Abstract
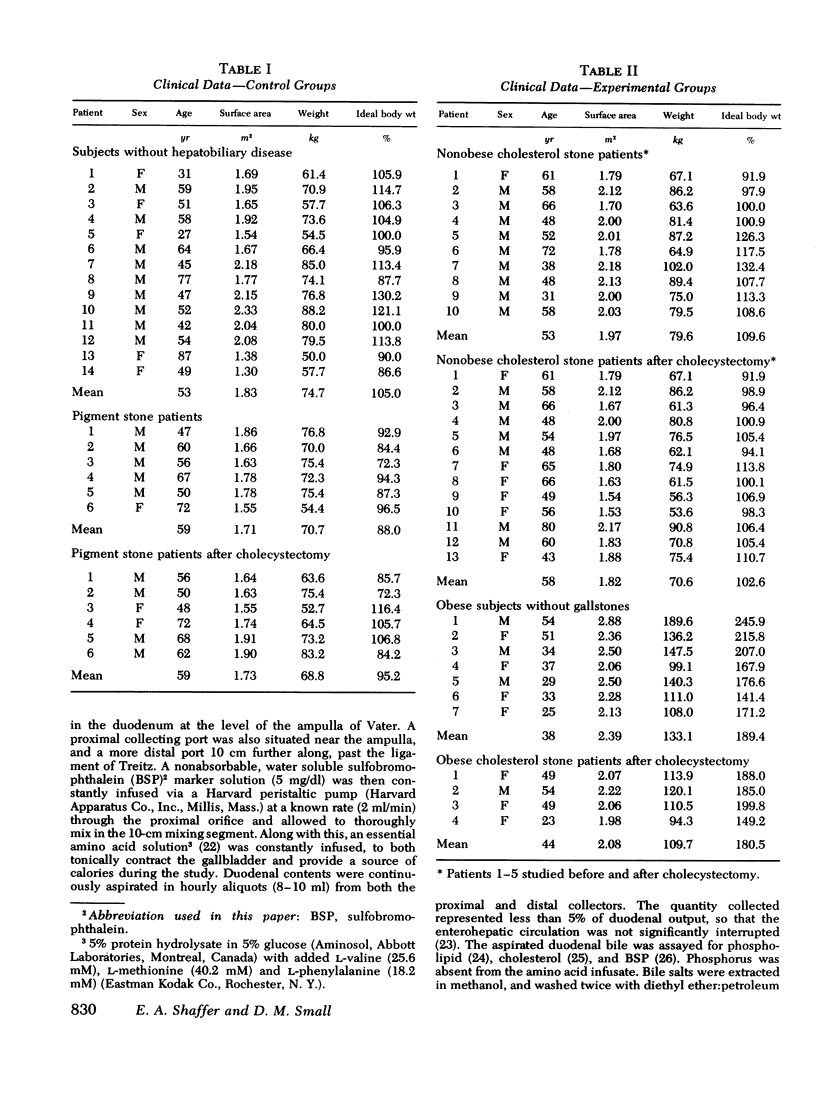
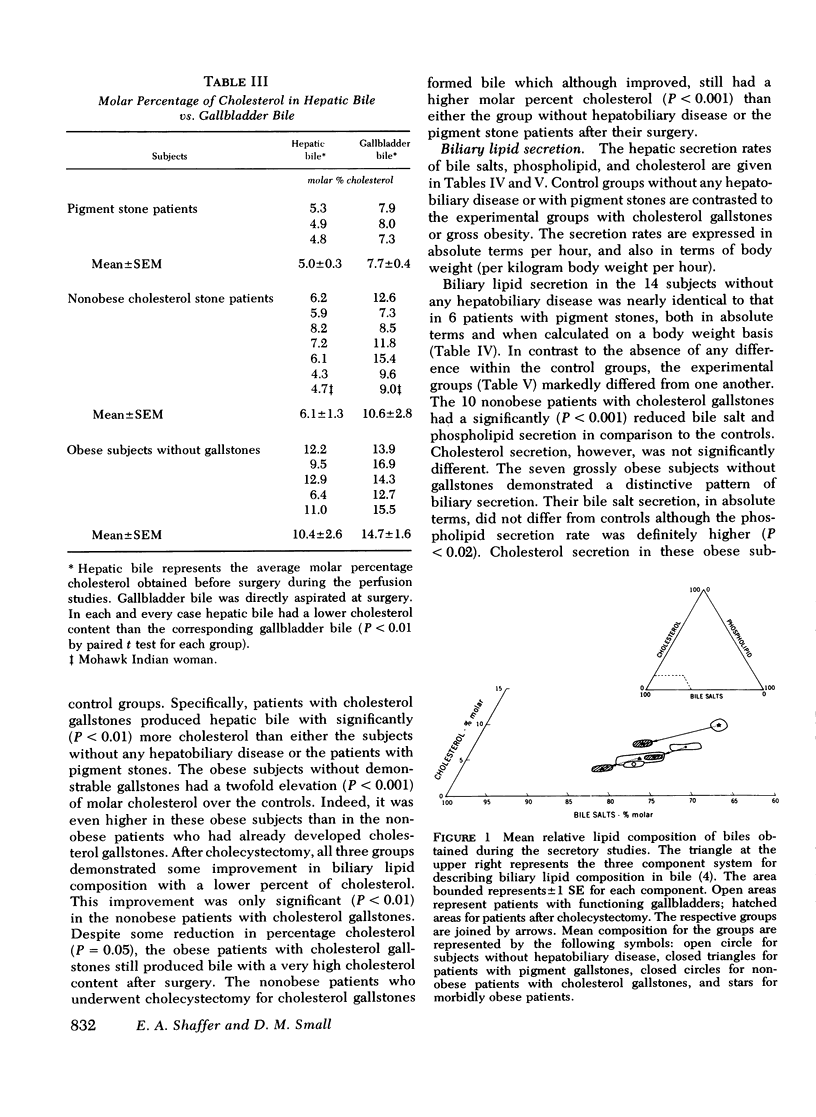
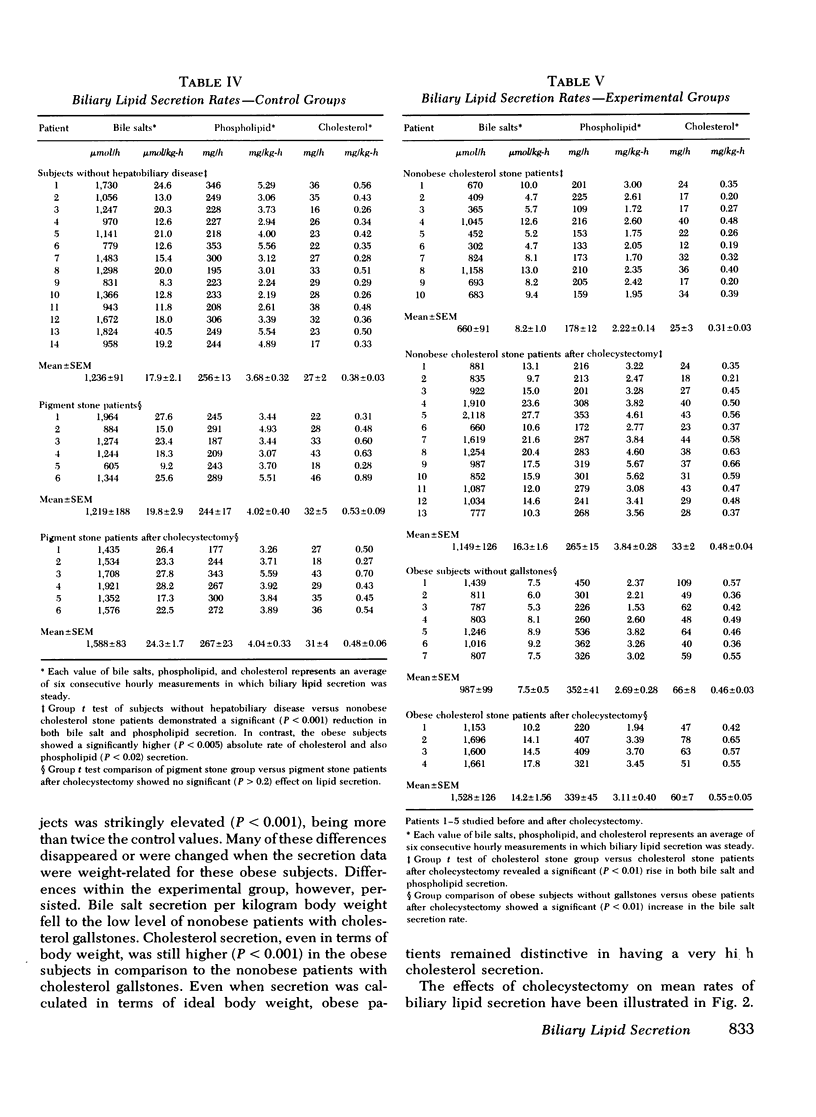
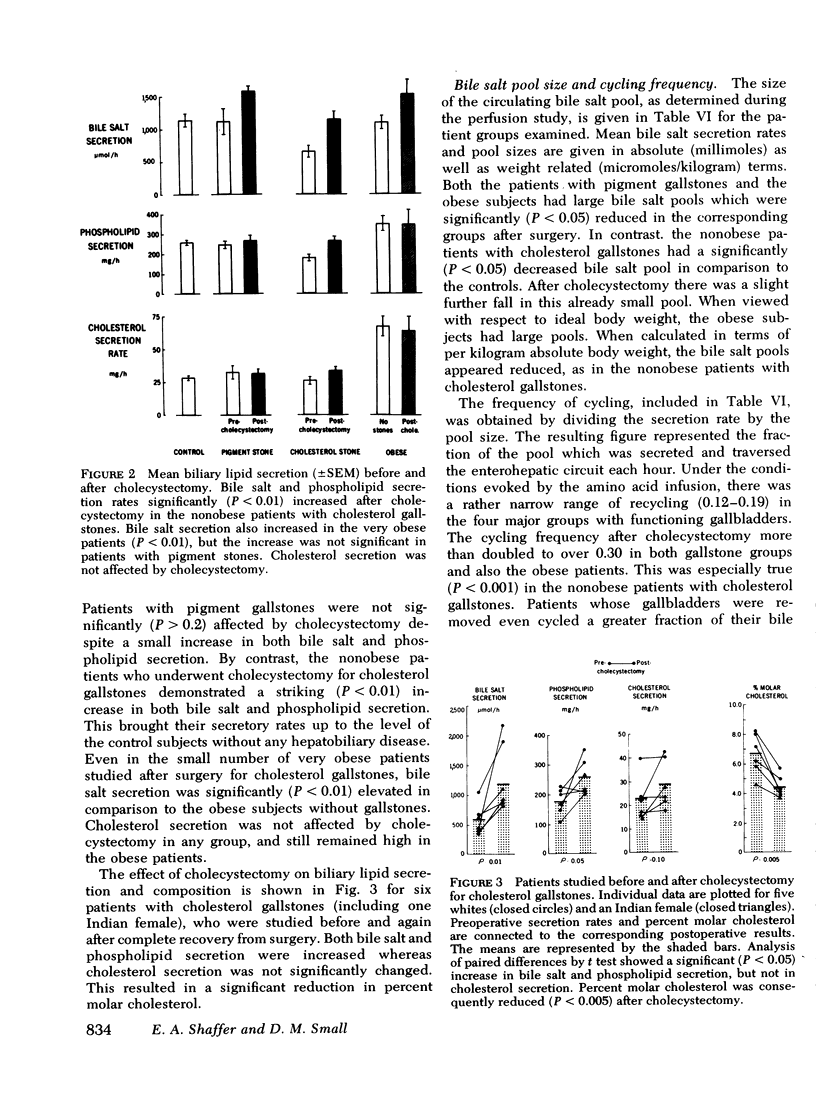
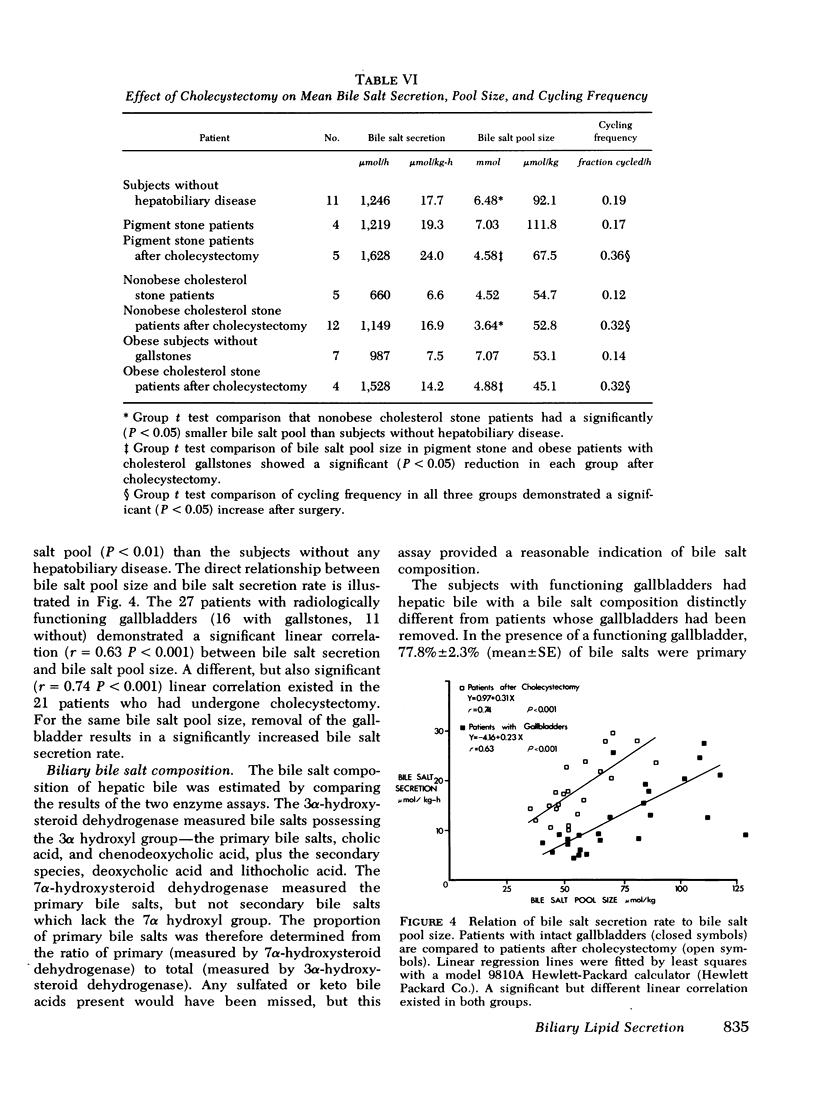
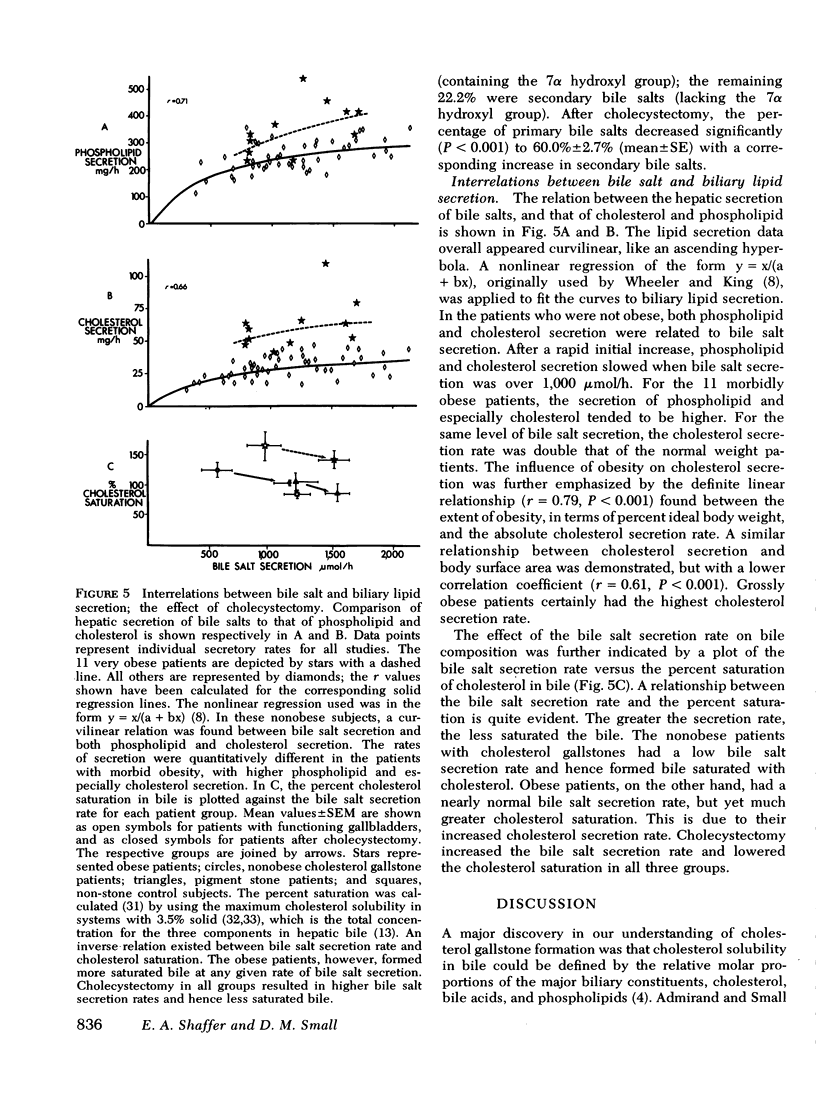
Cholesterol gallstone disease is initiated in a liver which produces abnormal bile with excess cholesterol relative to bile salts and phospholipid. To define the responsible secretory mechanism(s), the rate of biliary lipid secretion was measured by a duodenal marker perfusion technique, while the bile salt pool was simultaneously estimated by isotope dilution. Two groups of control patients expected to have normal biliary lipid composition--14 subjects without hepatobiliary disease and 6 patients with pigment gallstones, were compared to two experimental groups expected to have abnormal bile--10 nonobese patients with cholesterol gallstones and 7 obese subjects without gallstones. Both control groups had nearly identical biliary lipid secretion rates, and a corresponding low relative molar concentration of cholesterol. Two different secretory mechanisms were found to be responsible for the abnormal bile in the experimental groups. In the nonobese patients with cholesterol gallstones, bile salt and phospholipid secretion rates were both significantly reduced. Conversely, the grossly obese subjects had an increased cholesterol secretion. To determine how cholecystectomy improves biliary lipid composition, three groups of gallstone patients --6 with pigment stones, 4 grossly obese with cholesterol stones, and 13 nonobese with cholesterol stones --were all examined after full recovery from surgery. In the nonobese patients with cholesterol gallstones, both bile salt and phospholipid secretion significantly increased, causing a definite improvement in bile composition. Cholecystectomy produced a similar but less marked trend in the obese patients with cholesterol stones, and in the patients with pigment stones. Cholesterol secretion, however, was unaffected by surgery. The bile salt pool was definitely small in the nonobese patients with cholesterol gallstones and became slightly smaller after cholecystectomy. The pool was significantly reduced by cholecystectomyin the patients with cholesterol gallstones. Removal of the gallbladder in all three groups caused a greater fraction of the pool to cycle around the enterohepatic circulation each hour. This more rapid cycling produced the increase in bile salt and phospolipid secretion, and was responsible for the improved composition found after cholecystectomy.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. D., Metzger A. L., Grundy S. M. Biliary lipid secretion before and after cholecystectomy in American Indians with cholesterol gallstones. Gastroenterology. 1974 Jun;66(6):1212–1217. [PubMed] [Google Scholar]
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond H. R., Vlahcevic Z. R., Bell C. C., Jr, Gregory D. H., Swell L. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. N Engl J Med. 1973 Dec 6;289(23):1213–1216. doi: 10.1056/NEJM197312062892302. [DOI] [PubMed] [Google Scholar]
- Antsaklis G., Lewin M. R., Sutor D. J., Cowie A. G., Clark C. G. Gallbladder function, cholesterol stones, and bile composition. Gut. 1975 Dec;16(12):937–942. doi: 10.1136/gut.16.12.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bender S., Sauer H., Hoffmann D. Kinetics of bile acid metabolism in experimental blind loop syndrome. Gut. 1975 Dec;16(12):927–931. doi: 10.1136/gut.16.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion L. J., Grundy S. M. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest. 1975 Oct;56(4):996–1011. doi: 10.1172/JCI108180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman F., van der Linden W. Bile acid pool size in hamsters during gallstone formation and after cholecystectomy. Z Ernahrungswiss. 1974 Mar-Jun;13(1-2):37–42. doi: 10.1007/BF02025021. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Bloomer J. R., Maddrey W. C., Tilson D. Variation in hepatic bile composition following cholecystectomy in patients with previous gallstones. Yale J Biol Med. 1974 Dec;47(4):211–217. [PMC free article] [PubMed] [Google Scholar]
- Cahlin E., Jönsson J., Nilsson S., Scherstén T. Biliary lipid composition in normalipidemic and prebeta hyperlipoproteinemic gallstone patients. Influence of sucrose feeding of the patients on the biliary lipid composition. Scand J Gastroenterol. 1973;8(5):449–456. [PubMed] [Google Scholar]
- Crook J. N., Smith D. C., McAllister R. A., MacKay C. The effect of cholecystectomy on the size of the bile-salt pool in the dog. Br J Surg. 1971 Nov;58(11):867–867. [PubMed] [Google Scholar]
- Dowling R. H., Mack E., Small D. M. Effects of controlled interruption of the enterohepatic circulation of bile salts by biliary diversion and by ileal resection on bile salt secretion, synthesis, and pool size in the rhesus monkey. J Clin Invest. 1970 Feb;49(2):232–242. doi: 10.1172/JCI106232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S. Marker perfusion techniques for measuring intestinal absorption in man. Gastroenterology. 1966 Dec;51(6):1089–1093. [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Pancreozymin bioassay in man based on pancreatic enzyme secretion: potency of specific amino acids and other digestive products. J Clin Invest. 1970 Aug;49(8):1558–1564. doi: 10.1172/JCI106373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. R., Shaffer E. A., Sass-Kortsak A. Bilirubin secretion and conjujation in the Crigler-Najjar syndrome type II. Gastroenterology. 1976 May;70(5 PT1):761–765. [PubMed] [Google Scholar]
- Grundy S. M., Duane W. C., Adler R. D., Aron J. M., Metzger A. L. Biliary lipid outputs in young women with cholesterol gallstones. Metabolism. 1974 Jan;23(1):67–73. doi: 10.1016/0026-0495(74)90105-x. [DOI] [PubMed] [Google Scholar]
- Grundy S. M. Effects of polyunsaturated fats on lipid metabolism in patients with hypertriglyceridemia. J Clin Invest. 1975 Feb;55(2):269–282. doi: 10.1172/JCI107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Metzger A. L. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology. 1972 Jun;62(6):1200–1217. [PubMed] [Google Scholar]
- Grundy S. M., Metzger A. L., Adler R. D. Mechanisms of lithogenic bile formation in American Indian women with cholesterol gallstones. J Clin Invest. 1972 Dec;51(12):3026–3043. doi: 10.1172/JCI107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison W. G., Apter J. T. Micellar theory of biliary cholesterol excretion. Am J Physiol. 1972 Jan;222(1):61–67. doi: 10.1152/ajplegacy.1972.222.1.61. [DOI] [PubMed] [Google Scholar]
- Hepner G. W., Hofmann A. F., Malagelada J. R., Szczepanik P. A., Klein P. D. Increased bacterial degradation of bile acids in cholecystectomized patients. Gastroenterology. 1974 Apr;66(4):556–564. [PubMed] [Google Scholar]
- Hoffman N. E., Iser J. H., Smallwood R. A. Hepatic bile acid transport: effect of conjugation and position of hydroxyl groups. Am J Physiol. 1975 Aug;229(2):298–302. doi: 10.1152/ajplegacy.1975.229.2.298. [DOI] [PubMed] [Google Scholar]
- Kimball A., Pertsemlidis D., Panveliwalla D. Composition of biliary lipids and kinetics of bile acids after cholecystectomy in man. Am J Dig Dis. 1976 Sep;21(9):776–781. doi: 10.1007/BF01073029. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Williams C. N., Mahony D. E. A 3 alpha- and 7 alpha-hydroxysteroid dehydrogenase assay for conjugated dihydroxy-bile acid mixtures. Anal Biochem. 1974 Jan;57(1):127–136. doi: 10.1016/0003-2697(74)90059-1. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A. Cholesterol production in obesity. Circulation. 1971 Nov;44(5):842–850. doi: 10.1161/01.cir.44.5.842. [DOI] [PubMed] [Google Scholar]
- Nakayama F., van der Linden W. Role of gallbladder in gallstone formation. Acta Chir Scand. 1974;140(1):45–49. [PubMed] [Google Scholar]
- Nestel P. J., Schreibman P. H., Ahrens E. H., Jr Cholesterol metabolism in human obesity. J Clin Invest. 1973 Oct;52(10):2389–2397. doi: 10.1172/JCI107428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S., Scherstén T. Importance of bile acids for phospholipid secretion into human hepatic bile. Gastroenterology. 1969 Nov;57(5):525–532. [PubMed] [Google Scholar]
- Northfield T. C., Hofmann A. F. Biliary lipid output during three meals and an overnight fast. I. Relationship to bile acid pool size and cholesterol saturation of bile in gallstone and control subjects. Gut. 1975 Jan;16(1):1–11. doi: 10.1136/gut.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. H. Editorial: The gallbladder and bile composition. Am J Dig Dis. 1976 Sep;21(9):795–796. doi: 10.1007/BF01073033. [DOI] [PubMed] [Google Scholar]
- Redinger R. N., Small D. M. Bile composition, bile salt metabolism and gallstones. Arch Intern Med. 1972 Oct;130(4):618–630. [PubMed] [Google Scholar]
- Redinger R. N. The effect of loss of gallbladder function on biliary lipid composition in subjects with cholesterol gallstones. Gastroenterology. 1976 Sep;71(3):470–474. [PubMed] [Google Scholar]
- SELIGSON D., MARINO J., DODSON E. Determination of sulfobromophthalein in serum. Clin Chem. 1957 Oct;3(5):638–645. [PubMed] [Google Scholar]
- Schreibman P. H., Dell R. B. Human adipocyte cholesterol. Concentration, localization, synthesis, and turnover. J Clin Invest. 1975 May;55(5):986–993. doi: 10.1172/JCI108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer E. A., Braasch J. W., Small D. M. Bile composition at and after surgery in normal persons and patients with gallstones. Influence of cholecystectomy. N Engl J Med. 1972 Dec 28;287(26):1317–1322. doi: 10.1056/NEJM197212282872603. [DOI] [PubMed] [Google Scholar]
- Shaffer E. A., Small D. M. Gallstone disease: pathogenesis and management. Curr Probl Surg. 1976 Jul;13(7):3–72. doi: 10.1016/s0011-3840(76)80004-4. [DOI] [PubMed] [Google Scholar]
- Simmons F., Ross A. P., Bouchier I. A. Alterations in hepatic bile composition after cholecystectomy. Gastroenterology. 1972 Sep;63(3):466–471. [PubMed] [Google Scholar]
- Small D. M., Dowling R. H., Redinger R. N. The enterohepatic circulation of bile salts. Arch Intern Med. 1972 Oct;130(4):552–573. [PubMed] [Google Scholar]
- Small D. M., Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970 Jul 9;283(2):53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- Smallwood R. A., Jablonski P., Watts J. M. Intermittent secretion of abnormal bile in patients with cholesterol gall stones. Br Med J. 1972 Nov 4;4(5835):263–266. doi: 10.1136/bmj.4.5835.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway R. D., Schoenfield L. J. Effects of meals and interruption of enterohepatic circulation on flow, lipid composition, and cholesterol saturation of bile in man after cholecystectomy. Am J Dig Dis. 1975 Feb;20(2):99–109. doi: 10.1007/BF01072334. [DOI] [PubMed] [Google Scholar]
- Swell L., Bell C. C., Jr, Vlahcevic Z. R. Relationship of bile acid pool size to biliary lipid excretion and the formation of lithogenic bile in man. Gastroenterology. 1971 Nov;61(5):716–722. [PubMed] [Google Scholar]
- Trotman B. W., Ostrow J. D., Soloway R. D. Pigment vs cholesterol cholelithiasis: comparison of stone and bile composition. Am J Dig Dis. 1974 Jul;19(7):585–590. doi: 10.1007/BF01073011. [DOI] [PubMed] [Google Scholar]
- Trotman B. W., Petrella E. J., Soloway R. D., Sanchez H. M., Morris T. A., 3rd, Miller W. T. Evaluation of radiographic lucency or opaqueness of gallstones as a means of identifying cholesterol or pigment stones. Correlation of lucency or opaqueness with calcium and mineral. Gastroenterology. 1975 Jun;68(6):1563–1566. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Buhac I., Farrar J. T., Swell L. Diminished bile acid pool size in patients with gallstones. Gastroenterology. 1970 Aug;59(2):165–173. [PubMed] [Google Scholar]
- Wagner C. I., Trotman B. W., Soloway R. D. Kinetic analysis of biliary lipid excretion in man and dog. J Clin Invest. 1976 Feb;57(2):473–477. doi: 10.1172/JCI108299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H. O., King K. K. Biliary excretion of lecithin and cholesterol in the dog. J Clin Invest. 1972 Jun;51(6):1337–1350. doi: 10.1172/JCI106930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden W., Nakayama F. Occurrence of cholesterol crystals in human bile. Gut. 1974 Aug;15(8):630–635. doi: 10.1136/gut.15.8.630. [DOI] [PMC free article] [PubMed] [Google Scholar]



