Abstract
Background
We determined role of donor specific antibodies (DSA) and antibodies (Abs) to self-antigens, collagen-V (Col-V) and K-α1-Tubulin (KAT) in pathogenesis of acute antibody mediated rejection (AMR) and cardiac allograft vasculopathy (CAV) following human heart transplantation (HTx).
Methods
137 HTx recipients - 60 early period (≤ 12months) and 77 late period (> 12months) patients were enrolled. Circulating DSA was determined using LUMINEX. Abs against Col-I, II, IV, V and KAT were measured using ELISA. Frequency of CD4+T helper cells (CD4+Th) secreting IFN-γ, IL-5, IL-10 or IL-17 specific to self-antigens were determined using ELISPOT.
Results
A significant association between AMR and DSA was demonstrated. Development of DSA in AMR patients correlated well with the development of auto-Abs to Col-V(AMR(+): 383±72μg/mL, AMR(−): 172±49μg/mL, p=0.033) and KAT (AMR(+): 252±49μg/mL, AMR(−): 61±21μg/mL, p=0.014). Patients who developed AMR demonstrated increased frequencies of CD4+Th secreting IFN-γ and IL-5 with reduction in IL-10 specific for Col-V/KAT. Patients diagnosed with CAV also developed DSA and auto-Abs to Col-V (CAV(+): 835±142μg/mL, CAV(−): 242±68μg/mL, p=0.025) and KAT (CAV(+): 768±206μg/mL, CAV(−): 196±72μg/mL, p=0.001) with increased frequencies of CD4+Th secreting IL-17 with reduction in IL-10 specific for Col-V/KAT.
Conclusions
Development of Abs to HLA and self-antigens are associated with increases in CD4+Th secreting IFN-γ and IL-5 in AMR and IL-17 in CAV, with reduction in CD4+Th secreting IL-10 in both AMR and CAV.
Keywords: Self-antigens, cardiac transplantation, antibody mediated rejection, cardiac allograft vasculopathy
Introduction
Up to 40% of heart transplant (HTx) recipients demonstrate allograft dysfunction due to acute antibody mediated rejection (AMR) during early post-heart HTx period (1-5). Histopathological assessment of AMR is characterized by capillary injury, positive immunofluorescence for C4d, CD68 in endomyocardial biopsies and detection of donor specific antibodies (DSA) to mismatched HLA class I/II antigens (6, 7). Pretransplant sensitization to mismatched HLA has also been identified as an independent risk factor for development of AMR. Several studies have demonstrated a significant association between development of DSA and both acute as well as chronic cardiac allograft rejection (5, 7-9). Patients with AMR who develop antibodies (Abs) to donor HLA often progress to transplant associated cardiac allograft vasculopathy (CAV) early when compared to patients without anti-HLA (10, 11). A growing body of evidence suggests that increase in pro-inflammatory mediators including IFN-γ, IL-1, IL-12 and IL-17 during early posttransplant period is associated with development of DSA that subsequently leads to chronic allograft rejection (10, 12-14). Additionally, immune responses to non-HLA antigens have also been implicated in immunopathogensis of acute and chronic allograft rejection (15-19).
Both immune and non-immune factors contribute to chronic endothelial inflammation and fibroproliferation resulting in CAV (14, 15, 20). Recently, alloimmune responses to mismatched donor HLA have also been implicated in induction of immune responses to self antigens (15, 19, 21). A significant number of HTx recipients with histological evidence of rejection develop anti-skeletal muscle glycolipid, anti-muscle protein and anti-intracellular adhesion molecule-1 (17, 18, 22). Studies from our laboratory have shown immune responses to self antigens, collagen-V (Col-V), an extracellular matrix protein and K-α1-Tubulin (KAT), a gap junction intermediate filament cytoskeletal protein in lung transplant recipients undergoing chronic rejection (23, 24). We tested the possibility that these proteins may be antigenic targets in other transplanted organs besides the lung allograft. In cardiac tissue, endothelial cells have a large number of gap junctions (25) and given the increased levels of cyto skelatal KAT expression in gap junctions(26) and the demonstrated mutations of α-1-Tubulin in the pathogenesis of postcardiac transplant fatal cardiomyopathy, we studied KAT as an antigen target in HTx recipients. Collagen-V, on the other hand, is a protein that is selectively expressed in the human body and comprises up to 2% of the entire extracellular matrix protein in heart (27). Given that Col-V is found in interstitial connective tissue and has been shown to play an integral role in the structure and function of cardiac tissue, we examined Col-V as an antigenic target in HTx recipients (28). The objective of this study was to evaluate the role of DSA to mismatched HLA and serum levels of Abs against two novel cardiac self antigens, Col-V and KAT in post-HTx patients who were diagnosed with AMR and CAV. To define the mechanism for development of Abs, CD4+ T lymphocyte responses specific to individual self antigens and their cytokine secretion pattern were also determined.
Results
Patient Demographics
The characteristics of 137 HTx recipients in study cohort are detailed in Table 1, Supplemental Digital Content 1. Of 137 patients, 60 patients were monitored for development of acute AMR in early post-HTx period (early period (EP) ≤12 months) while 77 patients were followed for development of CAV in late post-HTx period (late period (LP) >12 months). Mean (± standard deviation) follow-up after HTx was 3.6±2.6 years while median follow-up was 3.4 years. Nine patients had diagnostic criteria consistent with acute AMR (AMR(+)) and 14 patients had evidence of moderate or severe CAV (CAV(+)). While patients with active infection at time of study enrollment met exclusion criteria, 5 patients developed systemic infections (3 with bacterial and 2 with viral) following study enrollment. Among the 9 AMR+ patients, 6 were C4d+. Four AMR+ patients had hemodynamic compromise. There was a mean 5.6 ± 8.8 units of blood transfusion to patients prior to heart transplant. Seven of nine patients were treated for AMR. All patients diagnosed with DSA were treated with IVIG and rituximab.
Development of Abs to self-antigens, Col-V and KAT are significantly increased in AMR(+)recipients
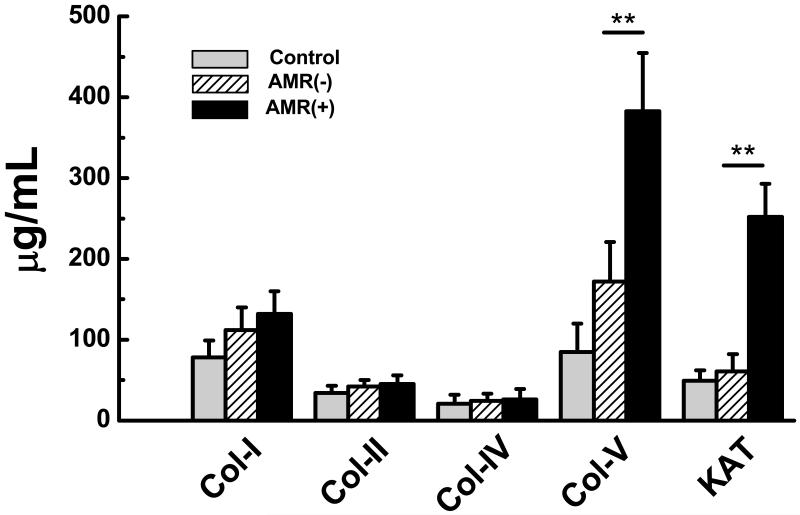
We analyzed EP HTx recipient sera for development of Abs to Col-V and KAT using ELISA. Patients with AMR develop increased Abs to Col-V (Figure 1: Control:85±35μg/mL, AMR(−):172±42μg/mL, AMR(+):383±72μg/mL) compared to stable HTx recipients without AMR (p=0.033). Antibodies to Col-V were detected 2.5±2.3 months before acute AMR was diagnosed. Donor specific Abs in AMR(+) patients was identified 3.3 ± 2.1 months prior to detection of anti-Col-V (Figure 5). Similarly, patients with AMR develop increased Abs to KAT (Figure 1: Control:49±23μg/mL, AMR(−):61±23μg/mL, AMR(+):252 ±57μg/mL) compared to stable HTx recipients without AMR (p=0.014). Antibodies to KAT were detected 1.2±2.3 months before acute AMR was diagnosed. Donor specific Abs in AMR(+) patients was identified 4.6±2.1 months prior to detection of anti-KAT (Figure 2). Antibodies to Col-I, II, and IV were not significantly elevated in AMR(+) patients compared to AMR(−) recipients (Figure 1).
Figure 1.
AMR(+) (n=9) recipients develop increased Abs to Col-V and KAT compared to controls and AMR (−) (n=51) recipients. There is no significant difference in Abs to Col-I, II, IV between the controls, AMR(−), and AMR(+) recipients (data presented as mean± S.D).
Figure 5.
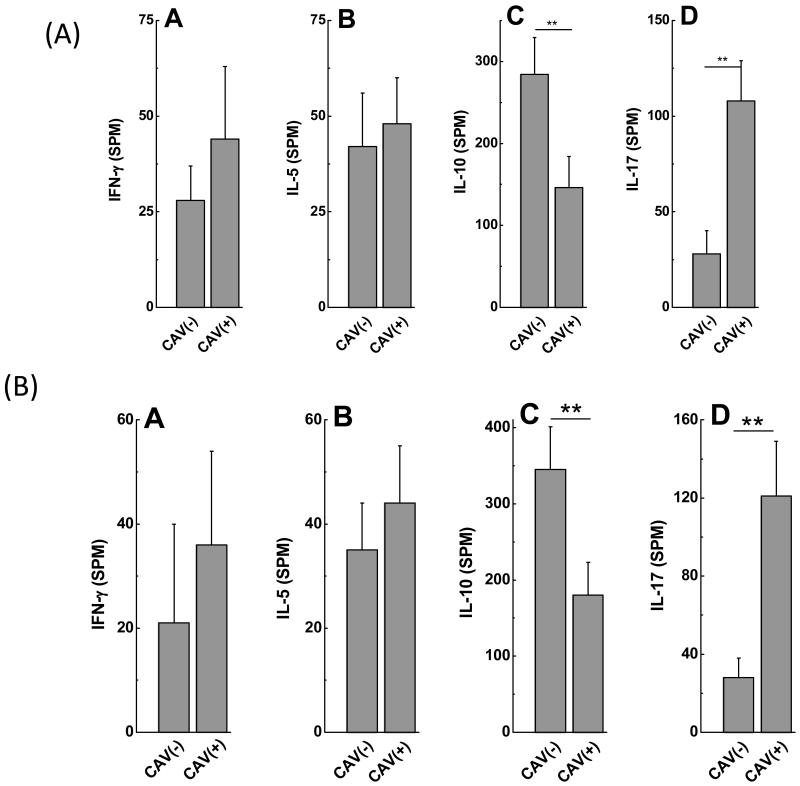
A: CAV(+)(n=14) recipients demonstrate increased frequency of IL-17 and decreased frequency of IL-10 secreting CD4+ T cells specific to Col-V compared to CAV(−) (n=63) recipients. There is no significant difference in the frequencies of IFN-γ and IL-5 secreting CD4+ T cells specific to Col-V between CAV(+) and CAV(−) recipients (data presented as mean± S.D).
B: CAV(+)(n=14) recipients demonstrate increased frequency of IL-17 and decreased frequency of IL-10 secreting CD4+ T cells specific to KAT compared to CAV(−)(n=63) recipients. There is no significant difference in the frequencies of IFN-γ and IL-5 secreting CD4+ T cells specific to KAT between CAV(+) and CAV(−) recipients (data presented as mean± S.D).
Figure 2.
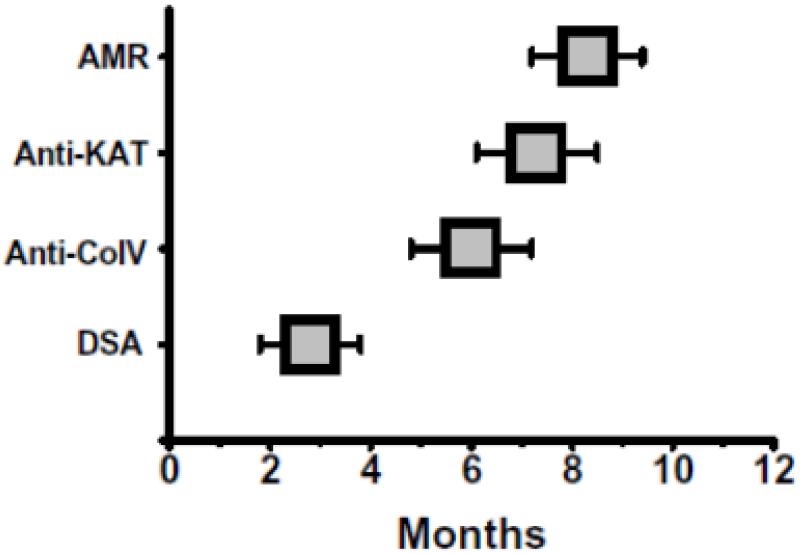
Serial monitoring of DSA (every month), Abs to Col-V and KAT in 7 EP DSA(+)AMR(+) recipients indicated that DSA was detectable at 2.7±0.9 months, followed by Col-V at 6.0±1.2 months, KAT at 7.3±1.2months in patients diagnosed with AMR at 8.5±1.1months.
AMR(+) recipients demonstrated increased frequencies of IL-5, IFN-γ and decreased frequencies of IL-10 secreting CD4+ T cells specific to Col-V and KAT
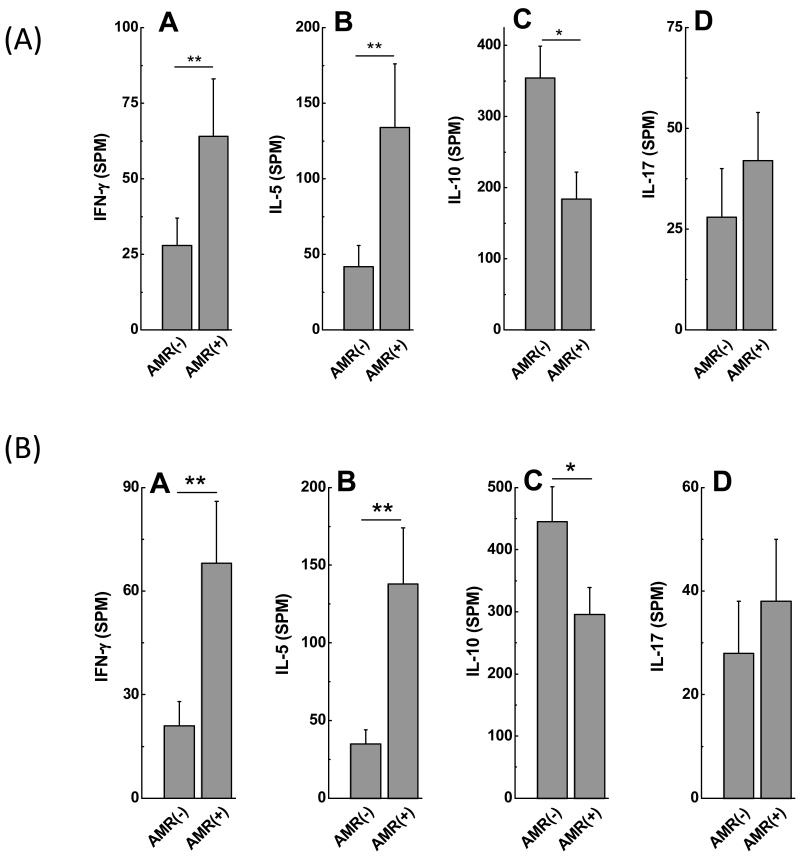
To identify immune responses that contribute to development of Abs to self antigens, frequency of CD4+ T cells secreting IFN-γ, IL-5, IL-17 and IL-10 specific to either Col-V or KAT were determined using ELISPOT. Patients with AMR demonstrated increased frequencies of IL-5 and IFN-γ against Col-V (Figure 3A: IFN-γ: AMR(−):28±9spm, AMR(+):64±19spm, p=0.008; IL-5: AMR(−): 42±14spm, AMR(+):134±42spm, p=0.003) and KAT (Figure 3B: IFN-γ: AMR(−):21±7spm, AMR(+): 68±18spm, p=0.03; IL-5: AMR(−):35±10spm, AMR(+):138±36spm, p=0.004). AMR(+) recipients demonstrated decreased frequencies of IL-10 secreting CD4+ T cells against Col-V (Figure 3A: AMR(−): 354 ±45spm, AMR(+):184±38spm, p=0.009) and KAT (Figure 3B: AMR(−):445±56spm, AMR(+): 296±43spm, p=0.03). There was no significant difference in frequencies of CD4+ T cells secreting IL-17 specific to Col-V (Figure 3A: AMR(−):28±12spm, AMR(+):42±12 spm, p=0.35) and KAT (Figure 3B: AMR(−):28±10spm, AMR(+):38±12spm, p=0.29) between AMR(−) and AMR(+) recipients. Patients with AMR demonstrate high frequencies of CD4+ T helper cells specific to self antigens which predominantly secrete IL-5 and IFN-γ. Induction of IL-5 and strong IFN-γ response by self antigen reactive CD4+T cells in AMR(+) recipients provides a mechanism which may contribute to activation and differentiation of B cells involved in autoantibody production.
Figure 3.
A: AMR(+) (n=9) recipients demonstrate increased frequencies of IL-5 and IFN-γ and decreased frequency of IL-10 secreting CD4+ T cells specific to Col-V compared to AMR(−) (n=51) recipients. There is no significant difference in the frequency of IL-17 secreting CD4+ T cells specific to Col-V between AMR(+) and AMR(−) recipients (data presented as mean± S.D).
B: AMR(+) (n=9) recipients demonstrate increased frequencies of IL-5 and IFN-γ and decreased frequency of IL-10 secreting CD4+ T cells specific to KAT compared to AMR(−) (n=51) recipients. There is no significant difference in the frequency of IL-17 secreting CD4+ T cells specific to KAT between AMR(+) and AMR(−) recipients (data presented as mean± S.D).
Development of Abs to Col-V and KAT are significantly associated with development of DSA in AMR(+) recipients
Patients who developed AMR and had DSA (DSA(+)AMR(+)) demonstrated increased Abs to Col-V (Table 2, Supplemental Digital Content 2: DSA(−)AMR(+):176±65μg/mL, DSA(+)AMR(+):344±94μg/mL, p=0.03) and KAT (Table 2, Supplemental Digital Content 2: DSA(−) AMR(+):91±42μg/mL, DSA(+)AMR(+):296 ±71μg/mL, p=0.003). There was no significant difference in Abs to Col-V (Table 2, Supplemental Digital Content 2: DSA(−)AMR(−):56±21μg/mL, DSA(+)AMR(−):61±28μg/mL, p=0.92) and KAT (Table 2, Supplemental Digital Content 2: DSA(−)AMR(−):59±18μg/mL, DSA(+)AMR(−):67±24μg/mL, p=0.89) in AMR(−) patients regardless of whether they were DSA(+) or DSA(−). These results demonstrate that development of DSA to mismatched HLA and alloimmune responses may play an important role in development of Abs to self antigens in AMR(+) recipients.
Development of Abs to self-antigens, Col-V and KAT are significantly increased in CAV(+) recipients
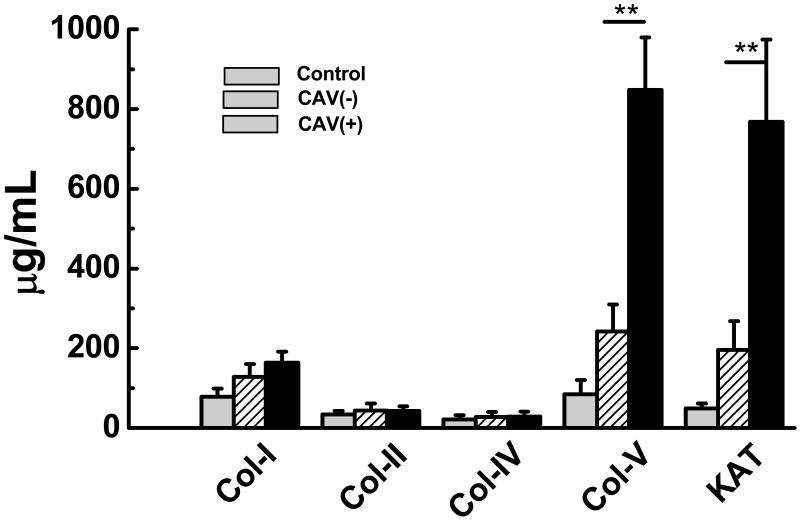
We tested LP HTx recipient sera for development of Abs to Col-V and KAT using ELISA. Patients with CAV develop increased Abs to Col-V (Figure 4: Control:85±35μg/mL, CAV(−):242±68μg/mL, CAV(+):848±132 μg/mL) compared to stable HTx recipients without CAV (p=0.025). Similarly, patients with CAV develop increased Abs to KAT (Figure 3: Control:49±13μg/mL, CAV(−):196±62μg/mL, CAV(+):768±206μg/mL) compared to stable HTx recipients without CAV (p=0.001). Antibodies to Col-I, II, and IV were not significantly elevated in CAV(+) patients compared to CAV(−) recipients (Figure 3). These results demonstrate that patients with moderate/severe CAV develop significant increased Abs to self antigens, Col-V and KAT compared to patients with none/minimal CAV.
Figure 4.
CAV(+) (n=14) recipients develop increased Abs to Col-V and KAT compared to controls and CAV(−)(n=63) recipients. There is no significant difference in Abs to Col-I, II, IV between the controls, CAV(−), and CAV(+) recipients (data presented as mean± S.D).
CAV(+) recipients demonstrated increased frequencies of IL-17 and decreased frequencies of IL-10 secreting CD4+ T cells specific to Col-V and KAT
To identify immune responses that contribute to development of Abs to self antigens, frequency of CD4+ T cells secreting IFN-γ, IL-5, IL-17 and IL-10 specific to either Col-V or KAT were determined using ELISPOT. Patients with CAV demonstrated increased frequencies of IL-17 secreting CD4+ T cells against Col-V (Figure 5A: CAV(−):28±12spm, CAV(+):108±21spm, p=0.002) and KAT (Figure 5B: CAV(−):28±10spm, CAV(+):121±31spm, p=0.003). CAV(+) recipients demonstrated decreased frequencies of IL-10 secreting CD4+ T cells against Col-V (Figure 5A: CAV(−):284±45spm, CAV(+):146±38spm, p=0.008) and KAT (Figure 5B: CAV(−):345±56spm, CAV(+):180±43spm, p=0.005). There was no significant difference in frequencies of CD4+ T cells secreting IFN-γ and IL-5 specific to Col-V (Figure 5A: IFN-γ: CAV(−): 28±9spm, CAV(+):44±19spm, p=0.55; IL-5: CAV(−):42±14spm, CAV(+):48±12spm, p=0.54) and KAT (Figure 5B: IFN-γ: CAV(−):21±19spm, CAV(+):36±18spm, p=0.64; IL-5: CAV(−):35±10spm, CAV(+):44±11spm, p=0.24) between CAV(−) and CAV(+) recipients. Patients with CAV demonstrate high frequencies of CD4+ T helper cells specific to self antigens which predominantly secrete the Th17 cytokine, IL-17. Induction of IL-17 by self reactive CD4+T cells in CAV(+) recipients indicate that IL-17 can facilitate germinal center formation and activation of B lymphocytes which can contribute to autoantibody development.
Development of Abs to Col-V and KAT are significantly associated with development of DSA in CAV(+) recipients
Patients who developed CAV and had DSA (DSA(+)CAV(+)) demonstrated increased Abs to Col-V (Table 3, Supplemental Digital Content 3: DSA(−)CAV(+):313±134μg/mL, DSA(+)CAV(+):812±142μg/mL, p=0.001) and KAT (Table 3, Supplemental Digital Content 3: DSA(−)CAV(+):220±78μg/mL, DSA(+)CAV(+):796±210μg/mL, p=0.02). There was no significant difference in Abs to Col-V (Table 3, Supplemental Digital Content 3: DSA(−)CAV(−):211±87μg/mL, DSA(+)CAV(−):264±76μg/mL, p=0.63) and KAT (Table 3, Supplemental Digital Content 3: DSA(−)CAV(−):134±104μg/mL, DSA(+)CAV(−):196±67μg/mL, p=0.78) in CAV(−) patients regardless of whether they were DSA(+) or DSA (−). As in AMR, DSA to mismatched HLA are significantly associated with development of Abs to self antigens in CAV(+) recipients supporting the conclusion that alloimmune responses can facilitate induction of immune responses to self antigens.
Discussion
The development of AMR and CAV following HTx is likely due to both cellular and humoral immune responses to mismatched donor HLA and/or cardiac specific self-antigens myosin and vimentin (29-31). Circulating DSA to mismatched HLA and non-HLA antigens including ABO, MICA and cardiac self antigens such as myosin and vimentin have been considered as major risk factors for development of AMR and CAV following HTx (29-31). Following transplantation, inflammation, alloimmune responses and subsequent tissue remodeling expose cryptic self antigens which lead to activation of self-reactive immune responses (32-34). Regulatory T cells (Tregs) are known to inhibit both autoreactive as well as alloreactive T cells (35). However, in the context of potent posttransplant immunosuppression including use of calcineurin inhibitors which are not conducive to Treg proliferation, there is loss of peripheral tolerance to self antigens resulting in immune responses to self antigens (36, 37). We assessed the temporal relationship between development of DSA and Abs to cardiac self antigens, Col-V and KAT and the concomitant T cell responses to these self antigens, in pathogenesis of AMR and CAV in post-HTx recipients. A significant association for development of Abs to mismatched HLA as well as to Col-V and KAT were identified in patients with AMR and CAV. Furthermore, induction of IL-5 and IFN- γ secreting self antigen reactive CD4+T cells with concomitant reduction in IL-10 leads to AMR whereas a cytokine switch to IL-17 pathway with a similar reduction in IL-10 leads to CAV.
In this study, AMR(+) patients developed Abs to both Col-V and KAT (Figure 1) and increased frequencies of IFN-γ and IL-5 secreting CD4+ T cells specific to these self antigens (Figures 2A, 2B). Concurrently, AMR+ patients demonstrated decreased frequency of IL-10 secreting CD4+ T cells, indicating a loss of peripheral tolerance to these self antigens (Figures 2A, 2B). These results provide evidence for the first time an important role for IL-5 in proliferation of B cells together with strong IFN-γ responses to self antigens which facilitates autoantibody production. A concomitant decline in IL-10 may also assist in induction of autoimmune response in AMR patients. AMR patients with DSA (AMR(+)DSA(+)) demonstrated significantly increased Abs to both Col-V and KAT compared to AMR patients without DSA (AMR(+)DSA(−), Table 2, Supplemental Digital Content 2). These results underline an important role for cross talk between alloimmune responses and autoimmune responses following HTx leading to AMR. The ligation of class I molecules on allograft epithelium or endothelium by anti-HLA class I initiates an inflammatory state characterized by neutrophil and macrophage infiltration as well as concomitant release of inflammatory cytokines, chemokines and fibrogenic growth factors (38, 39). The ensuring pro-inflammatory microenvironment can lead to development of immune responses against exposed domains of sequestered self antigens. Serial monitoring of DSA, anti-Col-V and anti-KAT in patients with AMR revealed that DSA precede development of anti-Col-V by 3.3±2.1 months, anti-KAT by 4.6±2.1 months and clinical diagnosis of AMR by 5.8±2.0 months. Taken together, these results strongly suggest an important role for alloimmune response as evidenced by development of DSA to mismatched HLA in development of autoimmunity to cardiac self antigens.
Antibodies to cardiac self antigens such as vimentin have been demonstrated to cause lesions consistent with coronary artery vasculopathy in a murine model and thus, provide preclinical evidence for role of autoimmune response in accelerating CAV (40). In this study, CAV(+) patients demonstrated development of Abs to Col-V and KAT (Figure 3). Furthermore, CAV+ patients demonstrated increased frequency of IL-17 secreting CD4+ T cells specific to Col-V and KAT (Figures 4A, 4B). Similar to AMR+ patients, CAV+ recipients demonstrated a decreased frequency of IL-10 secreting CD4+ T cells specific to self antigens. It has been shown that IFN-γ plays a significant role in alloimmune response (41, 42) while IL-17 plays a critical role in autoimmune response (43). Enhanced IFN-γ in AMR setting indicates a predominant role of IFN- γ and alloimmune responses in the antibody mediated rejection in the early period. However in the late period, development of CAV we propose an important role for IL-17 mediated autoimmune process and development of immune responses to self antigens. Studies have provided evidence for cross-talk between alloimmunity and autoimmunity in sold organ transplantation (44). However the relative low sample size of AMR (+) (n=9) and CAV (+) (n=14) might warrant further randomized multi-center studies to conclusively support this finding. Further although we demonstrate enhanced IFN-y levels in AMR and IL-17 levels in CAV to self-antigens, the mechanisms for the immunopathogenesis of AMR and CAV needs further studies.
Recent reports have suggested that the mechanisms contributing to the immunogenicity of Col-V leading to chronic rejection pathology following lung transplantation is predominantly mediated by the IL-17 dependent CD4+ T cell pathway (19). It has been reported that self antigen reactive T cells secreting IL-17 can induce extracellular matrix remodeling by modulating the MMP/TIMP and RANKL/osteoprotegerin complex (45). On the other hand, KAT as a gap junction protein is involved in signal transduction affecting cardiac contractility and autoimmune responses to this protein has the potential to significantly alter function of the transplanted heart. Previous studies from our laboratory have demonstrated that incubation of airway epithelial cells from BOS(+) patients who developed KAT Abs resulted in the increased expression of transcription factors (TCF5 and c-Myc), leading to increased expression of fibrogenic growth factors and fibroproliferation (24). Put together, these data suggest a signaling mechanism for anti-KAT playing a crucial role in pathogenesis of chronic rejection.
Therefore, we propose that early detection and abrogation of alloimmune response by serially monitoring of development of Abs to mismatched donor HLA antigens can identify patients who are at high risk for development of immune responses to self antigens. It is likely that prevention of development of immune responses to self antigens by early intervention of alloimmune responses will not only prevent AMR but also can prevent and or delay onset of CAV following human cardiac transplantation.
Material and Methods
Study Population
We enrolled 137 patients who underwent HTx at Barnes-Jewish Hospital/Washington University in an Institutional Review Board approved research study. Serum and peripheral blood mononuclear cells (PBMCs) were isolated from whole blood and stored at −135°C (46, 47). Endomyocardial biopsies were performed in all patients in early period (EP ≤/= 12 months) while coronary angiograms were performed in all patients in late period (LP >12 months) post-HTx. At time of endomyocardial biopsies or coronary angiograms, blood samples were collected from each patient. In accordance with International Society of Heart and Lung Transplantation (ISHLT) recommendations, a diagnosis of AMR was reached by assessment of clinical, histological and serological findings by the attending transplant cardiologist as described in our previous study (7, 48). Based on ISHLT guidelines, cardiac allograft vasculopathy was assessed based upon extent of allograft dysfunction and coronary artery stenosis detected by angiography (49). No detectable disease, left main disease < 50% or primary coronary vessel/branch stenosis < 70% without allograft dysfunction was termed none/mild (CAV(−)). Left main disease > 50%, single or multiple primary coronary vessel/branch stenosis > 70% with or without allograft dysfunction was termed moderate/severe (CAV(+)).
Detection of Abs to HLA
Presence of DSA in sera from HTx recipients was determined using Luminex technology (Biosource International Inc, CA) (46, 50). Before transplantation, all patients were screened for pre-formed anti-HLA antibodies using the LABScreen Single Antigen assay (One Lambda Inc, Canoga Park, CA). Donor hearts were accepted only if a virtual crossmatch with all previously identified antibodies was compatible. At transplantation, retrospectively, all recipients had a direct cytotoxicity crossmatch using serum obtained the day of surgery, and the results were available post-operatively. Thus, none of the patients who underwent transplant had DSA and all of the transplants were cytotoxity cross match negative with the donor. Mean fluorescence intensity (MFI) and ratio of MFI to positive control bead (MFI ratio) were recorded for all anti-HLA specificities. Anti-HLA were defined as donor specific (DSA) if donor for HTx shared same HLA allele. In addition, we performed follow-up testing for DSA every month during early period (</= 1 year) in AMR patients for the who developed DSA. Because of fluctuations in mean fluorescence intensity (MFI) between positive controls, our center’s HLA laboratory calculates a ratio of sample MFI to positive control MFI and defines a ratio of 0.2 or higher as positive. Most of the patients who developed DSA were positive within 4 months of transplant.
Detection of Abs to self antigens, Col-I, II, IV, V and KAT
Patient sera were tested for development of Abs to Col-I, II, IV, V and KAT by enzyme linked immunosorbent assay (ELISA) (15, 24). Sera were tested at dilutions of 1:500 for Abs against Col-I, II, IV, V and 1:1250 for Abs against KAT (24). To determine positive titers of Abs to self antigens, two standard deviations from mean concentration of Abs to Col-I, II, IV, V and KAT in healthy age-matched (n=11, age 47.8± 12.4, male=5 and female=6) control subjects was used as cutoff. The development of auto-Abs was monitored in the serum every 1 month in AMR patients and 6 months for CAV patients.
ELISPOT
Frozen PBMC collected from patients were cultured overnight in complete RPMI-1640 and viability was determined by trypan blue exclusion. PBMCs with viability of a minimum of 90% were used for ELISPOT assays. The CD4+T-cells were enriched by negative selection using immunomagnetic separation cocktails (Stem cell Technologies, Canada). Enriched CD4+ T cells (3×105) with approximately 95% purity were cultured in triplicate in presence of Col-V (20μg/ml) or KAT (20μg/ml) on the pre-coated ELISPOT plates (Multiscreen IP plate, Millipore, MA) with autologous irradiated CD4 depleted PBMCs as APCs (3×104) in complete RPMI-1640 medium. Cultures were placed for 48 to 72 hrs in humidified 5% CO2 incubator at 37°C and the plates were washed and developed to detect IFN-γ, IL-5, IL-10 and IL-17 using an ImmunoSpot analyzer (Cellular Technology). CD4+ T cells plus autologous APCs cultured in medium without antigens was negative control while CD4+ T cells plus autologous APCs cultured with PHA (5 μg/ml) was positive control. Number of spots in negative control subtracted from spots in experimental wells was reported as results in spots per million cells (spm). The AMR cohort was serially monitored once a month and CAV cohort every 6 months.
Statistical Analysis
Graphpad Prism version 4.03 software (GraphPad Software Inc, CA) was utilized. Mann-Whitney test assessed differences in CD4+ T cell responses specific to individual antigens between two groups (AMR(+) vs AMR(−); CAV(+) vs CAV(−)). Correlation analysis was performed using Spearman rank test. Two-sided level of significance was set at p>0.05 and results were expressed in mean ± standard deviation.
Supplementary Material
Abbreviations
- Abs
Antibodies
- AMR
Antibody Mediated Rejection
- CAV
Cardiac Allograft Vasculopathy
- Col-V
Collagen V
- ELISA
Enzyme Linked Immunosorbent Assay
- ELISPOT
Enzyme Linked Immunosorbent Spot
- EP
Early Period; </= 1 year post-heart transplant
- HLA
Human Leukocyte Antigens
- HTx
Heart Transplantation
- KAT
K-α1-Tubulin
- LP
Late Period; > 1 year post-heart transplant
- MHC
Major Histocompatibility Complex
Footnotes
Dilip S. Nath: Participated in research design, writing of paper, performance of research, data analysis. Supported by NIH Training Grant T32 HL07776. No conflict of interest.
Venkataswarup Tiriveedhi: Participated in research design, writing of paper, performance of research, data analysis. No conflict of interest.
Haseeb Ilias Basha: Participated in research design, writing of paper, performance of research, data analysis. No conflict of interest.
Donna Phelan: Contributed analytic tools. No conflict of interest.
Nader Moazami: Participated in research design. No conflict of interest.
Gregory A. Ewald: Participated in research design. No conflict of interest.
T. Mohanakumar: Participated in research design, writing of paper, contributed analytic tools. Supported by Barnes Jewish Christian (BJC) Healthcare Foundation. No conflict of interest.
Current Address:
Dilip S. Nath, M.D., Children’s National Medical Center, 111 Michigan Avenue NW, Washington DC 20010, dnath@cnmc.org
Venkataswarup Tiriveedhi, M.D., Ph.D., Washington University School of Medicine, Department of Surgery, Box 8109-3328 CSRB, 660 S. Euclid Avenue, St. Louis, MO 63110, swaruptv@yahoo.com
Haseeb Ilias Basha, M.D., Hurley Medical Center, One Hurley Plaza, Flint, MI 48503, convey2haseeb@yahoo.com
Donna Phelan, B.A., HLA Laboratory, Barnes-Jewish Hospital, One Barnes-Jewish Hospital Plaza, St. Louis, MO 63110, DLP2368@bjc.org
Nader Moazami, M.D., Minneapolis Cardiothoracic Surgery Consultants, 920 East 28th Street, Suite 610, Minneapolis, MN 55407, Nader.Moazami@allina.com
Gregory A. Ewald, M.D., Washington University School of Medicine, Cardiovascular Division, Box 8086, 660 S. Euclid Avenue, St. Louis, Mo 63110, GEWALD@DOM.wust.edu
T. Mohanakumar, Ph.D., Washington University School of Medicine, Department of Surgery, Box 8109-3328 CSRB, 660 S. Euclid Avenue, St. Louis, MO 63110, kumart@wustl.edu
References
- 1.Kfoury AG, Hammond ME. Controversies in defining cardiac antibody-mediated rejection: need for updated criteria. J Heart Lung Transplant. 29(4):389–394. doi: 10.1016/j.healun.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Kfoury AG, Hammond ME, Snow GL, Stehlik J, Reid BB, Long JW, Gilbert EM, Bader FM, Bull DA, Renlund DG. Early screening for antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2007;26(12):1264–1269. doi: 10.1016/j.healun.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Almuti K, Haythe J, Dwyer E, Itescu S, Burke E, Green P, Marboe C, Mancini D. The changing pattern of humoral rejection in cardiac transplant recipients. Transplantation. 2007;84(4):498–503. doi: 10.1097/01.tp.0000278094.41131.9f. [DOI] [PubMed] [Google Scholar]
- 4.Wu GW, Kobashigawa JA, Fishbein MC, Patel JK, Kittleson MM, Reed EF, Kiyosaki KK, Ardehali A. Asymptomatic antibody-mediated rejection after heart transplantation predicts poor outcomes. J Heart Lung Transplant. 2009;28(5):417–422. doi: 10.1016/j.healun.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez ER, Rose M, Stewart S, Suciu-Foca N, Zeevi A, Fishbein MC. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25(2):153–159. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007;7(9):2064–2074. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Stastny P, Ring S, Lu C, Arenas J, Han M, Lavingia B. Role of immunoglobulin (Ig)-G and IgM antibodies against donor human leukocyte antigens in organ transplant recipients. Hum Immunol. 2009;70(8):600–604. doi: 10.1016/j.humimm.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Kaczmarek I, Deutsch MA, Kauke T, Beiras-Fernandez A, Schmoeckel M, Vicol C, Sodian R, Reichart B, Spannagl M, Ueberfuhr P. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008;6(3):229–235. [PubMed] [Google Scholar]
- 10.Michaels PJ, Fishbein MC, Colvin RB. Humoral rejection of human organ transplants. Springer Semin Immunopathol. 2003;25(2):119–140. doi: 10.1007/s00281-003-0139-x. [DOI] [PubMed] [Google Scholar]
- 11.Hathout E, Beeson WL, Kuhn M, Johnston J, Fitts J, Razzouk A, Bailey L, Chinnock RE. Cardiac allograft vasculopathy in pediatric heart transplant recipients. Transpl Int. 2006;19(3):184–189. doi: 10.1111/j.1432-2277.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 12.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, Meyers B, Schuessler R, Trulock EP, Patterson GA, Mohanakumar T. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 13.Kobashigawa JA. Cardiac allograft vasculopathy in heart transplant patients: pathologic and clinical aspects for angioplasty/stenting. J Am Coll Cardiol. 2006;48(3):462–463. doi: 10.1016/j.jacc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Hornick PI, Mason PD, Yacoub MH, Rose ML, Batchelor R, Lechler RI. Assessment of the contribution that direct allorecognition makes to the progression of chronic cardiac transplant rejection in humans. Circulation. 1998;97(13):1257–1263. doi: 10.1161/01.cir.97.13.1257. [DOI] [PubMed] [Google Scholar]
- 15.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn MJ, Rose ML, Latif N, Bradd S, Lovegrove C, Seymour C, Pomerance A, Yacoub MH. Demonstration by western blotting of antiheart antibodies before and after cardiac transplantation. Transplantation. 1991;51(4):806–812. doi: 10.1097/00007890-199104000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Lawson C, Holder AL, Stanford RE, Smith J, Rose ML. Anti-intercellular adhesion molecule-1 antibodies in sera of heart transplant recipients: a role in endothelial cell activation. Transplantation. 2005;80(2):264–271. doi: 10.1097/01.tp.0000165433.88295.4c. [DOI] [PubMed] [Google Scholar]
- 18.Laguens RP, Argel MI, Chambo JG, Vigliano CA, San Martino JA, Perrone SV, Favaloro RR. Anti-skeletal muscle glycolipid antibodies in human heart transplantation as markers of acute rejection. Correlation with endomyocardial biopsy. Transplantation. 1996;62(2):211–216. doi: 10.1097/00007890-199607270-00011. [DOI] [PubMed] [Google Scholar]
- 19.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, Blum JS, Wilkes DS. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169(3):1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 20.Ramzy D, Rao V, Brahm J, Miriuka S, Delgado D, Ross HJ. Cardiac allograft vasculopathy: a review. Can J Surg. 2005;48(4):319–327. [PMC free article] [PubMed] [Google Scholar]
- 21.Fedoseyeva EV, Tam RC, Popov IA, Orr PL, Garovoy MR, Benichou G. Induction of T cell responses to a self-antigen following allotransplantation. Transplantation. 1996;61(5):679–683. doi: 10.1097/00007890-199603150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Gronen F, Ruprecht K, Weissbrich B, Klinker E, Kroner A, Hofstetter HH, Rieckmann P. Frequency analysis of HLA-B7-restricted Epstein-Barr virus-specific cytotoxic T lymphocytes in patients with multiple sclerosis and healthy controls. J Neuroimmunol. 2006;180(1-2):185–192. doi: 10.1016/j.jneuroim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Rao S, Aberg F, Nieves E, Band Horwitz S, Orr GA. Identification by mass spectrometry of a new alpha-tubulin isotype expressed in human breast and lung carcinoma cell lines. Biochemistry. 2001;40(7):2096–2103. doi: 10.1021/bi002323d. [DOI] [PubMed] [Google Scholar]
- 24.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hein S, Scheffold T, Schaper J. Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium. J Thorac Cardiovasc Surg. 1995;110(1):89–98. doi: 10.1016/S0022-5223(05)80013-3. [DOI] [PubMed] [Google Scholar]
- 26.Tiriveedhi V, Angaswamy N, Weber J, Mohanakumar T. Lipid raft facilitated ligation of K-alpha1-tubulin by specific antibodies on epithelial cells: Role in pathogenesis of chronic rejection following human lung transplantation. Biochem Biophys Res Commun. 399(2):251–255. doi: 10.1016/j.bbrc.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole WG, Chan D, Hickey AJ, Wilcken DE. Collagen composition of normal and myxomatous human mitral heart valves. Biochem J. 1984;219(2):451–460. doi: 10.1042/bj2190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linehan KA, Seymour AM, Williams PE. Semiquantitative analysis of collagen types in the hypertrophied left ventricle. J Anat. 2001;198(Pt 1):83–92. doi: 10.1046/j.1469-7580.2001.19810083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai J, Terasaki PI. Post-transplantation antibody monitoring and HLA antibody epitope identification. Curr Opin Immunol. 2008;20(5):602–606. doi: 10.1016/j.coi.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Kauke T, Kaczmarek I, Dick A, Schmoeckel M, Deutsch MA, Beiras-Fernandez A, Reichart B, Spannagl M. Anti-MICA antibodies are related to adverse outcome in heart transplant recipients. J Heart Lung Transplant. 2009;28(4):305–311. doi: 10.1016/j.healun.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 29(11):1277–1285. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177(8):5631–5638. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 34.Nath DS, Basha HI, Mohanakumar T. Antihuman leukocyte antigen antibody-induced autoimmunity: role in chronic rejection. Curr Opin Organ Transplant. 15(1):16–20. doi: 10.1097/MOT.0b013e3283342780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6(8):1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 36.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 38.Jaramillo A, Naziruddin B, Zhang L, Reznik SI, Smith MA, Aloush AA, Trulock EP, Patterson GA, Mohanakumar T. Activation of human airway epithelial cells by non-HLA antibodies developed after lung transplantation: a potential etiological factor for bronchiolitis obliterans syndrome. Transplantation. 2001;71(7):966–976. doi: 10.1097/00007890-200104150-00023. [DOI] [PubMed] [Google Scholar]
- 39.Jaramillo A, Fernandez FG, Kuo EY, Trulock EP, Patterson GA, Mohanakumar T. Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatr Transplant. 2005;9(1):84–93. doi: 10.1111/j.1399-3046.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 40.Leong HS, Mahesh BM, Day JR, Smith JD, McCormack AD, Ghimire G, Podor TJ, Rose ML. Vimentin autoantibodies induce platelet activation and formation of platelet-leukocyte conjugates via platelet-activating factor. J Leukoc Biol. 2008;83(2):263–271. doi: 10.1189/jlb.0607339. [DOI] [PubMed] [Google Scholar]
- 41.Rose ML. Interferon-gamma and intimal hyperplasia. Circ Res. 2007;101(6):542–544. doi: 10.1161/CIRCRESAHA.107.160911. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101(6):560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 43.Irmler IM, Gajda M, Brauer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007;179(9):6228–6236. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 44.Tiriveedhi V, Weber J, Seetharam A, Mohanakumar T. Cross-talk of alloimmune response and autoimmunity: role in pathogenesis of chronic rejection. Discov Med. 9(46):229–235. [PubMed] [Google Scholar]
- 45.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golocheikine A, Nath DS, Basha HI, Saini D, Phelan D, Aloush A, Trulock EP, Hachem RR, Alexander Patterson G, Ahearn JM, Mohanakumar T. Increased erythrocyte C4D is associated with known alloantibody and autoantibody markers of antibody-mediated rejection in human lung transplant recipients. J Heart Lung Transplant. 2009 doi: 10.1016/j.healun.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fields RC, Bharat A, Steward N, Aloush A, Meyers BF, Trulock EP, Chapman WC, Patterson GA, Mohanakumar T. Elevated soluble CD30 correlates with development of bronchiolitis obliterans syndrome following lung transplantation. Transplantation. 2006;82(12):1596–1601. doi: 10.1097/01.tp.0000241076.46033.4c. [DOI] [PubMed] [Google Scholar]
- 48.Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 29(7):717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T. Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: a 30-month analysis in living donor kidney transplantation. Hum Immunol. 71(3):268–273. doi: 10.1016/j.humimm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86(1):189–195. doi: 10.1016/j.athoracsur.2008.03.073. discussion 196-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.