Abstract
Background
Alloimmunization to minor red blood cell (RBC) antigens occurs commonly in sickle cell disease (SCD). Patients with alloimmunization demonstrate increased risk for new alloantibody formation with subsequent transfusion. Alloimmunization to human leukocyte antigens (HLA) can occur with RBC transfusion and may result in graft rejection during stem cell or organ transplantation. The prevalence and risk factors for HLA alloimmunization in multiply transfused pediatric SCD patients are unknown.
Procedure
A cross-sectional study of HLA alloimmunization in SCD patients aged 3–21 years with a history of ≥3 RBC transfusions was performed to test the hypothesis that HLA alloimmunization is associated with RBC alloimmunization. Antibodies to class I and class II HLA were measured by Flow Panel Reactive Antibody (FlowPRA®).
Results
Seventy-three SCD patients (30 with RBC antibodies) were tested. HLA antibodies were detected in 25/73 (34%) patients; class I HLA antibodies occurred in 24/73 (33%) and class II HLA antibodies occurred in 3 (4%). Among patients with RBC antibodies, 16/30 (53%) had HLA antibodies, while 9/43 (21%) patients without RBC antibodies had HLA antibodies (OR 4.32 [1.6–12.1]). In a multivariate analysis, antibodies to RBC antigens were an independent predictor of HLA alloimmunization (P =0.041). The association of RBC and HLA immunization was strongest among patients with no history of chronic transfusion therapy.
Conclusions
This analysis is the first description of HLA alloimmunization in pediatric SCD patients who receive primarily leukoreduced RBC transfusions and demonstrates that HLA alloimmunization tendency is associated with antibodies to RBC antigens.
Keywords: alloimmunization, human leukocyte antigen (HLA), sickle cell disease, transfusion
INTRODUCTION
Alloimmunization to minor red blood cell (RBC) antigens is a significant complication of transfusion therapy in sickle cell disease (SCD) that leads to increased risk of hemolytic transfusion reactions and limits the number of compatible RBC donors, thus restricting clinicians’ ability to rapidly and safely transfuse RBCs in many situations. Patients with SCD may have a prevalence of RBC alloimmunization as high as 35–40% in the absence of minor RBC antigen phenotype matching [1–3]. Although the risk of RBC alloimmunization in SCD may be related in part to a high number of lifetime transfusions and to minor RBC antigen phenotype disparity with blood donors [2,4–8], the fact that the majority of multiply transfused SCD patients do not form RBC alloantibodies suggests that other characteristics inherent to the individual contribute to alloimmunization. Patients who have become alloimmunized to one RBC antigen demonstrate an increased likelihood to form multiple alloantibodies to different antigens with subsequent transfusion [9,10]. Autoimmunization to RBC antigens in transfused patients is associated with alloimmunization, often occurring concurrently with RBC transfusion [11–14]. In contrast, factors that may contribute to a lower risk of RBC alloimmunization include initiation of chronic transfusion therapy at an early age and splenectomy [15–18]. Ultimately, however, it is not known why some patients have strong immunologic responses to allogeneic RBCs while other patients with similar transfusion exposures do not have the same immune response. This immunologic observation may extend to other important antigens including human leukocyte antigens (HLA).
HLA alloimmunization following transfusion of leukocyte-reduced (LR) RBCs is an under-recognized yet possible occurrence. Class I HLA alloimmunization due to RBC transfusion has been well documented in SCD and other transfused patients [19–21]. The simultaneous detection of anti-platelet glycoprotein antibodies in these patients and the past use of non-LR RBC transfusions has led investigators to believe that contaminating leukocytes and platelets are the main source of HLA exposure in RBC transfusions [22,23]; however, low numbers of class I HLA also are present on the surface of mature, anucleate RBCs [24,25]. Additionally, the Bennett-Goodspeed (Bg) group of minor RBC antigens has nearly identical homology to specific class I HLA molecules (A2, A68–69, B7, B57–58) [26,27]. Thus, it is possible that the RBC itself may be the primary source of HLA exposure and alloimmunization.
HLA antibodies may have serious adverse consequences in allogeneic hematopoietic cell transplantation (HCT), resulting in rejection of HLA-non-identical grafts [28–30] and platelet transfusion refractoriness during the pre-engraftment period [31]. HLA alloimmunization may also impact RBC transfusion therapy, as it has been associated with decreased RBC survival and hemolysis of allogeneic transfused RBC [32,33]. Although HLA antibodies may be inconsequential in most RBC transfusions, there is evidence that in rare circumstances including transfusion of Bg + RBCs, HLA alloantibodies may be associated with immune-mediated, delayed hyperhemolytic transfusion reactions (DHTR) [32,34,35].
Understanding the baseline prevalence of specific HLA antibodies and the risk factors for HLA alloimmunization is requisite to assessing their possible contributions to HCT complications and to DHTR. Based on the observations that individual SCD patients may have a tendency toward or against RBC alloimmunization, we hypothesized that patients with past immunization to RBC antigens either allo- or autoantibodies would also be more likely to demonstrate HLA alloimmunization. The primary aims of this study were to determine the association of HLA alloimmunization with RBC antibodies and to assess associations of HLA alloimmunization with transfusion and clinical history. The secondary aim was to assess HLA antibody specificity in order to determine the frequency of HLA antibodies with potential cross-reactivity to the Bg minor RBC antigens.
MATERIALS AND METHODS
Patient Selection
A cross-sectional observational study of HLA alloimmunization was undertaken in pediatric SCD patients. Institutional review board approval for this investigation was granted by Emory University and Children’s Healthcare of Atlanta (CHOA). Patients aged 3–21 years with a diagnosis of hemoglobin SS or S-β0 thalassemia SCD who had received three or more lifetime RBC transfusions were considered eligible for enrollment. Eligible subjects were identified from review of blood bank records and were offered study participation when presenting for routine health care, without acute complication or SCD crisis. Patients were excluded from study if they had past platelet transfusions, intravenous immunoglobulin infusions, HCTor organ transplantation, pregnancy, current fever or bacteremia, or systemic corticosteroids within the past 3 weeks.
Since 1993, all known patients with SCD transfused in Atlanta have received exclusively leukoreduced, non-irradiated, packed RBC units that are phenotypically matched for at least C, E, c, e, and K minor RBC antigens [36]. All RBC units transfused since the year 2000 were pre-storage leukoreduced; however, prior to 2000, LR was performed by filtration either at the patient’s bedside or prior to storage. Transfusion and medical records were reviewed to determine patients’ lifetime number of RBC transfusions, history of non-LR transfusions, and transfusion at hospitals without specific record of LR.
Patients were selected in a case–control fashion to assess the association of RBC immunization with HLA alloimmunization. Case subjects were defined as those with RBC alloantibodies and/or autoantibodies ever detected on routine indirect antiglobulin testing. Control subjects were defined as those with no history of RBC antibodies. Case and controls were matched for use of chronic transfusion therapy, defined as at least 6 months of routine RBC hypertransfusion for prophylaxis against SCD complications.
HLA Antibody Detection Methods
Patient serum was screened for anti-HLA immunoglobulin G (IgG) alloantibodies using FlowPRA® screening test (One Lambda, Inc., Canoga Park, CA), a flow cytometry-based test that enables simultaneous detection of class I and class II HLA antibodies [37]. Microparticles coated with purified HLA class I or class II molecules, representing the majority of common HLA antigens in the North American population [38], were incubated with patient serum. Class I and class II HLA-coated beads are distinguished by size and unique fluorescent emission properties. Incubated beads were stained with fluorescein isothiocyante (FITC)-conjugated goat anti-human IgG (Fe fragment specific; Jackson ImmunoResearch Laboratories, West Grove, PA). Particle fluorescence was analyzed on a FACScan (Becton-Dickinson, Mountain View, CA) flow cytometer. FITC emission, indicating HLA antibody binding, was detected by FL1 channel shift. Histograms of FL1 emission for FlowPRA I and FlowPRA II beads estimated the percentage of beads with bound antibody. The percentage of beads that react with antibody in the patient’s serum is termed the panel reactive antibody (PRA) activity, which, in broad terms, estimates the proportion of random population donors to which the patient is alloimmunized. FlowPRA results are considered positive when ≥3% of beads show FL1 channel shift emission. Positive FlowPRA screening assays were reflexed to LabScreen® Single Antigen Antibody Detection (One Lambda, Inc.) for result verification and identification of the specific HLA antibodies using fluorescent microparticles coated with single HLA antigens. Antibody binding to single antigen microparticles was assessed by addition of biotin-conjugated anti-human IgG and phycoerythrin-coated strepavidin and measurement of fluorescent FL2 channel shift.
Statistical Analysis
Based on estimated HLA alloimmunization rates in other transfused populations [19–21], to demonstrate a case–control odds ratio of ≥4.0 with 80% power and 95% confidence (α = 0.05), a minimum sample size of 72 patients was required. To assess for confounding in selection of cases and controls, characteristics of case and control patients were compared by chi-square or Fisher’s exact test for categorical variables and t-test for continuous variables. For univariate analysis, chi-square comparison and odds ratio calculation were used to compare the frequency of HLA alloimmunization in RBC antibody case and control groups as well as secondary categorical variables, including age, chronic transfusion therapy, lifetime number of transfusions, LR history, time since last transfusion, and splenectomy. Breslow–Day test was performed to detect interaction of secondary variables with RBC antibodies; stratified odds ratios were calculated in cases of significant interaction.
All variables were introduced into multivariate regression models, odds ratios for each variable were calculated by maximum likelihood estimates analysis, and statistical significance was determined by Wald chi-square test with significance level of 0.05. To assess for possible interaction with RBC antibodies, logistic regression was performed including terms for the interaction of RBC antibodies with current chronic transfusion therapy and with past chronic transfusion therapy.
RESULTS
Patient Characteristics
Children with SCD presenting to Children’s Healthcare of Atlanta for routine care from September 2005 to November 2008 were periodically reviewed to identify eligible study participants. Ninety-six eligible subjects were approached for study participation, and 73 (32 female, 41 male) consented for testing. Among participants, 30 (41%) had previously detected RBC antibodies (case patients): 16 with only RBC alloantibodies, 8 with only RBC autoantibodies, and 6 with both RBC allo- and autoantibodies. Fifty (68%) patients were transfused exclusively in Atlanta, while 23 patients had received past transfusions at other hospitals (6 with exclusive use of LR units, 7 without exclusive LR, and 10 in whom exclusive LR history was unknown). Characteristics of case and control groups are compared in Table I. Age was the only variable not equally distributed, with patients in the RBC antibody group tending to be older (14.5 ± 3.0 years) than those without RBC antibodies (11.7 ± 4.7 years) (P = 0.003).
TABLE I.
Patient Characteristics: Comparison of Case and Control Groups
| RBC antibody positive (n = 30) | RBC antibody negative (n = 43) | P-value | |
|---|---|---|---|
| Age (years) | 14.5 ± 3.0 (9.1–19.4) | 11.7 ± 4.7 (3.9–20.1) | 0.003 |
| Female | 12 (40.0) | 20 (46.5) | 0.58 |
| Male | 18 (60.0) | 23 (53.5) | |
| Lifetime number of RBC units | 0.40 | ||
| <25 | 8 (26.7) | 20 (46.5) | |
| 25–49 | 4 (13.3) | 4 (9.3) | |
| 50–99 | 11 (36.7) | 12 (27.9) | |
| ≥100 | 7 (23.3) | 7 (16.3) | |
| Chronic transfusion therapy | 0.69 | ||
| Never | 7 (23.3) | 10 (23.3) | |
| Past | 8 (26.7) | 8 (18.6) | |
| Current | 15 (50.0) | 25 (58.1) | |
| RBC leukoreduction (LR) history | 0.10 | ||
| Exclusively LR | 15 (50.0) | 32 (74.4) | |
| Not exclusively LR | 9 (30.0) | 7 (16.3) | |
| Incomplete LR history | 6 (20.0) | 4 (9.3) | |
| Past splenectomy | 5 (16.6) | 10 (23.3) | 0.49 |
| Days from last RBC transfusion | 234 ± 446 (21–2,265) | 313 ± 551 (12–2,280) | 0.52 |
Age in years and days from last RBC transfusion are expressed as the mean ± standard deviation (range). Within each case or control group, the number of female and male patients, the number of patients to receive <25, 25–49, 50–99, and ≥100 RBC units, the number of patients to have never received chronic transfusion therapy or to have received chronic transfusion therapy currently or in the past, the number of patients to have received exclusively leukoreduced (LR) transfusions or not exclusively LR transfusions, and the number of patients with splenectomy are given with percentage of each case or control group given in parentheses. The P-value is calculated by t-test or chi-square comparison, as appropriate.
HLA PRA and Specificity Results
HLA antibodies were detected in 25/73 (34%) subjects aged 5–20 years. Class I HLA antibodies were detected in 24 patients, and class II HLA antibodies were detected in 3 patients (2 patients had both class I and class II antibodies). The distribution of class I PRA results among patients with and without RBC antibodies is shown in Figure 1. High PRA ≥50% was more prevalent among patients with RBC antibodies.
Fig. 1.
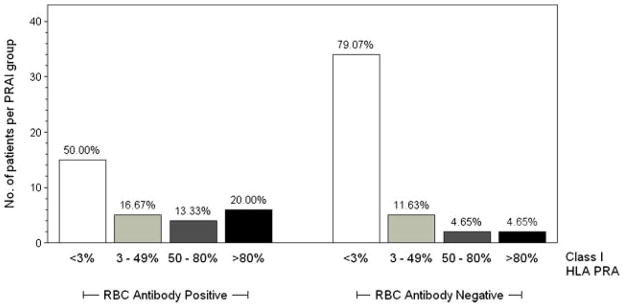
Panel reactive antibody (PRA) results for class I HLA antibodies in patients with red blood cell (RBC) antibodies (n = 30) and without RBC antibodies (n = 43). PRA ≥3% indicates the presence of HLA antibodies. PRA for class I antibodies was positive in 50% (n = 15) of patients with RBC antibodies and 21% (n = 9) of patients without RBC antibodies (P = 0.0093). High PRA >50% was more common among patients with RBC antibodies.
For patients with class I HLA antibodies, the median PRA was 57% (range: 3–99%); for class II HLA antibodies, the median PRA was 24% (range: 18–99%). Among those with HLA alloimmunization, 15 (60%) had HLA antibodies with known cross-reactivity with the Bg minor RBC antigen group. Six patients had HLA antibodies against one Bg antigen, six patients had antibodies to two Bg antigens, and three patients had antibodies to all three Bg antigens.
Univariate Associations With HLA Alloimmunization
HLA alloimmunization was detected in 16 of 30 (53%) patients with RBC antibodies and 9 of 43 (21%) patients without RBC antibodies (OR 4.32 [1.55–12.05], P = 0.0041). As shown in Table II, both RBC alloantibodies and autoantibodies were significantly associated with HLA alloimmunization, as compared to patients without RBC immunization (P = 0.021). Univariate analysis of other predictors of HLA alloimmunization is shown in Table III. Other than RBC antibodies, older age was the only additional variable to show a significant association with HLA alloimmunization; however, this finding is confounded by the association of age with RBC antibodies. The frequency of HLA alloimmunization did not differ significantly with gender (P = 0.63) or LR history (P = 0.26). Chronic transfusion therapy was not associated with HLA alloimmunization, however, demonstrated significant statistical interaction with the effect of RBC antibodies (Breslow–Day test, P = 0.013).
TABLE II.
Comparison of HLA Antibodies in Patients With RBC Autoantibodies and/or Alloantibodies
| RBC antibodies | HLA antibody positive | HLA antibody negative | P-value |
|---|---|---|---|
| Alloantibodies only | 7 (46.7) | 8 (53.3) | 0.021 |
| Autoantibodies only | 5 (55.6) | 4 (44.4) | |
| Allo- and autoantibodies | 4 (66.7) | 2 (33.3) | |
| No RBC antibodies | 9 (20.9) | 34 (79.1) |
The number of patients with HLA antibodies (n = 25) and without HLA antibodies (n = 48) is shown, stratified by RBC antibody history. The P-value is calculated by Fisher’s exact test.
TABLE III.
Univariate Analysis of HLA Alloimmunization
| n | HLA antibody positive | HLA antibody negative | OR (95% CI) | P-value | |
|---|---|---|---|---|---|
| RBC antibody positive | 30 | 16 (53.3) | 14 (46.7) | 4.32 (1.6–12.1) | 0.0041 |
| RBC antibody negative | 43 | 9 (20.9) | 34 (79.1) | ||
| Female | 32 | 10 (31.3) | 22 (68.8) | 1.27 (0.48–3.4) | 0.63 |
| Male | 41 | 15 (36.6) | 26 (63.4) | ||
| Age ≥13 years | 36 | 17 (47.2) | 19 (52.7) | 3.24 (1.2–9.0) | 0.021 |
| Age <13 years | 37 | 8 (21.6) | 29 (78.4) | ||
| Lifetime RBC units | n/a | 0.11 | |||
| <25 | 28 | 7 (25.0) | 21 (75.0) | ||
| 25–49 | 8 | 1 (12.5) | 7 (87.5) | ||
| 50–99 | 23 | 12 (52.2) | 11 (47.8) | ||
| ≥100 | 14 | 5 (35.7) | 9 (64.3) | ||
| Chronic transfusions | n/a | 0.93 | |||
| Never | 17 | 6 (35.3) | 11 (65.7) | ||
| Past | 16 | 6 (37.5) | 10 (62.5) | ||
| Current | 40 | 13 (32.5) | 27 (67.5) | ||
| LR history | n/a | 0.14 | |||
| Exclusively LR | 47 | 13 (27.7) | 34 (72.3) | ||
| Not exclusively LR | 16 | 6 (37.5) | 10 (62.5) | ||
| Incomplete history | 10 | 6 (60.0) | 4 (40.0) | ||
| Last RBC transfusion | 1.13 (0.4–3.1) | 0.82 | |||
| ≥90 days | 25 | 9 (36) | 16 (64) | ||
| <90 days | 48 | 16 (33) | 32 (67) | ||
| Splenectomy | 15 | 2 (13.3) | 13 (86.7) | 0.23 (0.05–1.1) | 0.056 |
| No splenectomy | 58 | 23 (39.7) | 35 (60.3) |
LR, leukoreduction; OR, odds ratio (95% confidence interval). The P-value is calculated by chi-squared comparison or Fisher’s exact test, as appropriate.
Multivariate Analysis
In a multivariate analysis (Table IV), RBC antibodies were the only factors associated with significantly increased odds of HLA alloimmunization (P = 0.041). Although chronic transfusion therapy was not an independent predictor of HLA alloimmunization, when interaction terms were introduced into multivariate analysis (data not shown), significant interaction was demonstrated between RBC antibodies and past chronic transfusion therapy (P = 0.011). In stratified univariate analysis, as shown in Table V, the association of RBC and HLA antibodies is shown to vary significantly depending upon the chronic transfusion history (a finding that is independent of the number of lifetime transfusions). Within the stratum of patients with a history of past (discontinued) chronic transfusion therapy, the relationship between RBC and HLA antibodies was not significant (OR 0.33 [0.04–2.77]); however, this association was significant for patients currently receiving chronic transfusion therapy (OR 7.88 [1.78–34.8]), and the association was greatest among those who had never received chronic transfusions (OR 22.5 [1.6–314.6]).
TABLE IV.
Multivariate Analysis of HLA Alloimmunization in SCD
| Variable | Odds ratio | Wald 95% CI | P-value |
|---|---|---|---|
| RBC antibodies | 3.61 | (1.05–12.3) | 0.041 |
| Age ≥13 | 3.08 | (0.69–13.8) | 0.14 |
| Lifetime number of RBC unitsa | |||
| 25–49 | 0.85 | (0.05–15.5) | 0.91 |
| 50–99 | 8.13 | (0.92–72.2) | 0.06 |
| ≥100 | 2.78 | (0.20–38.0) | 0.44 |
| Chronic transfusion therapyb | |||
| Current | 0.52 | (0.04–6.91) | 0.62 |
| Past | 0.35 | (0.03–3.97) | 0.39 |
| LR historyc | |||
| Not exclusively LR | 0.81 | (0.15–4.40) | 0.81 |
| Incomplete LR history | 1.78 | (0.28–11.4) | 0.54 |
| Days since last RBC transfusion | 1.00 | (0.999–1.002) | 0.63 |
| Splenectomy | 0.25 | (0.04–1.57) | 0.14 |
LR, leukoreduction. Odds ratios (95% confidence intervals) for each predictor variable are shown.
Reference group: <25 units;
Reference group: never received chronic transfusion therapy;
Reference group: exclusively LR units.
TABLE V.
Interaction of Chronic Transfusion Therapy and RBC Antibodies
| Chronic transfusion therapy
|
P-value | |||
|---|---|---|---|---|
| Current (n = 40) | Past (n = 16) | Never (n = 17) | ||
| RBC antibodies | 15 (37.5) | 8 (50.0) | 7 (41.2) | 0.73 |
| HLA antibodies | 13 (32.5) | 6 (37.5) | 6 (35.3) | 0.95 |
| Stratified odds ratio | 7.88 (1.78–34.8) | 0.33 (0.04–2.77) | 22.5 (1.6–314.6) | 0.013 |
The numbers (%) of patients in each chronic transfusion category (current, past, never) with RBC antibodies and HLA antibodies are given. The stratified odds ratios (95% confidence interval) reflect the association of RBC antibodies and HLA antibodies within each chronic transfusion category. The P-values for RBC antibodies and HLA antibodies are calculated by Fisher’s exact test. The P-value comparing stratified odds ratios is calculated by Breslow–Day test.
DISCUSSION
This analysis represents the first description of specific HLA antibody testing in SCD patients using techniques to detect antibodies against both class I and class II HLA. Fluorescence-activated sorting is a highly sensitive technique for detection of anti-HLA IgG, with a sensitivity 20–30% greater than conventional antiglobulin-enhanced lymphocyte cytotoxicity or ELISA assays, and false reactivity presumed near zero [30,39]. The HLA PRA can be regarded as an estimate of the percentage of people in the general population to which a patient may have anti-HLA alloantibodies. In combination with HLA antibody identification, this is a clinically relevant technique that may be used to determine if a patient is sensitized against specific transfusion or transplantation donors [37,38,40,41]. While HLA alloimmunization resulting from blood transfusion is a well-documented phenomenon in solid organ transplant recipients, it is relatively under-recognized among multiply transfused patients with hemoglobinopathies [42,43]. Awareness of HLA alloimmunization in SCD is important in multiple clinical scenarios and may help to identify individuals who will be at risk for a poor outcome from HCT due to either graft rejection or platelet transfusion refractoriness. Additionally, in rare cases, HLA alloantibodies may play a role in immune-mediated hyperhemolytic transfusion complications [34,44]. If HLA antibodies with high PRA are identified in SCD patients, blood transfusion therapy could potentially be modified by restricting blood donors to a pool of HLA-matched individuals to decrease further HLA alloimmunization in potential transplant patients.
The prevalence of HLA alloimmunization in SCD patients previously has been reported only in the context of non-LR RBC transfusions, and predictive factors have not been well-characterized [19]. In this study, the majority of patients have received exclusively LR RBC transfusions; although a small group of patients had previously received some non-LR RBC transfusions, it is important to note that the majority of transfusions received by these patients were LR and that exposure to non-LR transfusions were remote or infrequent occurrences. Thus, LR may not have had a significant influence on the frequency of HLA alloimmunization.
This study demonstrated a significant association between antibody responses to HLA and RBC antigens. Since the study cohort was intentionally selected in case–control fashion to compare groups of patients with and without RBC antibodies, this analysis does not allow for an estimate of HLA alloimmunization in the general pediatric SCD population. However, even in the control group of patients without past RBC antibodies, the rate of HLA alloimmunization exceeded 20%, a frequency that is high enough to consider screening multiply transfused SCD patients who are transplantation candidates or who have had recurrent hemolytic transfusion reactions. In contrast, large-scale studies of healthy (non-SCD) individuals have shown that <2% of previously transfused males and nulliparous females have HLA alloimmunization [45]. This suggests that SCD patients are at higher risk for transfusion-associated HLA alloimmunization than some other groups of patients.
Perhaps even more significant than the overall high frequency of HLA alloimmunization observed in this study is the observation of individuals who have not developed HLA alloimmunization despite numerous and recent exposures to foreign HLA antigens via transfusion. The finding that RBC antibodies are strongly associated with HLA alloimmunization does not suggest a causal link from one to another; rather, suggests that some individuals have immune responses that are associated with a greater risk of alloimmunization to a broad range of foreign blood antigens. The concept of immunologic responders is well established for RBC alloimmunization and appears to extend to HLA alloimmunization [46,47]. What remains unclear are the factors that determine an individual’s responder status or immunologic phenotype; however, the association of HLA and RBC antibodies helps to further characterize patients’ responder phenotypes, allowing investigators to seek common causative factors for alloimmunization.
In this analysis we identified individuals who appear to be highly prone to the formation of antibodies to multiple foreign antigens (demonstrating high PRA and multiple RBC antibodies) as well as individuals who demonstrate no alloimmunization responses despite similar transfusion histories. Chronic transfusion therapy, while not directly associated with HLA alloimmunization did have significant interaction with RBC antibodies, modulating the strength of association between RBC antibodies and HLA antibodies. Interestingly, the association between RBC and HLA alloimmunization was strongest in patients who had never received chronic transfusion therapy. From this one might speculate that the tendency to form both RBC and HLA antibodies is related not only to the frequency of foreign antigen exposure but also to the clinical context in which the blood transfusion occurs. Further large-scale investigations are needed to explore this interaction of chronic transfusion exposure and alloimmunization to antigens encountered during RBC transfusion.
Another finding worth noting was a trend (though non-significant) toward lower rates of HLA alloimmunization among splenectomized patients. This finding is not unexpected, since the spleen is primarily responsible for stimulating immunologic antibody responses to bloodstream antigens [48]. Our study is the first report to suggest an association between splenectomy and alloimmunization in SCD patients [15,16].
This analysis also represents the first characterization of HLA antibody specificity in SCD. Alloimmunization to class II HLAwas rare among SCD patients, occurring in only three children (all of whom had RBC antibodies). Since class II HLA is present only on a restricted group of leukocytes (antigen-presenting cells and B lymphocytes), this observation suggests that the majority of cases of transfusion-related HLA alloimmunization in SCD are a result of exposure to class I HLA present on contaminating cells such as platelets or T-lymphocytes or directly on the RBC surface. Bg antigens are variably expressed on the surface of RBCs and have close homology and serologic cross-reactivity to specific class I HLA (A2, A68–69, B7, and B57–58), thus are a possible cause of HLA alloimmunization responses [26,27,49–51]. In this study, 60% of patients with HLA alloimmunization demonstrated antibodies to Bg-like HLA antigens, leading us to speculate that a significant proportion of HLA antibodies in these patients are cross-reactive with RBC surface antigens and raising the intriguing possibility that such antibodies could result in hemolytic transfusion reactions under select circumstances. While the identification of these antibodies alone does not predict an adverse hemolytic transfusion reaction, previous RBC survival studies have demonstrated a direct association between RBC hemolysis and HLA antibodies; additionally, HLA alloantibodies have been detected in several cases of severe DHTR in which no causative RBC antibodies are identified, supporting the hypothesis that HLA antibodies as well as RBC antibodies may initiate DHTR [32–34,52]. Thus, gathering data on the frequency of HLA antibodies in healthy SCD patients is an important preliminary step to further investigations of the role that HLA antibodies may play in hyperhemolytic transfusion reactions.
Patients who undergo periodic HLA antibody screening may demonstrate a decrease in PRA or a loss of HLA antibody as the time from the immunologic challenge lengthens [20,53]. Thus, it is important for clinicians to recognize that multiply transfused patients with a negative HLA antibody screen may still be at risk for subsequent adverse sequelae of HLA alloimmunization. The cross-sectional design of this study limits generalizability to the broader pediatric SCD population. Larger scale prospective studies are needed to identify additional risk factors and underlying causes of HLA alloimmunization in SCD and other multiply transfused patients, so that ultimately transfusion therapy can be individualized for patients at greatest risk of HLA alloimmunization.
Acknowledgments
This study was supported by NIH Grant HL07001 (C. Josephson). The authors thank Patricia Brannon for assistance in HLA antibody testing and Paula Jessup for assistance in gathering transfusion data.
Footnotes
Conflict of Interest Statement: Marianne McPherson, Alan Anderson, Marta-Inés Castillejo, Christopher Hillyer, Cassandra Josephson: Nothing to disclose. Robert Bray and Howard Gebel: Unrestricted research support from One Lambda, Inc., Canoga Park, CA.
References
- 1.Coles SM, Klein HG, Holland PV. Alloimmunization in two multitransfused patient populations. Transfusion. 1981;21:462–466. doi: 10.1046/j.1537-2995.1981.21481276005.x. [DOI] [PubMed] [Google Scholar]
- 2.Vichinsky EP, Earles A, Johnson RA, et al. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 3.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–1437. [PubMed] [Google Scholar]
- 4.Castro O, Sandler SG, Houston-Yu P, et al. Predicting the effect of transfusing only phenotype-matched RBCs to patients with sickle cell disease: Theoretical and practical implications. Transfusion. 2002;42:684–690. doi: 10.1046/j.1537-2995.2002.00126.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Glynn SA, Schreiber GB, et al. First-time blood donors: Demographic trends. Transfusion. 2001;41:360–364. doi: 10.1046/j.1537-2995.2001.41030360.x. [DOI] [PubMed] [Google Scholar]
- 6.Shaz BH, Zimring JC, Demmons DG, et al. Blood donation and blood transfusion: Special considerations for African Americans. Transfus Med Rev. 2008;22:202–214. doi: 10.1016/j.tmrv.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Hoeltge GA, Domen RE, Rybicki LA, et al. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Arch Pathol Lab Med. 1995;119:42–45. [PubMed] [Google Scholar]
- 8.Aygun B, Padmanabhan S, Paley C, et al. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 9.Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999;39:763–771. doi: 10.1046/j.1537-2995.1999.39070763.x. [DOI] [PubMed] [Google Scholar]
- 10.Schonewille H, van de Watering LM, Brand A. Additional red blood cell alloantibodies after blood transfusions in a nonhematologic alloimmunized patient cohort: Is it time to take precautionary measures? Transfusion. 2006;46:630–635. doi: 10.1111/j.1537-2995.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 11.Castellino SM, Combs MR, Zimmerman SA, et al. Erythrocyte autoantibodies in paediatric patients with sickle cell disease receiving transfusion therapy: Frequency, characteristics and significance. Br J Haematol. 1999;104:189–194. doi: 10.1046/j.1365-2141.1999.01127.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahrens N, Pruss A, Kahne A, et al. Coexistence of autoantibodies and alloantibodies to red blood cells due to blood transfusion. Transfusion. 2007;47:813–816. doi: 10.1111/j.1537-2995.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 13.Garratty G. Autoantibodies induced by blood transfusion. Transfusion. 2004;44:5–9. doi: 10.1111/j.0041-1132.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 14.Young PP, Uzieblo A, Trulock E, et al. Autoantibody formation after alloimmunization: Are blood transfusions a risk factor for autoimmune hemolytic anemia? Transfusion. 2004;44:67–72. doi: 10.1046/j.0041-1132.2003.00589.x. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickson JE, Saakadze N, Cadwell CM, et al. The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells. Transfusion. 2009 Apr 28; doi: 10.1111/j.1537-2995.2009.02200.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrickson JE, Chadwick TE, Roback JD, et al. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 17.Michail-Merianou V, Pamphili-Panousopoulou L, Piperi-Lowes L, et al. Alloimmunization to red cell antigens in thalassemia: Comparative study of usual versus better-match transfusion programmes. Vox Sang. 1987;52:95–98. doi: 10.1111/j.1423-0410.1987.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 18.Spanos T, Karageorga M, Ladis V, et al. Red cell alloantibodies in patients with thalassemia. Vox Sang. 1990;58:50–55. doi: 10.1111/j.1423-0410.1990.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 19.Friedman DF, Lukas MB, Jawad A, et al. Alloimmunization to platelets in heavily transfused patients with sickle cell disease. Blood. 1996;88:3216–3222. [PubMed] [Google Scholar]
- 20.Lo SC, Chang JS, Lin SW, et al. Platelet alloimmunization after long-term red cell transfusion in transfusion-dependent thalassemia patients. Transfusion. 2005;45:761–765. doi: 10.1111/j.1537-2995.2005.04246.x. [DOI] [PubMed] [Google Scholar]
- 21.Buetens O, Shirey RS, Goble-Lee M, et al. Prevalence of HLA antibodies in transfused patients with and without red cell antibodies. Transfusion. 2006;46:754–756. doi: 10.1111/j.1537-2995.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 22.Sirchia G, Rebulla P, Mascaretti L, et al. The clinical importance of leukocyte depletion in regular erythrocyte transfusions. Vox Sang. 1986;51:2–8. doi: 10.1111/j.1423-0410.1986.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 23.Sniecinski I, O’Donnell MR, Nowicki B, et al. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988;71:1402–1407. [PubMed] [Google Scholar]
- 24.Rivera R, Scornik JC. HLA antigens on red cells. Implications for achieving low HLA antigen content in blood transfusions. Transfusion. 1986;26:375–381. doi: 10.1046/j.1537-2995.1986.26486262749.x. [DOI] [PubMed] [Google Scholar]
- 25.Everett ET, Kao KJ, Scornik JC. Class I HLA molecules on human erythrocytes. Quantitation and transfusion effects. Transplantation. 1987;44:123–129. doi: 10.1097/00007890-198707000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Morton JA, Pickles MM, Sutton L. The correlation of the Bga blood group with the HL-47 leucocyte group: Demonstration of antigenic sites on red cells and leucocytes. Vox Sang. 1969;17:536–547. doi: 10.1111/j.1423-0410.1969.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Morton JA, Pickles MM, Sutton L, et al. Identification of further antigens on red cells and lymphocytes. Association of B g b with W17 (Te57) and B g e with W28 (Da15, Ba) Vox Sang. 1971;21:141–153. doi: 10.1111/j.1423-0410.1971.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 28.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 29.McCann SR, Bacigalupo A, Gluckman E, et al. Graft rejection and second bone marrow transplants for acquired aplastic anaemia: A report from the Aplastic Anaemia Working Party of the European Bone Marrow Transplant Group. Bone Marrow Transplant. 1994;13:233–237. [PubMed] [Google Scholar]
- 30.Bray R, Rosen-Bronson S, Haagenson M, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. ASH Annual Meeting Abstracts. 2007;110:475. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPherson M, Anderson A, Jessup P, et al. Transfusion management of sickle cell patients during bone marrow transplantation with matched sibling donor. Transfusion. 2009;49:1977–1986. doi: 10.1111/j.1537-2995.2009.02213.x. [DOI] [PubMed] [Google Scholar]
- 32.Panzer S, Mueller-Eckhardt G, Salama A, et al. The clinical significance of HLA antigens on red cells. Survival studies in HLA-sensitized individuals. Transfusion. 1984;24:486–489. doi: 10.1046/j.1537-2995.1984.24685066806.x. [DOI] [PubMed] [Google Scholar]
- 33.Panzer S, Puchler K, Mayr WR, et al. Haemolytic transfusion reactions due to HLA antibodies. A prospective study combining red-cell serology with investigations of chromium-51-labelled red-cell kinetics. Lancet. 1987;1:474–478. doi: 10.1016/s0140-6736(87)92089-7. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi C, Ohto H, Miura S, et al. Delayed and acute hemolytic transfusion reactions resulting from red cell antibodies and red cell-reactive HLA antibodies. Transfusion. 2005;45:1925–1929. doi: 10.1111/j.1537-2995.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 35.Benson K, Agosti SJ, Latoni-Benedetti GE, et al. Acute and delayed hemolytic transfusion reactions secondary to HLA alloimmunization. Transfusion. 2003;43:753–757. doi: 10.1046/j.1537-2995.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- 36.Hillyer KL, Hare VW, Josephson CD, et al. Partners for life: The transfusion program for patients with sickle cell disease offered at the American Red Cross Blood Services, Southern Region, Atlanta, Georgia. Immunohematology. 2006;22:108–111. [PubMed] [Google Scholar]
- 37.Pei R, Wang G, Tarsitani C, et al. Simultaneous HLA class I and class II antibodies screening with flow cytometry. Hum Immunol. 1998;59:313–322. doi: 10.1016/s0198-8859(98)00020-2. [DOI] [PubMed] [Google Scholar]
- 38.Pei R, Lee JH, Shih NJ, et al. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43–49. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 39.Worthington JE, Robson AJ, Sheldon S, et al. A comparison of enzyme-linked immunoabsorbent assays and flow cytometry techniques for the detection of HLA specific antibodies. Hum Immunol. 2001;62:1178–1184. doi: 10.1016/s0198-8859(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 40.Gebel HM, Bray RA, Ruth JA, et al. Flow PRA to detect clinically relevant HLA antibodies. Transplant Proc. 2001;33:477. doi: 10.1016/s0041-1345(00)02100-x. [DOI] [PubMed] [Google Scholar]
- 41.Pei R, Lee J, Chen T, et al. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum Immunol. 1999;60:1293–1302. doi: 10.1016/s0198-8859(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 42.Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: Contraindication vs. risk. Am J Transplant. 2003;3:1488–1500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 43.Bray RA, Nolen JD, Larsen C, et al. Transplanting the highly sensitized patient: The Emory algorithm. Am J Transplant. 2006;6:2307–2315. doi: 10.1111/j.1600-6143.2006.01521.x. [DOI] [PubMed] [Google Scholar]
- 44.Petz LD. Bystander immune cytolysis. Transfus Med Rev. 2006;20:110–140. doi: 10.1016/j.tmrv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: Implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimring JC, Hendrickson JE. The role of inflammation in alloimmunization to antigens on transfused red blood cells. Curr Opin Hematol. 2008;15:631–635. doi: 10.1097/MOH.0b013e328313695e. [DOI] [PubMed] [Google Scholar]
- 47.Schonewille H, van de Watering LM, Loomans DS, et al. Red blood cell alloantibodies after transfusion: Factors influencing incidence and specificity. Transfusion. 2006;46:250–256. doi: 10.1111/j.1537-2995.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 48.Ware RE. Autoimmune hemolytic anemia. In: Orkin SH, Nathan DG, Ginsburg D, et al., editors. Nathan and Oski’s hematology of infancy and childhood. 7. Philadelphia: Saunders Elsevier; 2009. [Google Scholar]
- 49.Nordhagen R. Association between HLA and red cell antigens. VIII. Haemagglutinins in another series of cytotoxic anti-HLA-A2 sera. Vox Sang. 1978;35:375–377. doi: 10.1111/j.1423-0410.1978.tb02950.x. [DOI] [PubMed] [Google Scholar]
- 50.Nordhagen R, Aas M. Association between HLA and red cell antigens. VII. Survival studies of incompatible red blood cells in a patient with HLA-associated haemagglutinins. Vox Sang. 1978;35:319–323. doi: 10.1111/j.1423-0410.1978.tb02941.x. [DOI] [PubMed] [Google Scholar]
- 51.Giles CM, Darke C, Rowe GP, et al. HLA class I (Bg) antigens on red cells of SLE patients: A serological study with polyclonal and monoclonal antibodies. Vox Sang. 1989;56:254–261. doi: 10.1111/j.1423-0410.1989.tb02038.x. [DOI] [PubMed] [Google Scholar]
- 52.Win N, New H, Lee E, et al. Hyperhemolysis syndrome in sickle cell disease: Case report (recurrent episode) and literature review. Transfusion. 2008;48:1231–1238. doi: 10.1111/j.1537-2995.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 53.Gleichmann H, Breininger J. Over 95 per cent sensitization against allogeneic leukocytes following single massive blood transfusion. Vox Sang. 1975;28:66–73. doi: 10.1111/j.1423-0410.1975.tb02743.x. [DOI] [PubMed] [Google Scholar]


