Abstract
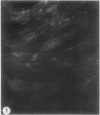
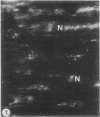
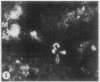
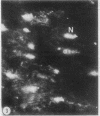
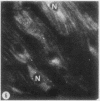
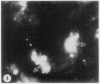
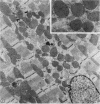
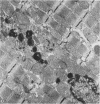
To determine the influence of cardiac ischemia on the activity and subcellular localization of lysosomal cathepsin D, anesthetized rabbits were subjected to ligation of the circumflex coronary artery. Total enzyme activity remained unchanged throughout the 2-h ischemic period, but the subcellular distribution of cathepsin D, as analyzed by biochemical and immunohistochemical techniques, was altered dramatically. A marked increase in nonsedimentable (i.e., 40,000-g supernate) activity developed by 30-45 min and increased further by 2 h. Simultaneously, the immunofluorescent localization of cathepsin D was also changed significantly. Within 30-60 min after occlusion, the fine, particulate staining observed in control myocytes was replaced by bright fluorescent patches composed of large granules. Many of these structures displayed prominent halos of diffuse fluorescent staining in the neighboring myocytic cytoplasm, apparently outside lysosomes per se. After 2 h, when nonsedimentable activity was maximally elevated, most of the fluorescent particles had disappeared completely. During this same interim there was no detectable change in the distribution of lysosomal cathepsin D within interstitial cells. These results are consistent with the hypothesis that an early feature of cardiac ischemia is the release of cathepsin D from myocytic lysosomes into the cytosol of damaged cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunk U. T., Ericsson J. L. Cytochemical evidence for the leakage of acid phosphatase through ultrastructurally intact lysosomal membranes. Histochem J. 1972 Nov;4(6):479–491. doi: 10.1007/BF01011128. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., BEAUFAY H. Tissue fractionation studies. 10. Influence of ischaemia on the state of some bound enzymes in rat liver. Biochem J. 1959 Dec;73:610–616. doi: 10.1042/bj0730610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franson R., Waite M., Weglicki W. Phospholipase A activity of lysosomes of rat myocardial tissue. Biochemistry. 1972 Feb 1;11(3):472–476. doi: 10.1021/bi00753a028. [DOI] [PubMed] [Google Scholar]
- Gottwik M. G., Kirk E. S., Hoffstein S., Weglicki W. B. Effect of collateral flow on epicardial and endocardial lysosomal hydrolases in acute myocardial ischemia. J Clin Invest. 1975 Oct;56(4):914–923. doi: 10.1172/JCI108171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERDSON P. B., SOMMERS H. M., JENNINGS R. B. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF NORMAL AND ISCHEMIC DOG MYOCARDIUM WITH SPECIAL REFERENCE TO EARLY CHANGES FOLLOWING TEMPORARY OCCLUSION OF A CORONARY ARTERY. Am J Pathol. 1965 Mar;46:367–386. [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Weissmann G., Hirsch J., Streuli F., Fox A. C. Cytochemical localization of lysosomal enzyme activity in normal and ischemic dog myocardium. Am J Pathol. 1975 May;79(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Ingwall J. S., DeLuca M., Sybers H. D., Wildenthal K. Fetal mouse hearts: a model for studying ischemia. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2809–2813. doi: 10.1073/pnas.72.7.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Sommers H. M., Herdson P. B., Kaltenbach J. P. Ischemic injury of myocardium. Ann N Y Acad Sci. 1969 Jan 31;156(1):61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- Kao R., Rannels D. E., Morgan H. E. Effects of anoxia and severe ischemia on the turnover of myocardial proteins. Acta Med Scand Suppl. 1976;587:117–123. doi: 10.1111/j.0954-6820.1976.tb05873.x. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson B. D. Hepatic lysosome and serum enzyme alterations in rats exposed to high altitude. Am J Physiol. 1966 Sep;211(3):651–655. doi: 10.1152/ajplegacy.1966.211.3.651. [DOI] [PubMed] [Google Scholar]
- Pollack M. S., Bird J. W. Distribution and particle properties of acid hydroase in denervated muscle. Am J Physiol. 1968 Sep;215(3):716–722. doi: 10.1152/ajplegacy.1968.215.3.716. [DOI] [PubMed] [Google Scholar]
- Reville W. J., Goll D. E., Stromer M. H., Robson R. M., Dayton W. R. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Subcellular localization of the protease in porcine skeletal muscle. J Cell Biol. 1976 Jul;70(1):1–8. doi: 10.1083/jcb.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciutti M. A. Lysosomes and myocardial cellular injury. Am J Cardiol. 1972 Oct;30(5):498–502. doi: 10.1016/0002-9149(72)90040-9. [DOI] [PubMed] [Google Scholar]
- Ricciutti M. A. Myocardial lysosome stability in the early stages of acute ischemic injury. Am J Cardiol. 1972 Oct;30(5):492–497. doi: 10.1016/0002-9149(72)90039-2. [DOI] [PubMed] [Google Scholar]
- Spath J. A., Jr, Lane D. L., Lefer A. M. Protective action of methylprednisolone on the myocardium during experimental myocardial ischemia in the cat. Circ Res. 1974 Jul;35(1):44–51. doi: 10.1161/01.res.35.1.44. [DOI] [PubMed] [Google Scholar]
- Topping T. M., Travis D. F. An electron cytochemical study of mechanisms of lysosomal activity in the rat left ventricular mural myocardium. J Ultrastruct Res. 1974 Jan;46(1):1–22. doi: 10.1016/s0022-5320(74)80018-3. [DOI] [PubMed] [Google Scholar]
- VAN LANCKER J. L., HOLTZER R. L. The release of acid phosphatase and beta-glucuronidase from cytoplasmic granules in the early course of autolysis. Am J Pathol. 1959 May-Jun;35(3):563–573. [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K., Mueller E. A. Increased myocardial cathepsin D activity during regression of thyrotoxic cardiac hypertrophy. Nature. 1974 May 31;249(456):478–479. doi: 10.1038/249478a0. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Poole A. R., Dingle J. T. Influence of starvation on the activities and localization of cathepsin D and other lysosomal enzymes in hearts of rabbits and mice. J Mol Cell Cardiol. 1975 Nov;7(11):841–855. doi: 10.1016/0022-2828(75)90135-2. [DOI] [PubMed] [Google Scholar]