Abstract
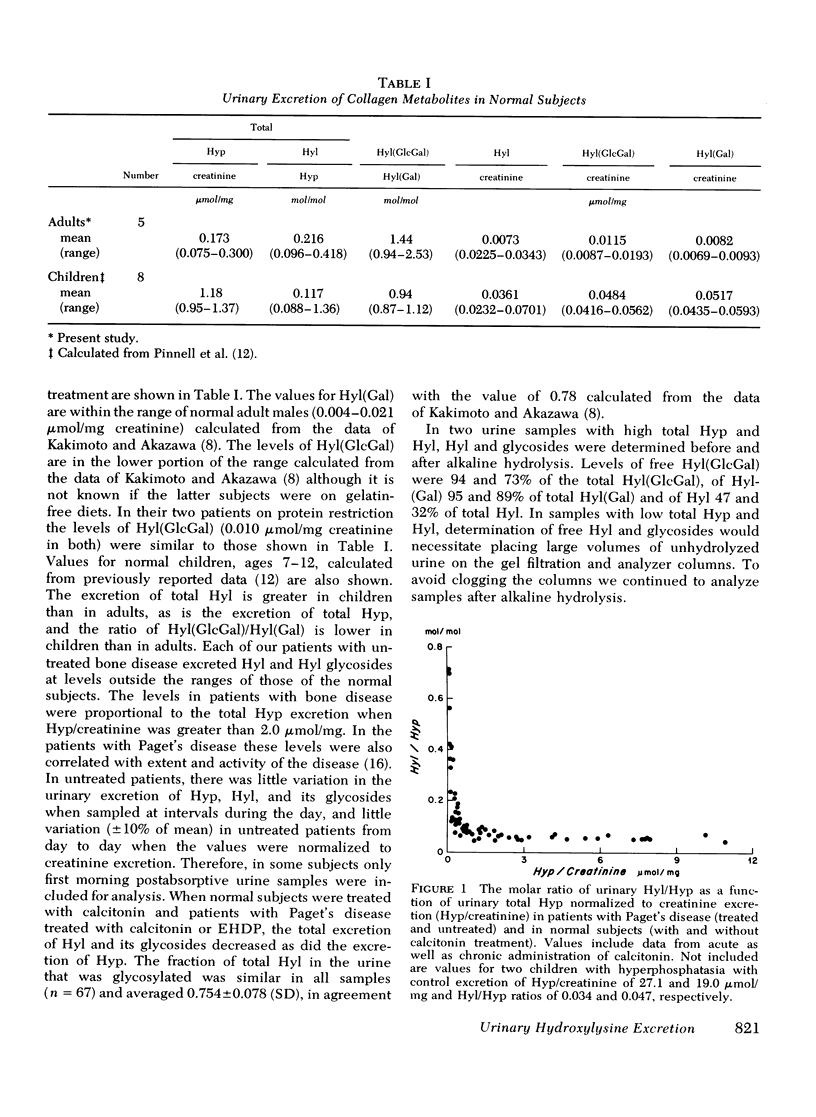
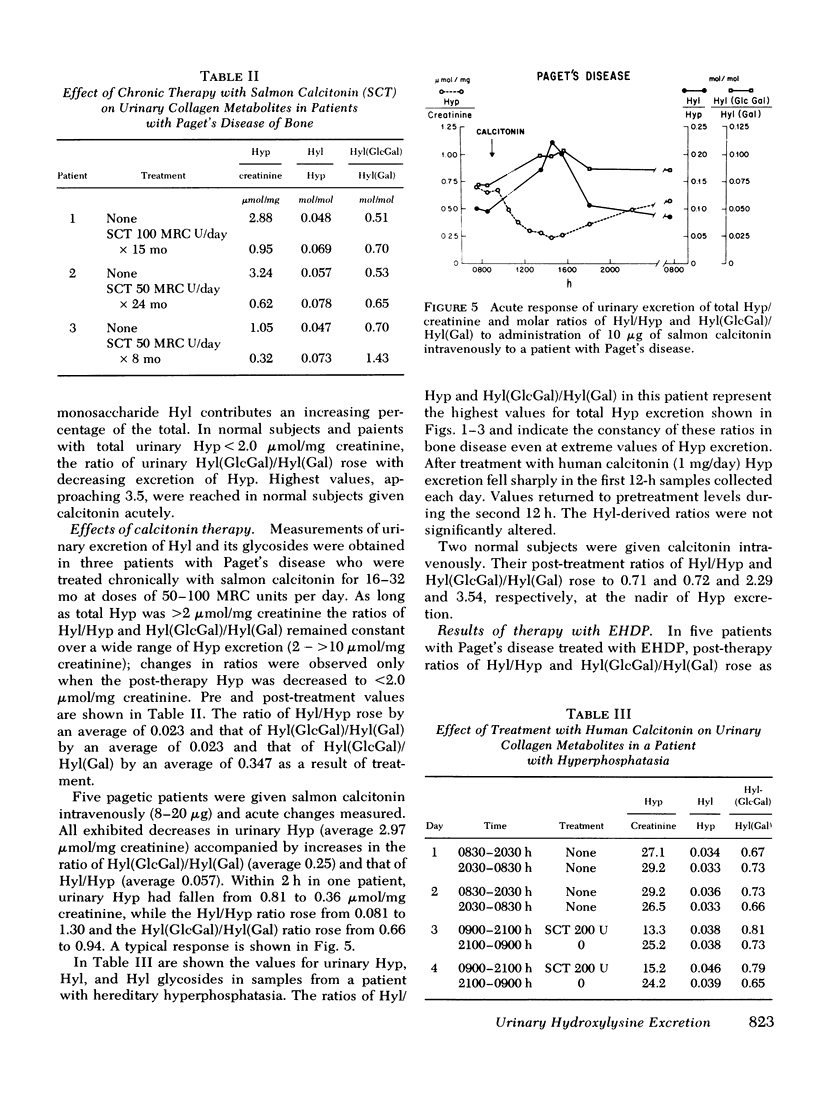
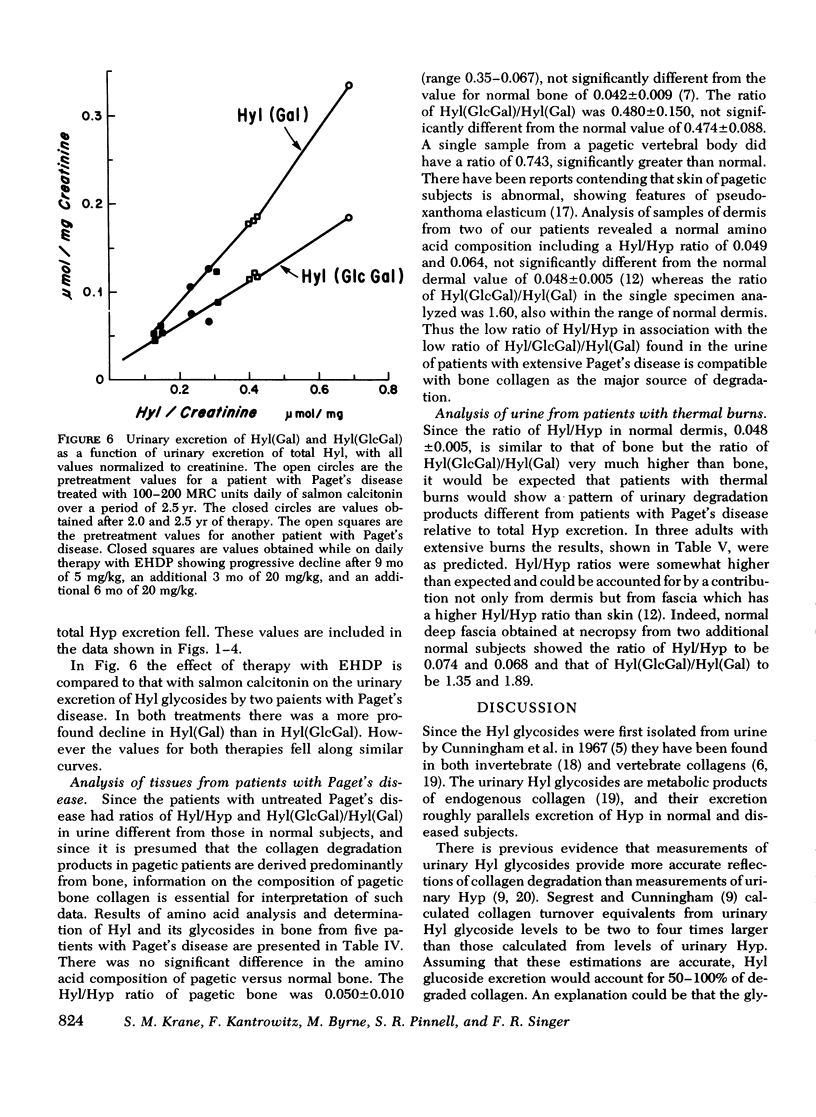
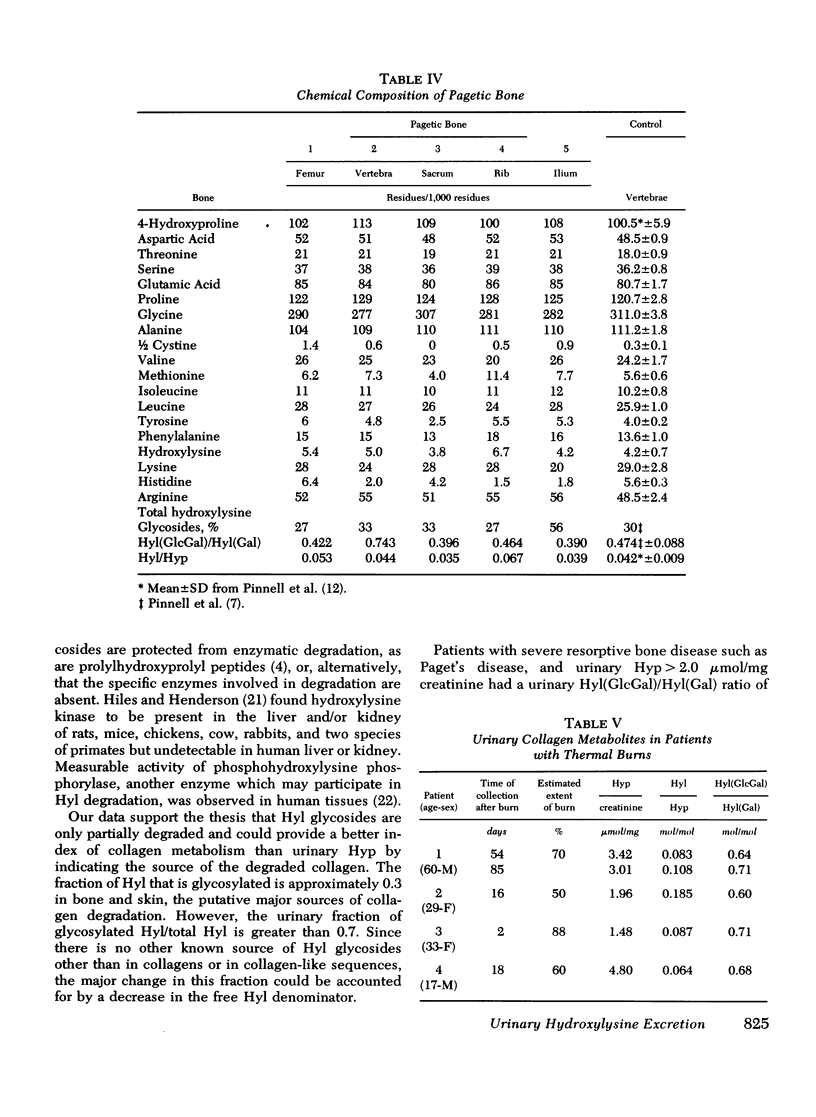
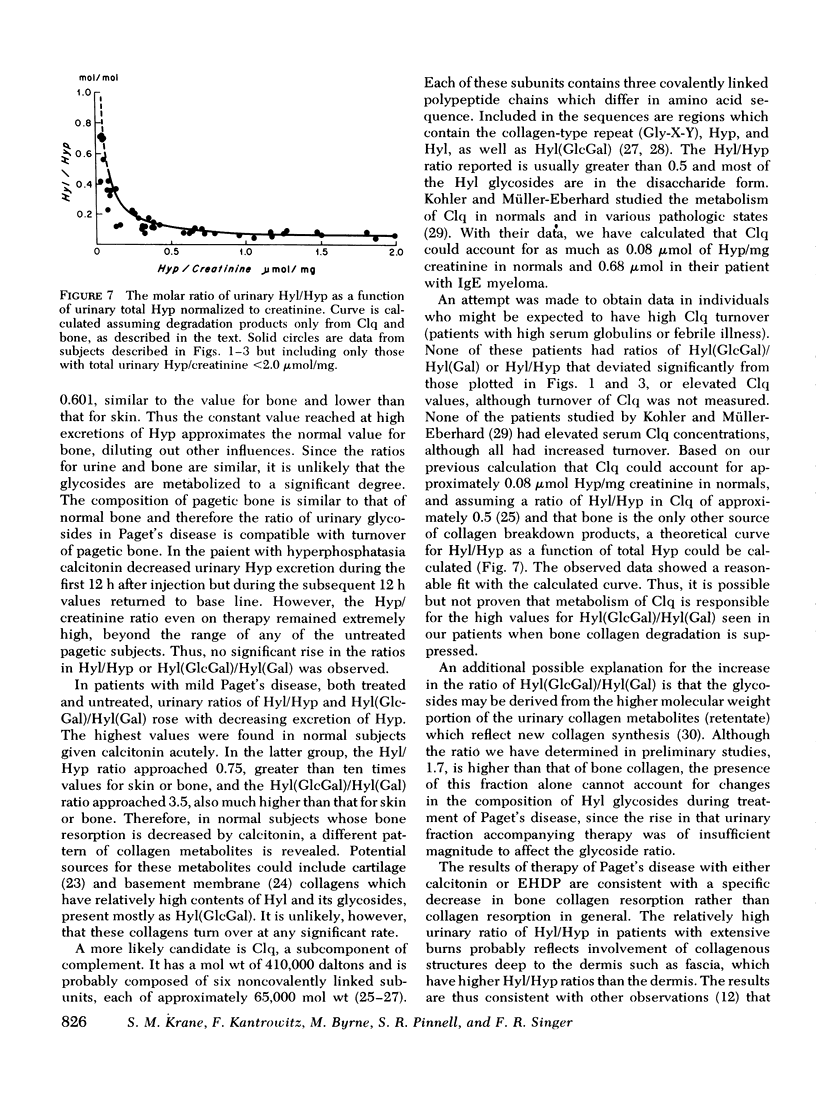
Urimary excretion of hydroxyprolin (Hyp) is one index of total collagen degradation, from all sources. Since some of the Hyp released from collagen may be further metabolized before it is excreted, other markers are necessary to measure collagen breakdown. Excretion of the glycosides of hydroxylysine (Hyl), glucosyl galactosyl hydroxylysine (Hy1[Gl)cGa1]), and galactosyl hydroxylysine (Hyl[Ga)]), more accurately reflects collagen metabolism since these products occur in specificratios in different tissue collagens and are themselves metabolized only to a minor degree. The ratios of total Hy1/Hyp and Hyl(GlcGal)/Hyl(Ga1) were measured in the urine of norma. subjects and of patients with Paget's disease of bone, hyperphosphatasia, and extensive thermal burns. In patients with extensive thermal burns the pattern of urinary Hy1 and its glycosides was consistent with degradation of collagen in dermis and fascia. When bone collagen degradation was dominant, the pattern of urinary metabolites reflected that source. Pagetic bone collagen has an amino acid composition similar to normal bone and Hy1(G1cGa1/Hyl(G1) of 0.396-0.743,vs. normal of 0.474+/-0.088. In untreated patients with severe Paget's disease of bone or hyperphosphatasia (urinary Hyp greater than 2.0 micronmol/mg creatinine) urinary Hyl/Hyp averaged 0.052+/-0.042 (0.042+/-0.009 in normal bone) and Hy1(G1cGa1)/Hy1(Ga1) 0.601+/-0.017 (0.47+/-0.009 in normal bone). When bone resorption was decreased sufficiently with calcitonin or disodium etidronate in these patients, both the urinary ratios of Hy1/Hyp and Hy1(G1cGa1)/Hyl(Gal) rose. In normal subjects treated with calcitonin and excreting relatively little Hyp, the ratio of Hy1/H)P approached 0.7 and Hy1(G1ycGa1)/Hy1(Ga1) approached 3.5. There increased ratios reveal the existence of a source of collagen breakdown other than skin or bone. The first subcompoent of complement, Clq, which has collagen-like sequences, relatively high amounts of Hy1, and most of the glycosylated Hy1 as Hy1(G1cGa1), could be the source of these metabolites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenasi R. Urinary hydroxylysine and hydroxylysyl glycoside excretions in normal and pathologic states. J Lab Clin Med. 1974 Apr;83(4):673–679. [PubMed] [Google Scholar]
- Calcott M. A., Müller-Eberhard H. J. C1q protein of human complement. Biochemistry. 1972 Aug 29;11(18):3443–3450. doi: 10.1021/bi00768a018. [DOI] [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D., Segrest J. P. The isolation of identical hydroxylysyl glycosides from hydrolysates of soluble collagen and from human urine. J Biol Chem. 1967 May 25;242(10):2570–2571. [PubMed] [Google Scholar]
- EFRON M. L., BIXBY E. M., PRYLES C. V. HYDROXYPROLINEMIA. II. A RARE METABOLIC DISEASE DUE TO A DEFICIENCY OF THE ENZYME "HYDROXYPROLINE OXIDASE". N Engl J Med. 1965 Jun 24;272:1299–1309. doi: 10.1056/NEJM196506242722501. [DOI] [PubMed] [Google Scholar]
- Franck W. A., Bress N. M., Singer F. R., Krane S. M. Rheumatic manifestations of Paget's disease of bone. Am J Med. 1974 May;56(5):592–603. doi: 10.1016/0002-9343(74)90629-9. [DOI] [PubMed] [Google Scholar]
- Hammerstedt R. H., Swan P. B., Henderson L. M. Degradation of 5-hydroxylysine in the rat and in the perfused liver. Arch Biochem Biophys. 1968 Oct;128(1):243–251. doi: 10.1016/0003-9861(68)90028-3. [DOI] [PubMed] [Google Scholar]
- Hiles R. A., Henderson L. M. The partial purification and properties of hydroxylysine kinase from rat liver. J Biol Chem. 1972 Feb 10;247(3):646–651. [PubMed] [Google Scholar]
- Kakimoto Y., Akazawa S. Isolation and identification of N-G,N-G- and N-G,N'-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem. 1970 Nov 10;245(21):5751–5758. [PubMed] [Google Scholar]
- Kefalides N. A. Biochemical properties of human glomerular basement membrane in normal and diabetic kidneys. J Clin Invest. 1974 Feb;53(2):403–407. doi: 10.1172/JCI107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. F., Müller-Eberhard H. J. Metabolism of human C1q. Studies in hypogammaglobulinemia, myeloma, and systemic lupus erythematosus. J Clin Invest. 1972 Apr;51(4):868–875. doi: 10.1172/JCI106881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane S. M., Muñoz A. J., Harris E. D., Jr Urinary polypeptides related to collagen synthesis. J Clin Invest. 1970 Apr;49(4):716–729. doi: 10.1172/JCI106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. A review of biochemical studies on the genetically distinct collagens of the skeletal system. Clin Orthop Relat Res. 1973 May;(92):260–280. doi: 10.1097/00003086-197305000-00024. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Piez K. A. An accelerated single-column procedure for the automatic analysis of amino acids in collagen and elastin hydrolyzates. Anal Biochem. 1966 Aug;16(2):320–326. doi: 10.1016/0003-2697(66)90161-8. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Fox R., Krane S. M. Human collagens: differences in glycosylated hydroxylysines in skin and bone. Biochim Biophys Acta. 1971 Jan 19;229(1):119–122. doi: 10.1016/0005-2795(71)90325-4. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Krane S. M., Kenzora J. E., Glimcher M. J. A heritable disorder of connective tissue. Hydroxylysine-deficient collagen disease. N Engl J Med. 1972 May 11;286(19):1013–1020. doi: 10.1056/NEJM197205112861901. [DOI] [PubMed] [Google Scholar]
- Reid K. B. A collagen-like amino acid sequence in a polypeptide chain of human C1q (a subcomponent of the first component of complement). Biochem J. 1974 Jul;141(1):189–203. doi: 10.1042/bj1410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARICCO R. J., PINKUS H. Quantitative and qualitative data on the pigment cells of adult human epidermis. J Invest Dermatol. 1957 Jan;28(1):33–45. doi: 10.1038/jid.1957.4. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Cunningham L. W. Variations in human urinary O-hydroxylysyl glycoside levels and their relationship to collagen metabolism. J Clin Invest. 1970 Aug;49(8):1497–1509. doi: 10.1172/JCI106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Russell R. G., Bishop M. Diphosphonates and Page's disease of bone. Lancet. 1971 May 8;1(7706):945–947. doi: 10.1016/s0140-6736(71)91447-4. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem J. 1962 May;83:304–314. doi: 10.1042/bj0830304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P. H., Klein L. The quantitative relationship of urinary peptide hydroxyproline excretion to collagen degradation. J Clin Invest. 1969 Jan;48(1):1–10. doi: 10.1172/JCI105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M., Niedermeier W., Butler W. T. Chemical studies of Clq; a modulator of immunoglobulin biology. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1388–1394. doi: 10.1016/s0006-291x(71)80028-1. [DOI] [PubMed] [Google Scholar]



