Abstract
In this paper we provide a detailed description of an experimental method for investigating the induction and resolution of recall immune response to antigen in humans in vivo. This involves the injection of tuberculin purified protein derivative (PPD) into the skin, followed by inducing suction blisters at the site of injection, from which leucocytes and cytokines that are involved in the response can be isolated and characterized. Using this technique we found that although the majority of CD4+ T cells in the skin that are present early in the response express cutaneous lymphocyte antigen (CLA), the expression of this marker is reduced significantly in later phases. This may enable these cells to leave the skin during immune resolution. Furthermore, interleukin (IL)-2 production can be detected both in CD4+ T cells and also in the blister fluid at the peak of the response at day 7, indicating that mediators found in the blister fluid are representative of the cytokine microenvironment in vivo. Finally, we found that older humans have defective ability to respond to cutaneous PPD challenge, but this does not reflect a global immune deficit as they have similar numbers of circulating functional PPD-specific CD4+ T cells as young subjects. The use of the blister technology enables further characterization of the skin specific defect in older humans and also general mechanisms that govern immune regulation in vivo.
Keywords: ageing, DTH, human immunity
Introduction
Animal models have provided important insight into immune mechanisms in health and disease; however, the results obtained are often not reproducible in humans due to species-specific differences 1,2. It is therefore essential to develop new technology and experimental systems for investigating immunity in humans in vivo. Obvious barriers to this ambition are ethical constraints relating to in vivo manipulation of leucocytes, which dictates that most human studies are dependent upon the use of leucocytes isolated from the peripheral blood. However, investigation of the function of these cells in vitro loses the tissue-specific context within which immune responses operate. This, together with the possibility that leucocytes that are activated in cell culture, may behave differently to those that are activated in vivo, limits the relevance of data that are generated. Furthermore, it has been very difficult to investigate the kinetics of a human immune response from initiation to resolution in vivo, and most studies rely upon investigating snapshot changes of leucocyte phenotype or function after a response has been initiated 3.
The lack of a robust human experimental system prompted us to develop technology that allowed the study of the initiation and resolution phases of an antigen-specific CD4+ memory T cell recall response in vivo 4–7. To achieve this we injected intradermally one of three different clinical-grade antigens, namely tuberculin purified protein derivative (PPD), Candida albicans antigens (Candin) or varicella zoster virus (VZV) antigens 5–7. The kinetics of cellular infiltration is assessed by performing punch biopsies at the site of the cutaneous immune response at different times after antigenic challenge that are analysed subsequently by immunohistology 4–6. Furthermore, to analyse the function of the cells and cytokines that were present during the course of the response, we created suction blisters at the site of antigen injection, from which leucocytes and soluble mediators that were involved could be isolated and analysed 4,5,8. This experimental system has provided data on the kinetics of specific effector and regulatory T cell (Treg) infiltration into the skin and how this is altered during ageing 4,5,7,8.
In this paper we present a detailed critique of the suction blister technique, including the relationship between size of the response and the number of cells that are recovered and the assessment of possible contamination with blood leucocyte populations. We also demonstrate that the previously described decreased cutaneous immune response to PPD in older subjects is not representative of a global defect of immunity during ageing, as there are comparable numbers of interferon (IFN)-γ-secreting antigen-specific CD4+ T cells in the blood in both young and older subjects. This skin-specific decrease in immunity during ageing may, in part, explain the increased incidence of cutaneous malignancy and infection in older subjects 9,10. The methods for investigating skin immunity that we describe in this paper will facilitate understanding of the age-associated defect in immunity in humans in vivo.
Materials and methods
Human volunteers
This work was approved by the Ethics Committee of the Royal Free Hospital. Healthy young individuals below the age of 40 years and older individuals aged more than 70 years were recruited for the study. Exclusion criteria based on a modified version of the SENIEUR (SENIorEURopean) protocol were employed to recruit older people in order to reduce confounding factors due to associated significant co-morbidity 11. All volunteers provided written informed consent and study procedures were performed in accordance with the principles of the Declaration of Helsinki.
Mantoux test
Mantoux tests were performed on the volar aspect of each forearm by the intradermal injection of tuberculin PPD (Evans Vaccines Ltd, Liverpool, UK) 0·1 ml 100 units/ml 5. Baseline skin erythema was measured using a DermaSpectrometer (Cortex Technology, Hadsund, Denmark) (Fig. S1). The mean of three measurements was recorded. Skin erythema index (EI), size of induration and palpability were also recorded 3 days after MT induction and at the time of sampling (if different). The change in skin EI was calculated by subtracting the baseline from the MT measurement. The induration size was determined by calculating the mean of two measured perpendicular planes. The change in erythema index, size of induration and palpability were scored and the sum of these scores were then used to give an overall clinical score at 3 days and the time of sampling (example shown in Table 1). Non-responding individuals were defined as having a clinical score of less than 1 at 3 days. The experimental protocol involved either raising a skin suction blister over the Mantoux test or taking a 5-mm punch biopsy. Samples were collected between 2 and 19 days after Mantoux test induction during the induction, peak or resolution phases of the immune response. Each volunteer was allotted to a specific sample time-point.
Table 1.
Assessment of the Mantoux test (MT) clinical score
 |
An example showing how induration diameter, change in erythema index and palpability were scored and added together to give an overall clinical score on day 3 post-MT induction.
Suction blister induction
Skin suction blister induction involves separating the epidermis from the dermis at the lamina lucida by the application of prolonged negative pressure using a VP25 Eschmann suction pump (Fig. S2a) that is attached to a suction cup that is placed on the site of injection (Fig. S2b). These suction chambers were constructed by the Medical Engineering Department of the Royal Free Hospital, UK as described (Fig. S2c). Suction blisters were raised over normal or PPD-injected skin 18–24 h prior to sampling (Fig. 1a,b). The MT induration size determined the aperture of the suction cup template used, i.e. 15-mm diameter template if >15 mm induration, 12·5-mm diameter template if 10–15 mm and 10-mm diameter template if <10 mm. A negative pressure of 25–40 kPa (200–300 mmHg) below atmospheric pressure was applied to the skin for 2–4 h using a clinical suction pump (VP25; Eschmann, Lancing, UK; Fig. S1). The procedure was performed at warm room temperature (∼22°C) until a single unilocular blister was formed (Fig. 1c). The blister was then protected overnight with a rigid adhesive dressing assembled using Comfeel Plus Ulcer Dressing (Coloplast Ltd, Peterborough, UK; Fig. 1d), a Universal top (Sterilin, Fisher Scientific UK Ltd, Loughborough, UK; Fig. 1e), Micropore tape (3M Healthcare, Loughborough, UK; Fig. 1f,g) and Tubigrip bandaging (Seton Healthcare Group PLC, Oldham, UK; Fig. 1h).
Fig. 1.
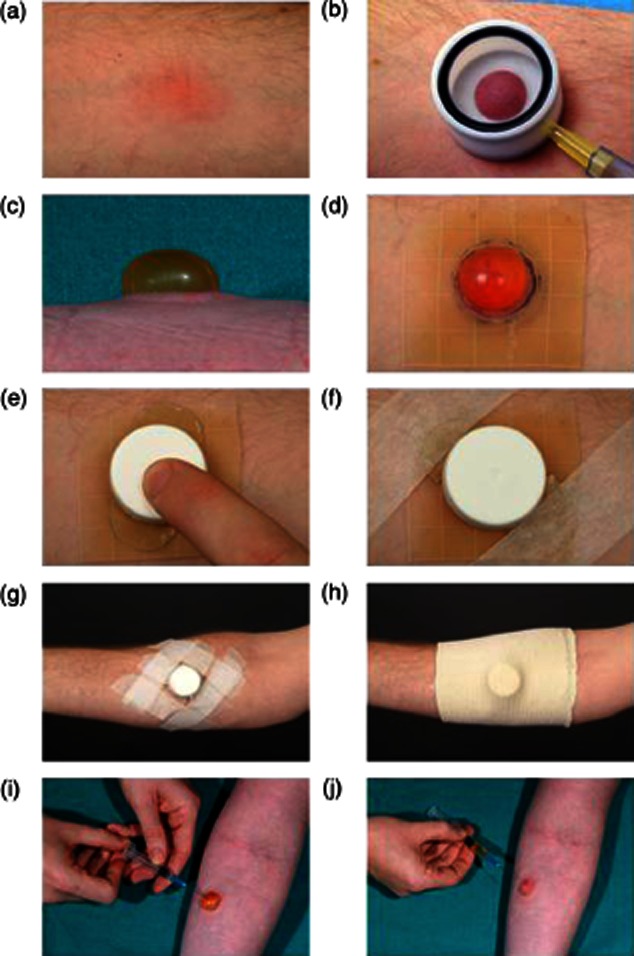
Practical aspects of skin suction blister induction. (a) Skin suction blisters were raised on Mantoux tests or normal skin 18–24 h prior to sampling. (b) A suction cup was centred on the Mantoux test and a negative pressure of 25–40 kPa (200–300 mmHg) below atmospheric pressure was applied to the skin until (c) a unilocular blister was formed. The blister was dressed with (d) a 5 × 5-cm piece of Comfeel Plus ulcer dressing, (e) a trimmed universal container top, (f,g) Micropore tape and (h) a Tubigrip bandage. (i–j) Blister fluid was aspirated the following day using a sterile 23-G needle and 2-ml syringe.
Blister fluid was aspirated using a sterile 23-G needle and 2-ml syringe (Tyco Healthcare UK Ltd, Gosport, UK; Fig. 1i,j). The volume of fluid recovered was recorded and suspended in 1·5-ml conical microtubes (Alpha Laboratories Ltd, Eastleigh, UK). The aspirated blister was dressed with Betadine dry powder spray (Seton Healthcare Group PLC) and a Mepore dressing (Mölnlycke Health Care Ltd, Dunstable, UK). Volunteers were advised to keep this covered and dry for 24–48 h before leaving open to the air. Suction cups were dismantled and disinfected in Barrycidal 36 for at least 24 h after use (Heraeus Instruments Ltd, Brentwood, Essex, UK).
Suction blister cell isolation
The blister fluid was microcentrifuged at 650 g for 4 min (MicroCentaur, MSE Sanyo, London, UK) to pellet the cellular contents. The supernatant was removed and stored in cryogenic tubes (Nalge Europe Ltd, Hereford, UK) at −70°C. The blister cell pellet was then resuspended in 1 ml RPMI-1640 (Gibco, BRL Life Technologies, Paisley, UK) containing 10% human serum supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine (all from Sigma-Aldrich, Gillingham, Dorset, UK) until analysed.
Estimation of blister cell peripheral blood contamination
Optimization of the skin suction blister technique was carried out on normal non-inflamed skin. Data relating to parameters measured during the collection of blister fluid from these experiments are shown in Table 2. Blister red and white blood cell numbers were quantified using a haemocytometer and viability was assessed by trypan blue exclusion. An estimation of the percentage of peripheral blood (PB) white cells that were contaminating the blister leucocytes was performed. The skin suction blister technique causes relatively little trauma, especially with small blisters on non-inflamed skin that are harvested immediately 12,13. Our intention was to induce suction blisters on inflamed skin to obtain as many skin leucocytes as possible, and this meant delaying the aspiration of the blister fluid for up to 24 h. The presence of red blood cells (RBC) in blister fluid points to haemorrhage from the underlying dermal blood vessels. This may allow peripheral blood leucocytes to enter the suction blister directly, independent of the selective recruitment of leucocytes across cutaneous post-capillary venules. Previous studies that examined lymphocytes isolated from delayed-type hypersensitivity responses using skin suction blisters have not monitored RBC contamination routinely 14–17. The total number of red and white cells in the aspirated fluid of each blister was quantified using a haemocytometer and the absolute number of PB RBC and white blood cell (WBC) counts were measured by the Department of Haematology, Royal Free Hospital. Because there are 1000-fold more RBC than WBC in the peripheral blood of healthy volunteers, for every 1000 RBC in the blister fluid we can estimate that there would be one contaminating peripheral blood WBC that was not part of the underlying cutaneous inflammatory infiltrate. The estimate was calculated as follows:
Table 2.
Parameters measured on skin suction blisters induced on normal control skin
| Blister diameter (mm) | Volume blister fluid (μl) | Number of RBC | Number of WBC | RBC/μl blister fluid | WBC/μl blister fluid | % PB WBC contaminated | |
|---|---|---|---|---|---|---|---|
| 1 | 7·5 | 50 | 3000 | 12 600 | 60 | 252 | 0·02 |
| 2 | 10 | 150 | 16 800 | 31 200 | 112 | 208 | 0·05 |
| 3 | 11 | 200 | 8000 | 217 500 | 40 | 1088 | 0·004 |
| 4 | 11·5 | 350 | 14 400 | 40 800 | 41 | 117 | 0·04 |
| 5 | 11·5 | 250 | 20 800 | 134 400 | 83 | 538 | 0·02 |
| 6 | 12 | 400 | 0 | 3600 | 0 | 9 | 0 |
| 7 | 13·5 | 700 | 17 500 | 51 300 | 25 | 73 | 0·03 |
| 8 | 12·5 | 350 | 4200 | 21 600 | 12 | 62 | 0·02 |
| 9 | 15·5 | 750 | 46 200 | 98 400 | 62 | 131 | 0·05 |
| 10 | 10 | 250 | 11 000 | 13 000 | 44 | 52 | 0·09 |
| 11 | 14·5 | 750 | 57 000 | 72 000 | 76 | 96 | 0·08 |
| 12 | 12 | 250 | 600 | 10 200 | 2 | 41 | 0·006 |
| 13 | 13 | 500 | 0 | 5500 | 0 | 11 | 0 |
| Mean | 11·88 | 380·77 | 15 346 | 54 777 | 42 | 206 | 0·03 |
| s.d. | 2·07 | 231 | 17 707 | 62 876 | 35 | 300 | 0·03 |
The diameter and volume of fluid recovered from each blister (numbered 1–13) were measured. The total blister leucocyte and red cell count was determined using a haemocytometer. Peripheral blood red blood cell (RBC) and white blood cell (WBC) counts were also measured. This allowed an estimate of the percentage of peripheral blood leucocytes contaminating the blister due to micro-haemorrhages to be made. PB: peripheral blood; s.d.: standard deviation.
 |
The mean number of leucocytes isolated per blister raised on normal skin was 54 777 s.d. ± 62 876 (range 3600–217 500) (Table 2). The number of red blood cells isolated per blister was consistently lower than the number of leucocytes [mean 15 346 s.d. ± 17 707 (range 0–57 000)]. Overall, this meant that the estimated percentage of contaminating PB WBC in the blister leucocytes was very low in suction blisters raised on normal non-inflamed skin [mean 0·03% s.d. ± 0·03 (range 0–0·09)] (Table 2). Additionally, leucocyte viability as assessed by trypan blue exclusion was greater than 95%.
We also analysed the effect of clinical responsiveness to the MT on cell recovery. The data obtained from the day 7 MT volunteers are presented as a representative example. Larger clinical responses generally allowed bigger suction blisters to be induced (Fig. 2a) (r = 0·44, P = 0·015). Against this background, a trend of increasing numbers of leucocytes isolated from suction blisters was observed with increasing clinical responsiveness (r = 0·49, P = 0·0056, Fig. 2b). Despite this trend, a larger clinical response did not necessarily ensure the isolation of a large number of leucocytes. When the number of leucocytes isolated per microlitre of blister fluid was analysed to control for blister size the correlation with clinical response was less predictive (r = 0·27, P = 0·16, Fig. 2c). Overall, however, larger MT responses generally resulted in more leucocytes being isolated.
Fig. 2.
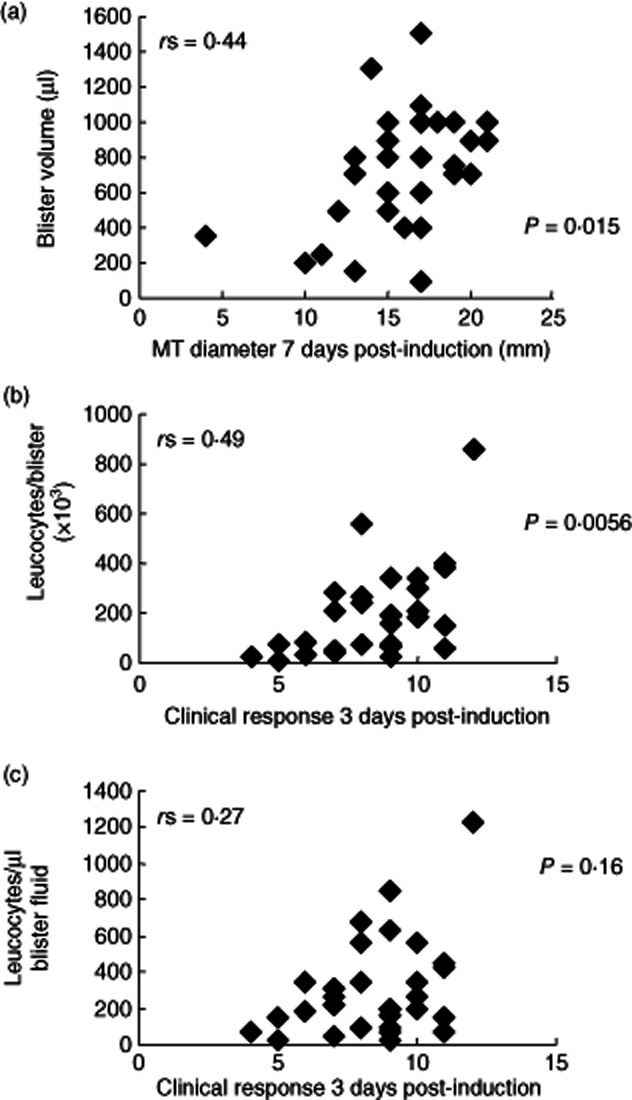
Blister volumes and leucocyte numbers isolated from skin suction blisters raised over Mantoux tests (MT) 7 days after induction. Figures show data collected from 30 skin suction blisters raised on day 7 following MT induction. (a) Blister fluid volume aspirated compared to MT induration diameter (mm) at day 7. (b) Number of leucocytes isolated from skin suction blisters at day 7 compared to the clinical response measured at day 3. (c) Number of leucocytes isolated per microlitre (μl) of skin suction blister fluid at day 7 compared to the clinical response measured at day 3. The Spearman's rank correlation (r) and P-values are shown for each graph.
PBMC preparation and proliferation experiments
Heparinized blood was collected from young and old volunteers at the time of blister aspiration or as specified. Peripheral blood mononuclear cells (PBMCs) were prepared by density centrifugation on Ficoll-Paque (Amersham Biosciences UK Ltd, Chalfont St Giles, UK) and resuspended in complete medium. For the measurement of cellular proliferation by [3H]-thymidine incorporation, PBMCs were added to 96-well round-bottomed tissue culture plates (Falcon; Becton Dickinson Labware, Oxford, UK) at a concentration of 105 cells/well. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 5 days before adding [3H]-thymidine (Amersham Biosciences UK Ltd). The mean of triplicate wells was calculated and used for the purpose of data analysis.
Flow cytometric analysis
Four-parameter analysis of blister and blood T cell phenotypes was performed on a fluorescence activated cell sorter (FACS)Calibur™ (Becton Dickinson). PBMCs and blister cells were stained with antibodies to CD3, CD4, CLA, CD45RA, CD69 or Ki67 (all from BD Pharmingen). Appropriate isotype controls were used. For Ki67 staining, cells were first fixed and permeabilized using the Caltag Fix & Perm® Cell Permeabilization Kit (Caltag Laboratories, Burlingame, CA, USA). To compare CD4 cell numbers in the suction blisters we used Trucount tubes (Becton Dickinson) in combination with staining for CD3, CD4 and CD8 (Multitest; Becton Dickinson).
Antigen-specific CD4+ T cells were identified by intracellular accumulation of effector cytokines following short-term in-vitro stimulation with antigen in the presence of brefeldin A, as described 8. Following surface staining, cells were fixed and permeabilized using a modified protocol of the Caltag Fix & Perm® Cell Permeabilization Kit (Caltag Laboratories), as described 5,7. Unstimulated PBMC and blister cell controls were undertaken to determine background staining. Isotype-matched negative controls in conjunction with the unstimulated controls were used to verify the staining specificity and as a guide for setting markers to delineate positive and negative populations. Purified tetanus toxoid (Aventis Pasteur MSD Ltd, Maidenhead, UK) was used as a control antigen at a final dilution of 1 : 1000. Gating was set on the live lymphocyte population using forward- and side-scatter profiles to include lymphocytic blasts.
Multiple cytokine assay by multiplex bead immunoassay
The multiplex assay of cytokines and chemokines in blister fluid was performed using Luminex 100 and cytokine Beadlyte assay kit (both from Upstate Biotechnology, Lake Placid, NY, USA), as described previously 7.
Statistics
Statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Dr Richard Morris, Department of General Practice and Population Sciences, Royal Free and University College Medical School provided statistical advice. Non-parametric tests were used predominantly. The Kruskal–Wallis test was used to compare three or more unpaired groups and the Mann–Whitney U-test was used when comparing only two unpaired groups. The Wilcoxon matched-pairs test was used when comparing two groups of paired data. The use of parametric tests, such as one-way analysis of variance, unpaired and paired t-tests, were used only if the data passed a normality test.
Results
CLA expression of CD4+ T cells isolated from skin during the Mantoux reaction
CLA is expressed on a subset of memory T cells that home to the skin 16,18,19. We have shown previously that CD4+ T cells proliferate at the site of the Mantoux reaction 5; however, it was not clear if this cellular expansion affected CLA expression. In Fig. 3a we show a representative staining profile of CD4+ memory T cells in the PBMC and skin, isolated by suction blisters, that were positive for CLA expression at the peak of the clinical response on day 3 after PPD injection. No significant change in the percentage of CLA positivity was observed on PB CD4+ memory T cells during the course of the MT (Fig. 3b; Kruskal–Wallis test, P = 0·3). Intriguingly, in suction blisters the percentage of CLA expression on CD4+ memory T cells in the skin decreased from 76·04% at 3 days to 27·06% by 14 days post-injection (Kruskal–Wallis test, P = 0·03). In addition, the median fluorescence intensity (MFI) of CLA on CD4+ T cells in skin decreased during the MT (544·2 and 233·7 on days 3 and 19, respectively). In contrast, there was no significant change in CLA expression by CD8+ (CD4−CD3+) T cells that were found in the PB or skin during the MT (Kruskal–Wallis test, P = 0·88 and 0·86, respectively). The data are consistent with the recruitment of skin-homing CLA+ T cells into the skin during the induction phase of the immune response. This is followed subsequently by the progressive loss of CLA expression on CD4+ T cells coinciding with the proliferation and clonal expansion of PPD-specific CD4+ T cells. The loss of CLA expression could be a mechanism to allow emigration of antigen-specific cells from the skin during the resolution phase of the response.
Fig. 3.

Characteristics of skin-infiltrating T cells following Mantoux test (MT) induction. (a) Cutaneous lymphocyte antigen (CLA) expression on peripheral blood and normal skin blister CD3+ T cells. Peripheral blood mononuclear cells (PBMC) and blister cells were isolated and stained with anti-CD3, anti-CD4- and anti-CLA antibodies and analysed by flow cytometry. Samples were analysed by gating on the CD3+CD4+ CD45RA− subset of cells within the live lymphocyte/lymphoblast gate. Gating strategy was used to exclude cell debris, but include lymphocyte blasts. Dot-plots showing CLA expression on CD3+ T cells isolated from the peripheral blood (PB) and a suction blister raised on day 3 following MT induction (b) PBMC (◊) and suction blister (SB) (▪) cells isolated from skin following MT induction were analysed using flow cytometry for the percentage of CD4+ memory T cells positive for CLA expression. The mean ± standard error of the mean of three experiments per time-point is shown.
Comparison of cytokine responses identified by histology and in suction blisters
Previous studies have shown that the leucocytes that are found in the suction blisters after PPD injection are representative of the cells identified by histology in skin sections 5. However, it is not clear if the cytokines in the blister fluid are representative of those that are present during the response in situ. In a previous study, maximum cellular expression of interleukin (IL)-2 in histological sections was observed 7 days after intradermal PPD injection 6. We now show that cutaneous CD4+ T cells isolated from MTs can produce IL-2 following restimulation with PPD ex vivo, while minimal interferon (IFN)-γ or IL-2 was produced in the absence of PPD restimulation (Fig. 4a). We found that maximal numbers of IL-2-secreting cells in the skin were found 7 days after PPD injection, coinciding with the time of maximal expression of this cytokine by histological analysis 6. The proportion of PPD-specific CD4+ T cells capable of producing IL-2 was low during the clinical peak at day 3 (0·3%), but showed a marked increase at day 7 (8·8%) that was maintained during resolution at day 19 (9·8%) (Kruskal–Wallis test, P = 0·01). Virtually all IL-2-producing CD4+ T cells also expressed IFN-γ (Fig. 4a). We next investigated the presence of IL-2 in the blisters that were induced at different times after PPD injection. We found detectable levels of this cytokine at day 7 after PPD challenge, but not at the other time-points investigated (Fig. 4b). Collectively, this indicates that cytokine production during the Mantoux reaction can be assessed irrespective of whether histological, intracellular cytokine analysis or blister fluid assays were used for detection.
Fig. 4.
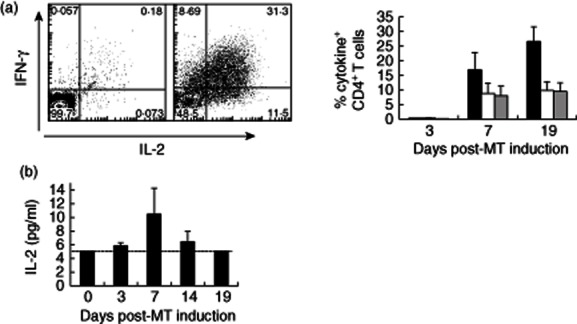
Antigen-specific cells accumulating following Mantoux test (MT) induction make interleukin (IL)-2 and interferon (IFN)-γ. (a) Blister cells were stimulated with purified protein derivative (PPD) ex vivo for 15 h in the presence of brefeldin A. CD4+ T cells were then examined for intracellular IFN-γ and IL-2 expression by flow cytometry. Representative fluorescence activated cell sorter (FACS) plots are shown on the left. The percentage of CD4+ T cells expressing IFN-γ (black bars), IL-2 (white bars) and both IFN-γ and IL-2 (grey bars) are shown. The mean ± standard error of the mean (s.e.m.) of three to four experiments per time-point is shown. (b) IL-2 expression in suction blister (SB) supernatants was assayed by multiplex bead immunoassay using a Luminex 100 and cytokine Beadlyte assay kit. The graph shows the mean ± s.e.m. of three to 18 experiments per time-point. The dotted line on the graph showing IL-2 data denotes the lower limit of detection in the assay.
Decreased Mantoux response in older humans
We investigated whether old and young subjects responded equally to PPD injection. None of our volunteers had a previous history evidence of tuberculosis infection. We found that 72 h after injection with the antigen, older subjects had a significantly decreased clinical score (Fig. 5a). However, when we investigated the proportions of PPD-specific CD4+ T cells in the blood that were identified by IFN-γ secretion after PPD stimulation in vitro there were similar numbers in both groups (Fig. 5b). Furthermore, the ability of T cells from blood to proliferate after PPD stimulation was the same between both groups (Fig. 5c). This indicates that decreased responsiveness of older subjects to PPD in the skin does not reflect a universal reduction of numbers or function of PPD-specific CD4+ T cells in these individuals. In addition, as vaccination with bacille Calmette–Guérin (BCG) only began in the 1950s, it is possible that the differences observed in the skin response were due to the different vaccination history of both groups of subjects. However, in a previous study, when Candida albicans or varicella zoster skin-test antigens were used, we found a similar defect in older subjects 7. This suggests that the defect in cutaneous response to antigen challenge is likely to be age-related, and not to whether they had had previous BCG vaccination.
Fig. 5.
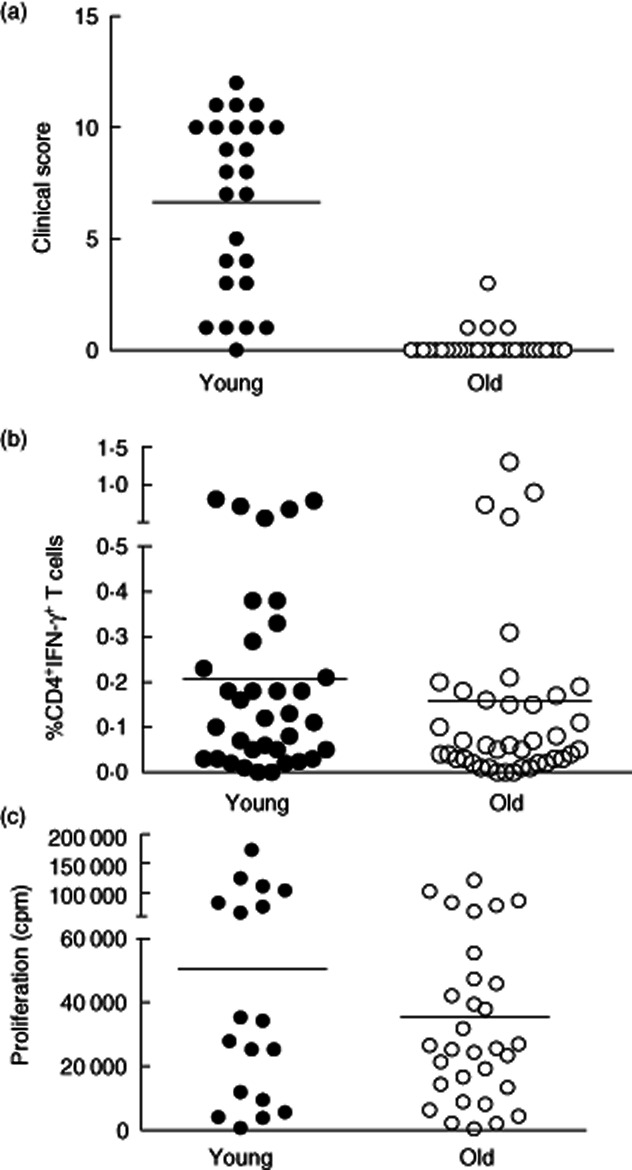
Old individuals do not mount a good clinical response to Mantoux test despite showing equivalent responses in circulating CD4 T cells. (a) Comparison of clinical scores between young and old. Twenty-five young (under 40) and 32 old (aged more than 70 years) individuals had a Mantoux test. Induration diameter, erythema index and palpability of the lesion were assessed and graded at day 3 and a clinical score assigned based on these parameters. (b) Comparison of the proportion of PPD-specific cells in peripheral blood of old and young individuals. PBMCs were collected from 35 young and 40 old individuals and stimulated with PPD ex vivo for 15 h in the presence of brefeldin A. CD4+ T cells were then examined for intracellular interferon (IFN)-γ. Representative fluorescence activated cell sorter (FACS) plots are shown on the left. The percentage of CD4+ T cells expressing IFN-γ in young and old individuals is shown in the graph (n = 18 young, 31 old). (c) PBMC from young and old individuals were stimulated ex vivo with PPD (1 μg/ml) for 6 days. Proliferation was assessed by [3H]-thymidine incorporation.
We next investigated how the decreased clinical response to PPD in older subjects was reflected in changes in the leucocytes in the skin that were isolated from the site of injection using the suction blister technology. We found that the number of CD4+ T cells on day 7 after injection of PPD, at the peak of the response, was significantly lower in old compared to young subjects (Fig. 6a). Furthermore, when we investigated the activation (CD69 expression) and proliferative activity (Ki67 expression) of CD4+ T cells that were isolated from the skin we found that there were significantly reduced levels of both molecules in older compared to younger subjects (Fig. 6b,c). Importantly, the percentage of Ki67+ cells we observed in blisters by flow cytometry was always well matched with the percentage observed by histological analysis 4,5. Finally when we investigated the kinetics of infiltration of PPD-specific CD4+ T cells, identified by IFN-γ stimulation after 16 h PPD rechallenge of blister-isolated cells in vitro, we found that older subjects had significantly lower numbers of specific cells present at all the time-points tested (Fig. 6d). This supports the data shown in Fig. 5, indicating that older individuals have a deficient ability to mount an optimal response to PPD in the skin, despite the fact that they have similar numbers of PPD-responsive T cells in the blood. This may indicate a lack of early inflammatory signals, defects in antigen presentation or impairment of antigen-specific T cell function.
Fig. 6.
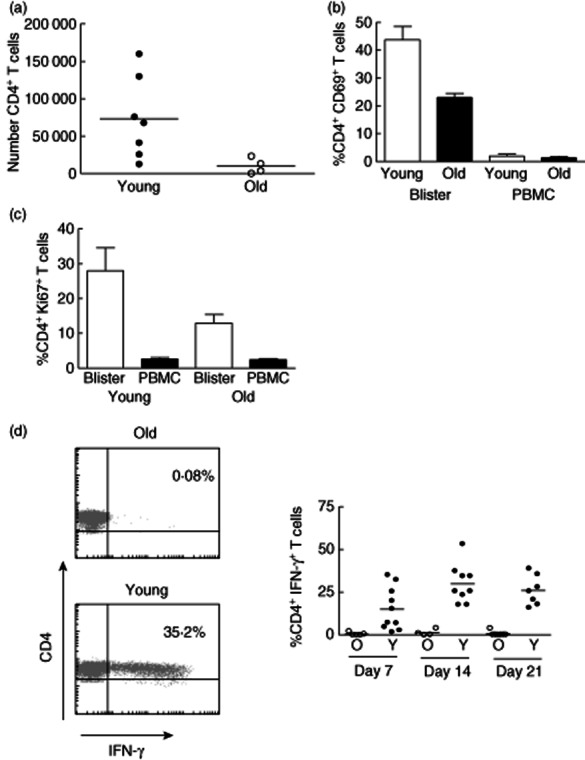
Reduced clinical responses to Mantoux test (MT) in old individuals are associated with reduced T cell accumulation and activation in the skin. (a) Skin suction blisters were raised on day 7 following MT induction in young and old individuals. Absolute number of CD4+ T cells in each blister was calculated using fluorescence activated cell sorter (FACS) analysis using Truecount tubes (n = 7 young and n = 4 old). (b) Cells isolated from skin suction blisters and peripheral blood on day 7 after the MT induction were stained with antibodies for CD3, CD4 and CD69 immediately ex vivo and analysed by flow cytometry. Graph shows the mean ± standard error of the mean of the percentage of CD4+ T cells expressing CD69 in blisters and peripheral blood mononuclear cells (PBMC) (Mann–Whitney U-test P = 0·0357; n = 4 young, 5 old). (c) Blister cells and PBMCs isolated on day 7 following MT were stained with antibodies for CD4 and Ki67 in order to identify CD4+Ki67+ T lymphocytes by flow cytometry (Mann–Whitney U-test P = 0·095; n = 5, mean ± standard error of the mean is shown). (d) Blister cells were collected at different times following MT induction and stimulated with purified protein derivative (PPD) ex vivo for 15 h in the presence of brefeldin. CD4+ T cells were then examined for intracellular interferon (IFN)-γ expression by flow cytometry. Representative FACS plots are shown on the left. The percentage of CD4+ T cells producing IFN-γ at different time-points is shown in the graph (horizontal lines represent the mean).
Discussion
The importance of the skin immune system, including the large number of T cells resident in the skin, in responses to both non-pathogenic and pathogenic organisms as well as chemical and physical challenges, is now accepted widely 20–23. In this study we have used skin suction blister technology coupled to challenge with the recall antigen PPD to probe a human memory T cell response in vivo. This has enabled investigation of the functionality of the responsive CD4+ T cells in the skin at different times after the initial antigen challenge. These methods enable the assessment of human immunity in vivo, in the context of the tissue where the immune response is taking place. An important observation was that the activation, proliferative activity and cytokine production that was seen in CD4+ T cells in the site of antigenic challenge was not observed in CD4+ T cells from the blood of the same individuals when investigated directly ex vivo. This highlights the fact that we are able to study multiple parameters at the local site of an immune response at different times after the reaction is initiated that are not reflected in the blood of the same donors. Apart from investigating the effects of ageing, this method could be used to investigate the status of immune responsiveness in the uninvolved skin of patients with skin diseases such as psoriasis, and also those with skin malignancies such as melanoma, which is the main impetus for presenting the extensive technological detail in this paper.
Although the numbers of cells that can be isolated from suction blisters are relatively low, we have been able to investigate T cell receptor clonality using the heteroduplex method 5, telomere length, telomerase activity and also cytokine production in the isolated T cells 4,5,8. It is also possible to use T cells recovered from skin blisters for long-term culture and cloning of antigen-specific cells 4. In addition, by investigating the characteristics of the isolated cells in parallel with histological analyses of the context of cellular infiltration, a picture of the functional cellular interactions that are taking place in the immune response in this tissue can be constructed.
The data we present in this paper extend our previous observations that older humans have a localized inability to respond to antigens in the skin, while the numbers of functional antigen-specific T cells in the blood of the same individuals are similar to those of younger subjects. These data question the utility of the clinical use of the Mantoux test in older humans, as the cutaneous response does not appear to reflect overall global immune responsiveness in these individuals. Although not investigated in this study, our previous data using Candida skin-test antigens suggests that the defect lies in the inability of older humans to amplify the response to antigen in the skin, resulting in the lack of local endothelial cell activation that prevents the recruitment of circulating T cells to the response 7. One hypothesis that is currently under investigation is that forkhead box protein 3 (FoxP3)+ regulatory T (Treg) cells that are increased in the skin of older humans 7 may inhibit the initial cascade of immune stimulatory events in the skin and therefore the amplification phase of the response does not occur. It is of interest that increased numbers of Tregs are also found in the skin as well as other tissues of mice during ageing 24. While older humans also have increased numbers of circulating Tregs 3, it is not known if there is also an over-representation of these cells in tissues other than skin. Another unanswered question is why these Tregs are increased in the first place. One possibility is that damage to skin by sun exposure or changes in the skin integrity during ageing predisposes this tissue to excessive non-specific inflammation, and that the Tregs are increased in this tissue to control this inflammation. This hypothesis remains to be tested. Ultraviolet B (UVB) exposure has been shown to induce Treg cells and immunosuppression 25,26. However, using the methods described, questions such as the antigen specificity of Tregs that are found in the skin during a recall response to antigen can now be addressed in humans in vivo 3,4,8.
The technology we have developed will allow the investigation of how specific vaccination in older humans, e.g. using Zostavax alters VZV-specific CD4+ T cell infiltration into the skin after challenge with varicella zoster skin test antigens. Previous studies have shown that skin responses to this virus are decreased during ageing 7,27,28 and are boosted after vaccination in some, but not all, subjects 28–30, but it is not known which component of defective immunity is reconstituted and how this correlates with vaccine efficacy in these individuals. Finally, these methods can also be applied to the study of other situations where altered cutaneous immunity occurs in humans in vivo.
Acknowledgments
This work was funded by the BBSRC to A.N.A., the MRC to M.V.S. and Dermatrust. We would like to thank all the volunteers who have taken part in this study.
Disclosure
The authors have no conflicting financial interests.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Clinical measurement of the Mantoux test (MT). (a) The Mantoux test is characterized clinically by the presence of a localized area of erythematous indurated oedematous skin. MTs were induced on the volar aspect of the forearm. The MT peaks clinically 2–3 days after induration. (b) The erythema index was measured at baseline, on day 3 and at the time of sampling using a DermaSpectrometer. The mean of three measurements was recorded.
Fig. S2. Suction blister equipment. (a) A clinical grade suction pump was used for skin suction blister induction. The pump has a gauge allowing a consistent negative pressure to be applied to the skin. The VP25 Eschmann suction pump has sterile disposable tubing and liners that can be changed between volunteers. (b) The suction cup is applied to the skin and connected to the pump using a cut-down pipette tip. (c) Suction cups consisted of three main components: a template and cup made of nylon plus a see-through Perspex lid. Rubber O-rings fit around the lid and template so that the three components fit together, forming airtight seals. The size of template used can be changed according to the size of Mantoux test (MT) induration, i.e. 15-mm diameter template if >15 mm induration, 12·5-mm diameter template if 10–15 mm and 10-mm diameter template if <10 mm. The template apertures have bevelled edges. This is to ensure that skin is drawn comfortably into the suction cup during blister induction. The see-through lid allows the induction process to be observed and monitored.
References
- 1.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayday AC, Peakman M. The habitual, diverse and surmountable obstacles to human immunology research. Nat Immunol. 2008;9:575–580. doi: 10.1038/ni0608-575. [DOI] [PubMed] [Google Scholar]
- 3.Vukmanovic-Stejic M, Reed JR, Lacy KE, Rustin MH, Akbar AN. Mantoux test as a model for a secondary immune response in humans. Immunol Lett. 2006;107:93–101. doi: 10.1016/j.imlet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Vukmanovic-Stejic M, Agius E, Booth N, et al. The kinetics of CD4Foxp3 T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed JR, Vukmanovic-Stejic M, Fletcher JM, et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 2004;199:1433–1443. doi: 10.1084/jem.20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orteu CH, Poulter LW, Rustin MH, Sabin CA, Salmon M, Akbar AN. The role of apoptosis in the resolution of T cell-mediated cutaneous inflammation. J Immunol. 1998;161:1619–1629. [PubMed] [Google Scholar]
- 7.Agius E, Lacy KE, Vukmanovic-Stejic M, et al. Decreased TNF-{alpha} synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vukmanovic-Stejic M, Sandhu D, Sobande TO, et al. Varicella zoster-specific CD4+Foxp3+ T cells accumulate after cutaneous antigen challenge in humans. J Immunol. 2013;190:977–986. doi: 10.4049/jimmunol.1201331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laube S. Skin infections and ageing. Ageing Res Rev. 2004;3:69–89. doi: 10.1016/j.arr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Vukmanovic-Stejic M, Rustin MH, Nikolich-Zugich J, Akbar AN. Immune responses in the skin in old age. Curr Opin Immunol. 2011;23:525–531. doi: 10.1016/j.coi.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 12.Kiistala U, Mustakallio KK, Rorsman H. Suction blisters in the study of cellular dynamics of inflammation. Acta Derm Venereol. 1967;47:150–153. doi: 10.2340/0001555547150153. [DOI] [PubMed] [Google Scholar]
- 13.Black AK, Greaves MW, Hensby CN, Plummer NA, Eady RA. A new method for recovery of exudates from normal and inflamed human skin. Clin Exp Dermatol. 1977;2:209–216. doi: 10.1111/j.1365-2230.1977.tb02560.x. [DOI] [PubMed] [Google Scholar]
- 14.Pitzalis C, Kingsley GH, Covelli M, Meliconi R, Markey A, Panayi GS. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991;21:369–376. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- 15.Pitzalis C, Cauli A, Pipitone N, et al. Cutaneous lymphocyte antigen-positive T lymphocytes preferentially migrate to the skin but not to the joint in psoriatic arthritis. Arthritis Rheum. 1996;39:137–145. doi: 10.1002/art.1780390118. [DOI] [PubMed] [Google Scholar]
- 16.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- 17.Sellati TJ, Waldrop SL, Salazar JC, Bergstresser PR, Picker LJ, Radolf JD. The cutaneous response in humans to Treponema pallidum lipoprotein analogues involves cellular elements of both innate and adaptive immunity. J Immunol. 2001;166:4131–4140. doi: 10.4049/jimmunol.166.6.4131. [DOI] [PubMed] [Google Scholar]
- 18.Picker LJ, Terstappen LW, Rott LS, Streeter PR, Stein H, Butcher EC. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990;145:3247–3255. [PubMed] [Google Scholar]
- 19.Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–753. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaerli P, Ebert L, Willimann K, et al. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199:1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naik S, Bouladoux N, Wilhelm C, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lages CS, Suffia I, Velilla PA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz A, Maeda A, Wild MK, et al. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz A, Schwarz T. UVR-induced regulatory T cells switch antigen-presenting cells from a stimulatory to a regulatory phenotype. J Invest Dermatol. 2010;130:1914–1921. doi: 10.1038/jid.2010.59. [DOI] [PubMed] [Google Scholar]
- 27.Schmader K. Herpes zoster in older adults. Clin Infect Dis. 2001;32:1481–1486. doi: 10.1086/320169. [DOI] [PubMed] [Google Scholar]
- 28.Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 29.Trannoy E, Berger R, Hollander G, et al. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: a randomized, controlled, dose–response trial. Vaccine. 2000;18:1700–1706. doi: 10.1016/s0264-410x(99)00510-1. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M, Okada S, Miyagawa H, et al. Enhancement of immunity against VZV by giving live varicella vaccine to the elderly assessed by VZV skin test and IAHA, gpELISA antibody assay. Vaccine. 2003;21:3845–3853. doi: 10.1016/s0264-410x(03)00303-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


