Simple correlational analyses were used to determine parameters associated with P1NP and CTX for the group as a whole, and within girls and boys. For data not normally distributed, we used Spearman’s correlation. Finally, stepwise regression modelling was performed to determine independent predictors of P1NP and CTX. We used a P value of 0·10 to enter and to leave the model.
Results
Table 1 shows the clinical and biochemical characteristics of all subjects separated by gender. Overall, there was no difference in mean age. However, when subjects were separated into groups based on the timing of expected peak pubertal growth, there was a significant difference between the ages of boys and girls as expected, given that boys have their peak growth velocity later in puberty compared with girls. The genders did not differ for GH parameters, IGF-1, P1NP, CTX or 25(OH) vitamin D levels. Vitamin D levels were normal in the majority of our subjects. Only 17% had vitamin D levels in the insufficient range (<20 ng/ml; <50 nmol/l) and 6% in the deficient range (<15 ng/ml; <37·5 nmol/l).
Table 1.
Clinical and biochemical characteristics of boys versus girls
| Boys (n = 45) | Girls (n = 41) | P value | |
|---|---|---|---|
| Age (years) | 13·6 ± 2·3 | 13·2 ± 2·2 | 0·38 |
| Age (years) Group 1 | 11·3 ± 1·3 | 10·4 ± 0·7 | 0·03 |
| Age (years) Group 2 | 13·8 ± 0·9 | 12·2 ± 1·2 | 0·0004 |
| Age (years) Group 3 | 15·9 ± 1·4 | 14·9 ± 1·3 | 0·03 |
| BMI-SDS | 0·07 ± 0·66 | 0·04 ± 0·77 | 0·83 |
| GH baseline (ng/ml)* | 1·77 ± 2·68 | 1·77 ± 2·92 | 0·92 |
| GH nadir (ng/ml)* | 0·17 ± 0·15 | 0·24 ± 0·23 | 0·14 |
| GH AUC (ng/ml)* | 98·7 ± 131·7 | 152·4 ± 179·6 | 0·09 |
| IGF-1 (ng/ml) | 227·1 ± 123·7 | 248·5 ± 119·3 | 0·44 |
| Aminoterminal propeptide of type 1 procollagen (ng/ml) | 513·3 ± 250·2 | 448·3 ± 339·6 | 0·33 |
| Carboxy-terminal collagen crosslinks (ng/ml) | 1·8 ± 0·7 | 1·5 ± 1·0 | 0·11 |
| 25(OH) vitamin D (ng/ml) | 29·6 ± 13·9 | 31·3 ± 11·9 | 0·60 |
Means ± SDS.
Boys: Group 1: pre- to early puberty (Tanner stages 1–2); Group 2: mid–late puberty (Tanner stages 3–4); Group 3: late puberty (Tanner stage 5). Girls: Group 1: prepuberty (Tanner stage 1); Group 2: early–mid puberty (Tanner stages 2–3); Group 3: mid–late puberty (Tanner stages 4–5).
Comparisons performed with log-transformed values to approximate a normal distribution.
When subjects were separated based on timing of peak growth and gender, in girls (Table 2), GH nadir was higher in Group 2 compared with Groups 1 and 3 (Table 3). IGF-1 was the highest in Group 3. Similar to GH nadir, both P1NP and CTX were highest in Group 2. In boys (Table 3), both GH nadir and GH AUC were significantly higher in Group 2 compared with Groups 1 and 3. IGF-1 followed the trend seen in the girls with the highest levels in Group 3. Similar to GH, both P1NP and CTX were higher in Group 2 than in Groups 1 and 3. Vitamin D levels did not differ between the groups in either gender.
Table 2.
Clinical and biochemical characteristics of girls by grouping based on timing of peak growth
| Girls | Group 1 (Tanner stage 1) (n = 7) | Group 2 (Tanner stages 2–3) (n = 14) | Group 3 (Tanner stages 4–5) (n = 20) | P value |
|---|---|---|---|---|
| Age (yrs) | 10·4 ± 0·7*,† | 12·2 ± 1·2*,‡ | 14·9 ± 1·3†,‡ | <0·0001 |
| BMI-SDS | −0·13 ± 0·81 | −0·22 ± 0·61 | 0·28 ± 0·81 | NS |
| GH baseline (ng/ml)§ | 1·55 ± 2·05 | 1·72 ± 2·48 | 1·88 ± 3·50 | NS |
| GH nadir (ng/ml)§ | 0·10 ± 0·05† | 0·37 ± 0·31‡ | 0·19 ± 0·15 | 0·02 |
| GH AUC (ng/ml) | 82·5 ± 86·5 | 235·9 ± 235·0 | 114·9 ± 135·2 | 0·09 |
| IGF-1 (ng/ml) | 155·3 ± 67·2* | 229·5 ± 132·0 | 312·8 ± 92·0‡ | 0·008 |
| P1NP (ng/ml)¶ | 381·9 ± 178·8 (216·6–547·3) | 692·5 ± 364·0* (472·5–912·5) | 239·9 ± 211·6† (239·9–211·6) | 0·0009 |
| CTX (ng/ml)¶ | 1·1 ± 0·6† (0·42–1·77) | 2·4 ± 1·3*,‡ (1·37–3·37) | 1·1 ± 0·6† (0·77–1·47) | 0·006 |
| 25(OH) vitamin D (ng/ml) | 34·6 ± 6·8 | 33·9 ± 15·9 | 26·9 ± 9·3 | NS |
Means ± SDS.
P < 0·05 compared with Group 3.
P < 0·05 compared with Group 2.
P < 0·05 compared with Group 1.
Comparisons performed with log-transformed values to approximate a normal distribution.
Ranges provided for aminoterminal propeptide of type 1 procollagen (P1NP) and carboxy-terminal collagen crosslinks (CTX) indicate the 5th and 95th confidence intervals.
Table 3.
Clinical and biochemical characteristics of boys by grouping based on timing of peak growth
| Boys | Group 1 (Tanner stage 1–2) (n = 17) | Group 2 (Tanner stages 3–4) (n = 13) | Group 3 (Tanner stage 5) (n = 15) | P value |
|---|---|---|---|---|
| Age (years) | 11·3 ± 1·3*,† | 13·8 ± 0·9*,‡ | 16·0 ± 1·4†,‡ | <0·0001 |
| BMI-SDS | 0·23 ± 0·60 | −0·30 ± 0·67 | 0·21 ± 0·62 | NS |
| GH baseline (ng/ml)§ | 2·02 ± 2·80 | 2·37 ± 3·50 | 0·92 ± 1·36 | NS |
| GH nadir (ng/ml)§ | 0·13 ± 0·10† | 0·26 ± 0·20*,‡ | 0·14 ± 0·14† | 0·008 |
| GH AUC (ng/ml)§ | 41·6 ± 37·7† | 186·7 ± 163·0*,‡ | 99·0 ± 148·4† | 0·003 |
| IGF-1 (ng/ml) | 161·5 ± 71·8* | 253·9 ± 134·4 | 278·2 ± 134·1‡ | 0·02 |
| P1NP (ng/ml)¶ | 540·5 ± 125·1 (476·2–604·9) | 653·3 ± 242·2* (507·0–799·7) | 361·1 ± 291·0† (199·9–522·2) | 0·005 |
| CTX (ng/ml)¶ | 1·81 ± 0·63 (1·48–2·15) | 2·23 ± 0·76* (1·74–2·71) | 1·50 ± 0·66† (1·08–1·92) | 0·04 |
| 25(OH) vitamin D (ng/ml) | 28·9 ± 9·0 | 30·3 ± 19·1 | 30·0 ± 15·1 | NS |
Means ± SDS.
P < 0·05 compared with Group 3.
P < 0·05 compared with Group 2.
P < 0·05 compared with Group 1.
Comparisons performed with log-transformed values to approximate a normal distribution.
Ranges provided for aminoterminal propeptide of type 1 procollagen (P1NP) and carboxy-terminal collagen crosslinks (CTX) indicate the 5th and 95th confidence intervals.
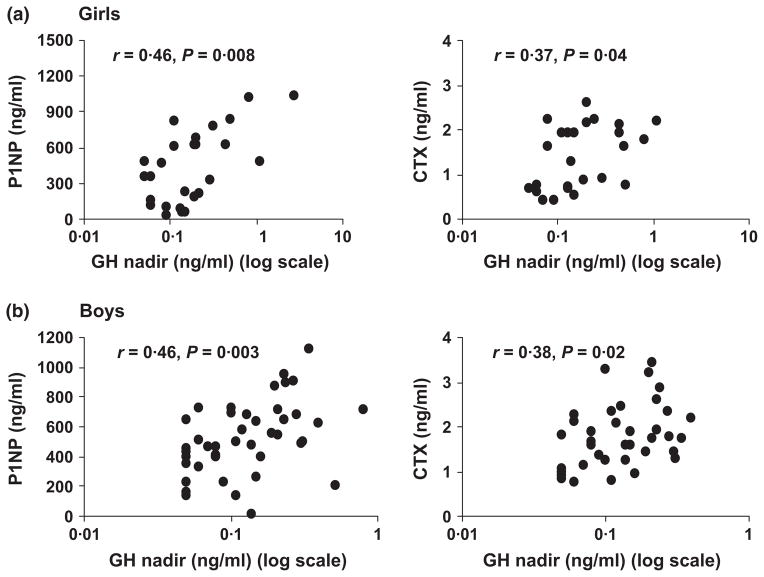
Table 4 shows correlations of P1NP and CTX with clinical and biochemical parameters. GH nadir and GH AUC were positively associated with P1NP and CTX (Fig. 1), whereas age was negatively associated with P1NP in boys. BMI-SDS and IGF-1 were not significantly associated with P1NP or CTX overall. However, we did find a positive association of P1NP with IGF-1 in Group 3 girls (r = 0·54, P = 0·05). We also examined associations of season of blood draw and vitamin D with levels of P1NP and CTX and found no associations in either group (data not reported). Of interest, GH levels did not correlate with IGF-1 levels.
Table 4.
Associations of aminoterminal propeptide of type 1 procollagen (P1NP) and carboxy-terminal collagen crosslinks (CTX) with various clinical and biochemical parameters in girls and boys
| P1NP
|
CTX
|
|||
|---|---|---|---|---|
| r | P | r | P | |
| Girls | ||||
| Age | −0·30 | 0·08 | −0·14 | 0·50 |
| BMI-SDS | −0·10 | 0·70 | −0·12 | 0·50 |
| GH nadir* | 0·46 | 0·008 | 0·37 | 0·04 |
| GH AUC* | 0·48 | 0·006 | 0·44 | 0·02 |
| GH baseline* | 0·20 | 0·28 | 0·14 | 0·50 |
| IGF-1 | −0·29 | 0·10 | −0·16 | 0·40 |
| 25(OH) vitamin D | 0·20 | 0·96 | −0·01 | 0·96 |
| Boys | ||||
| Age | −0·45 | 0·001 | −0·25 | 0·11 |
| BMI-SDS | −0·17 | 0·28 | −0·29 | 0·07 |
| GH nadir* | 0·46 | 0·003 | 0·38 | 0·02 |
| GH AUC* | 0·38 | 0·01 | 0·35 | 0·03 |
| GH baseline* | 0·48 | 0·002 | 0·30 | 0·08 |
| IGF-1 | −0·00 | 0·99 | 0·25 | 0·12 |
| 25(OH) vitamin D | 0·02 | 0·90 | −0·13 | 0·40 |
Comparisons performed with log-transformed values to approximate a normal distribution.
Significant P values are listed in bold font.
Fig. 1.
Aminoterminal propeptide of type 1 procollagen (P1NP) and carboxy-terminal collagen cross-links (CTX) associations with GH nadir: GH nadir (plotted on a logarithmic scale) was positively associated with P1NP and CTX in girls (panel a) (r = 0.46 and 0.37, P = 0.008 and 0.04, respectively) and boys (panel b) (r = 0.46 and 0.38, P = 0.003 and 0.02, respectively).
On regression modelling with age, BMI-SDS, IGF-1 and either GH nadir (Table 5a) or GH AUC (Table 5b) entered into the model, GH nadir and GH AUC were independent and positive determinants of both P1NP and CTX. These findings did not change when IGF-1 was removed from the model. IGF-1 levels did not determine P1NP levels in these regression models and were found to be a significant determinant of CTX only in boys when GH parameters were not added to the model (r2 = 0·25; P = 0·04).
Table 5.
Regression modelling to determine independent predictors of aminoterminal propeptide of type 1 procollagen (P1NP) and carboxy-terminal collagen crosslinks (CTX), with age, (a) BMI-SDS, GH nadir and IGF-1 entered into the model (b) BMI-SDS, GH AUC and IGF-1 entered into the model
| P1NP | Parameter estimate | F ratio | P | r2 | CTX | Parameter estimate | F ratio | P | r2 |
|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||
| Girls | |||||||||
| Intercept | 1668·1 | Intercept | 2·2 | ||||||
| Age | −64·6 | 7·9 | 0·01 | 0·37 | Age | 0 | 0·9 | 0·60 | |
| BMI-SDS | 0 | 0·1 | 0·80 | BMI-SDS | 0 | 0·3 | 0·60 | ||
| Log GH nadir | 492·9 | 13·3 | 0·001 | 0·20 | Log GH nadir | 1·0 | 3·6 | 0·07 | 0·13 |
| IGF-1 | 0 | 1·1 | 0·30 | IGF-1 | 0 | 1·6 | 0·20 | ||
| Boys | |||||||||
| Intercept | 1406·5 | Intercept | 3·7 | ||||||
| Age | −44·7 | 9·8 | 0·003 | 0·37 | Age | −0·1 | 3·0 | 0·09 | |
| BMI-SDS | 0 | 0·0 | 0·95 | BMI-SDS | 0 | 0·5 | 0·50 | ||
| Log GH nadir | 324·2 | 10·4 | 0·003 | 0·21 | Log GH nadir | 0·8 | 4·3 | 0·05 | 0·14 |
| IGF-1 | 0 | 0·8 | 0·37 | IGF-1 | 0 | 2·5 | 0·13 | ||
| (b) | |||||||||
| Girls | |||||||||
| Intercept | 118·6 | Intercept | −0·2 | ||||||
| Age | −59·1 | 6·1 | 0·02 | 0·36 | Age | 0 | 0·5 | 0·50 | |
| BMI-SDS | 0 | 0·1 | 0·75 | BMI-SDS | 0 | 0·2 | 0·65 | ||
| Log GH AUC | 327·3 | 11·9 | 0·002 | 0·23 | Log GH AUC | 0·9 | 4·9 | 0·04 | 0·17 |
| IGF-1 | 0 | 0·6 | 0·46 | IGF-1 | 0 | 0·8 | 0·37 | ||
| Boys | |||||||||
| Intercept | 885·7 | Intercept | 2·3 | ||||||
| Age | −48·9 | 11·2 | 0·002 | 0·20 | Age | −0·1 | 3·8 | 0·06 | 0·20 |
| BMI-SDS | 0 | 0·2 | 0·66 | BMI-SDS | 0 | 0·7 | 0·40 | ||
| Log GH AUC | 171 | 8·0 | 0·007 | 0·34 | Log GH AUC | 0·5 | 4·2 | 0·04 | 0·12 |
| IGF-1 | 0 | 0·6 | 0·40 | IGF-1 | 0 | 2·5 | 0·12 | ||
Significant P values are listed in bold font.
Discussion
We show that during puberty, GH status, but not IGF-1, is positively associated with markers of both bone formation and resorption, which peak at the pubertal stages associated with peak growth in both boys and girls. Our data suggest that rising levels of GH in puberty (independent of systemic IGF-1) are likely responsible for the increase in bone turnover associated with puberty.
Previous studies have shown that bone turnover markers are higher at times of rapid growth, such as in infancy and puberty.16,20,21 Our study evaluated the associations of GH and IGF-1 with levels of bone turnover markers at different stages of puberty based on the timing of peak growth. GH, P1NP and CTX were highest at the pubertal stages that correspond with peak pubertal growth for both genders. Our data are consistent with those of other studies showing that boys have the highest GH levels at Tanner stage 4 of puberty 17,19 and also have the highest P1NP and CTX levels at Tanner stage 4.14,22 In girls, we found that GH, PINP and CTX were highest in Tanner stages 2–3 of puberty, similar to findings in other studies.14,23,24 However, these earlier studies examined either markers of bone turnover or GH status, and none reported levels of bone turnover markers in relation to GH.
We found a significant positive correlation of markers of both bone formation and resorption with GH parameters in boys and girls. In addition, GH remained a positive predictor of bone turnover markers even after controlling for age, BMI-SDS and IGF-1 in a regression model. In contrast to findings reported by Blumsohn et al.,24 we did not find a significant correlation of IGF-1 levels with bone turnover markers. IGF-1 was a significant predictor of CTX only in boys in the regression model that did not include GH parameters (data not shown). Although GH and IGF-1 levels were not significantly associated in this study, regression models were also run both with and without IGF-1 to determine whether bone trophic effects of IGF-1 may affect the significance of GH associations with bone turnover markers. GH remained a positive and independent predictor of P1NP and CTX in both models. The limited variability in vitamin D levels within our subjects may explain the lack of association of vitamin D levels or season of blood draw with markers of bone turnover.
Based on our data, we conclude that the rise in GH during peak pubertal growth may be a stronger determinant for increasing bone turnover than systemic IGF-1. We did not find significant associations of IGF-1 with bone turnover markers, and this may indicate that direct GH effects, or autocrine–paracrine effects of tissue IGF-1 (which could not be assessed in this study), are more important for bone modelling and statural growth than is systemic IGF-1 (which was assessed in this study). Animal studies have shown that tissue IGF-1 is more important for statural growth than is systemic IGF-1, whereas systemic IGF-1 is an important determinant of structure and density of bone.25,26 Markers of bone turnover peaked in our study at the stage of puberty associated with peak growth and peak GH secretion, suggesting that our findings primarily reflect the impact of GH on statural growth, mediated by direct GH effects or autocrine–paracrine effects of tissue IGF-1, and not systemic IGF-1.
Previous studies have shown that following peak pubertal growth, bone turnover markers decrease but bone mass accrual continues and that peak bone mass accumulation occurs approximately 0·7 years after peak height velocity.14,27 This suggests that IGF-1 along with sex steroids, both of which remain elevated when compared with GH levels in late puberty, may significantly contribute to continued bone mass accrual in the latter part of puberty. We found that IGF-1 levels were, in fact, highest in late puberty, and there was a positive association between P1NP and IGF-1 in late puberty within girls. Although sex steroid levels were not evaluated in this study, other studies have reported that adult levels in late puberty are positively correlated with bone mass accumulation.14 In addition, sex steroids, particularly oestrogen, have important effects on skeletal growth and maturation, with stimulating effects on growth velocity in early puberty, and inhibitory effects at the higher sex steroid levels associated with later puberty.14,24 The decrease in levels of bone turnover markers observed in our study in late puberty likely reflects the antiresorptive effect of higher levels of sex steroids.
We used the GH nadir following an oral glucose load as an indicator of GH status in this study, because GH nadir is strongly associated with GH secretory parameters as assessed by frequent sampling overnight.11 It will be important in future studies to determine whether findings are similar when other methods of GH evaluation are used. Of note, GH nadir did not correlate with IGF-1 levels, and this may be because (i) GH and IGF-1 levels peak at different pubertal stages and (ii) nadir GH levels are not an integrated measure of GH secretion, which may correlate better with systemic IGF-1 levels.
There are limitations of our study. First, this is a cross-sectional evaluation of subjects at different pubertal stages, and hence, we can only infer correlation, but not causation. Second, bone turnover markers have a diurnal variation, with peak concentrations occurring in the morning followed by a nadir in the late afternoon.28 All of our subjects were evaluated during the morning in the fasting state to account for this diurnal variation. In addition, P1NP and CTX reflect bone changes at the growth plate and at the periosteum throughout the skeleton. Thus, levels of these markers likely reflect the aggregate of these changes. Third, GH is known to increase muscle mass. The force of muscle pull on bone stimulates bone metabolism, which could lead to increases in bone turnover markers independent of direct GH effects on bone. This was not possible to evaluate in our study. Finally, we did not evaluate bone mineral content in our subjects. However, associations of bone mass and bone turnover markers have been evaluated in previous studies,14 and in this study, we were primarily interested in determining the hormonal control of bone turnover in puberty. However, it would have been interesting to assess whether IGF-1 was a stronger predictor of bone mineral content than of markers of bone turnover, consistent with animal models that indicate that systemic IGF-1 drives bone mass and structure while tissue IGF-1 is an important determinant of statural growth.25,26
Our study provides evidence that GH status is strongly associated with bone metabolism during peak pubertal growth in boys and girls, independent of IGF-1. Additional studies are needed to further investigate the role of GH and IGF-1 on statural growth versus changes in bone mass and structure in puberty.
Acknowledgments
We thank the research nurses and bionutritionists at the Clinical Research Center at Massachusetts General Hospital and our subjects, without whom this study would not have been possible. The project described was supported by Grant Numbers 1 UL1 RR025758-03 and M01-RR-01066, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Jurimae J, Cicchella A, Jurimae T, et al. Regular physical activity influences plasma ghrelin concentration in adolescent girls. Medicine and Science in Sports and Exercise. 2007;39:1736–1741. doi: 10.1249/mss.0b013e31812e5294. [DOI] [PubMed] [Google Scholar]
- 2.Jurimae J, Latt E, Haljaste K, et al. Influence of puberty on ghrelin and BMD in athletes. International Journal of Sports Medicine. 2009;30:403–407. doi: 10.1055/s-0028-1105937. [DOI] [PubMed] [Google Scholar]
- 3.Leger J, Mercat I, Alberti C, et al. The relationship between the GH/IGF-I axis and serum markers of bone turnover metabolism in healthy children. European Journal of Endocrinology. 2007;157:685–692. doi: 10.1530/EJE-07-0402. [DOI] [PubMed] [Google Scholar]
- 4.Pomerants T, Tillmann V, Jurimae J, et al. The influence of serum ghrelin, IGF axis and testosterone on bone mineral density in boys at different stages of sexual maturity. Journal of Bone and Mineral Metabolism. 2007;25:193–197. doi: 10.1007/s00774-006-0744-6. [DOI] [PubMed] [Google Scholar]
- 5.Pomerants T, Tillmann V, Karelson K, et al. Impact of acute exercise on bone turnover and growth hormone/insulin-like growth factor axis in boys. Journal of Sports Medicine and Physical Fitness. 2008;48:266–271. [PubMed] [Google Scholar]
- 6.Tuchman S, Thayu M, Shults J, et al. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. Journal of Pediatrics. 2008;153:484–490. doi: 10.1016/j.jpeds.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seibel MJ. The use of molecular markers of bone turnover in the management of patients with metastatic bone disease. Clinical endocrinology (Oxford) 2008;68:839–849. doi: 10.1111/j.1365-2265.2007.03112.x. [DOI] [PubMed] [Google Scholar]
- 8.Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporosis International. 2009;20:843–851. doi: 10.1007/s00198-009-0838-9. [DOI] [PubMed] [Google Scholar]
- 9.Vestergaard P. Bone metabolism in type 2 diabetes and role of thiazolidinediones. Current Opinion in Endocrinology, Diabetes, and Obesity. 2009;16:125–131. doi: 10.1097/MED.0b013e328325d155. [DOI] [PubMed] [Google Scholar]
- 10.Misra M, Cord J, Prabhakaran R, et al. Growth hormone suppression after an oral glucose load in children. Journal of Clinical Endocrinology and Metabolism. 2007;92:4623–4629. doi: 10.1210/jc.2007-1244. [DOI] [PubMed] [Google Scholar]
- 11.Misra M, Miller KK, Herzog DB, et al. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. Journal of Clinical Endocrinology and Metabolism. 2004;89:1605–1612. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- 12.Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. Journal of Clinical Endocrinology and Metabolism. 2007;92:443–449. doi: 10.1210/jc.2006-1706. [DOI] [PubMed] [Google Scholar]
- 13.Rotteveel J, Schoute E, Delemarre-van de Waal HA. Serum procollagen I carboxyterminal propeptide (PICP) levels through puberty: relation to height velocity and serum hormone levels. Acta Paediatrica. 1997;86:143–147. doi: 10.1111/j.1651-2227.1997.tb08855.x. [DOI] [PubMed] [Google Scholar]
- 14.van Coeverden SC, Netelenbos JC, de Ridder CM, et al. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clinical endocrinology (Oxford) 2002;57:107–116. doi: 10.1046/j.1365-2265.2002.01573.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Sluis IM, Hop WC, van Leeuwen JP, et al. A cross-sectional study on biochemical parameters of bone turnover and vitamin d metabolites in healthy dutch children and young adults. Hormone Research. 2002;57:170–179. doi: 10.1159/000058378. [DOI] [PubMed] [Google Scholar]
- 16.Szulc P, Kaufman JM, Delmas PD. Biochemical assessment of bone turnover and bone fragility in men. Osteoporosis International. 2007;18:1451–1461. doi: 10.1007/s00198-007-0407-z. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg H, Williams RH. Williams Textbook of Endocrinology. Saunders/Elsevier; Philadelphia, PA: 2008. [Google Scholar]
- 18.Rose SR, Municchi G, Barnes KM, et al. Spontaneous growth hormone secretion increases during puberty in normal girls and boys. Journal of Clinical Endocrinology and Metabolism. 1991;73:428–435. doi: 10.1210/jcem-73-2-428. [DOI] [PubMed] [Google Scholar]
- 19.Albertsson-Wikland K, Rosberg S, Karlberg J, et al. Analysis of 24-hour growth hormone profiles in healthy boys and girls of normal stature: relation to puberty. Journal of Clinical Endocrinology and Metabolism. 1994;78:1195–1201. doi: 10.1210/jcem.78.5.8175978. [DOI] [PubMed] [Google Scholar]
- 20.Eastell R, Hannon RA. Biomarkers of bone health and osteoporosis risk. Proceedings of the Nutrition Society. 2008;67:157–162. doi: 10.1017/S002966510800699X. [DOI] [PubMed] [Google Scholar]
- 21.Mora S, Prinster C, Proverbio MC, et al. Urinary markers of bone turnover in healthy children and adolescents: age-related changes and effect of puberty. Calcified Tissue International. 1998;63:369–374. doi: 10.1007/s002239900542. [DOI] [PubMed] [Google Scholar]
- 22.Sorva R, Anttila R, Siimes MA, et al. Serum markers of collagen metabolism and serum osteocalcin in relation to pubertal development in 57 boys at 14 years of age. Pediatric Research. 1997;42:528–532. doi: 10.1203/00006450-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Libanati C, Baylink DJ, Lois-Wenzel E, et al. Studies on the potential mediators of skeletal changes occurring during puberty in girls. Journal of Clinical Endocrinology and Metabolism. 1999;84:2807–2814. doi: 10.1210/jcem.84.8.5905. [DOI] [PubMed] [Google Scholar]
- 24.Blumsohn A, Hannon RA, Wrate R, et al. Biochemical markers of bone turnover in girls during puberty. Clinical endocrinology (Oxford) 1994;40:663–670. doi: 10.1111/j.1365-2265.1994.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 25.Courtland HW, Sun H, Beth-On M, et al. Growth hormone mediates pubertal skeletal development independent of hepatic IGF-1 production. Journal of Bone and Mineral Research. 2011;26:761–768. doi: 10.1002/jbmr.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yakar S, Canalis E, Sun H, et al. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. Journal of Bone and Mineral Research. 2009;24:1481–1492. doi: 10.1359/JBMR.090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. International Journal of Sports Medicine. 1997;18(Suppl 3):S191–S194. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 28.Saggese G, Baroncelli GI, Bertelloni S, et al. Twenty-four-hour osteocalcin, carboxyterminal propeptide of type I pro-collagen, and aminoterminal propeptide of type III procollagen rhythms in normal and growth-retarded children. Pediatric Research. 1994;35:409–415. [PubMed] [Google Scholar]