Abstract
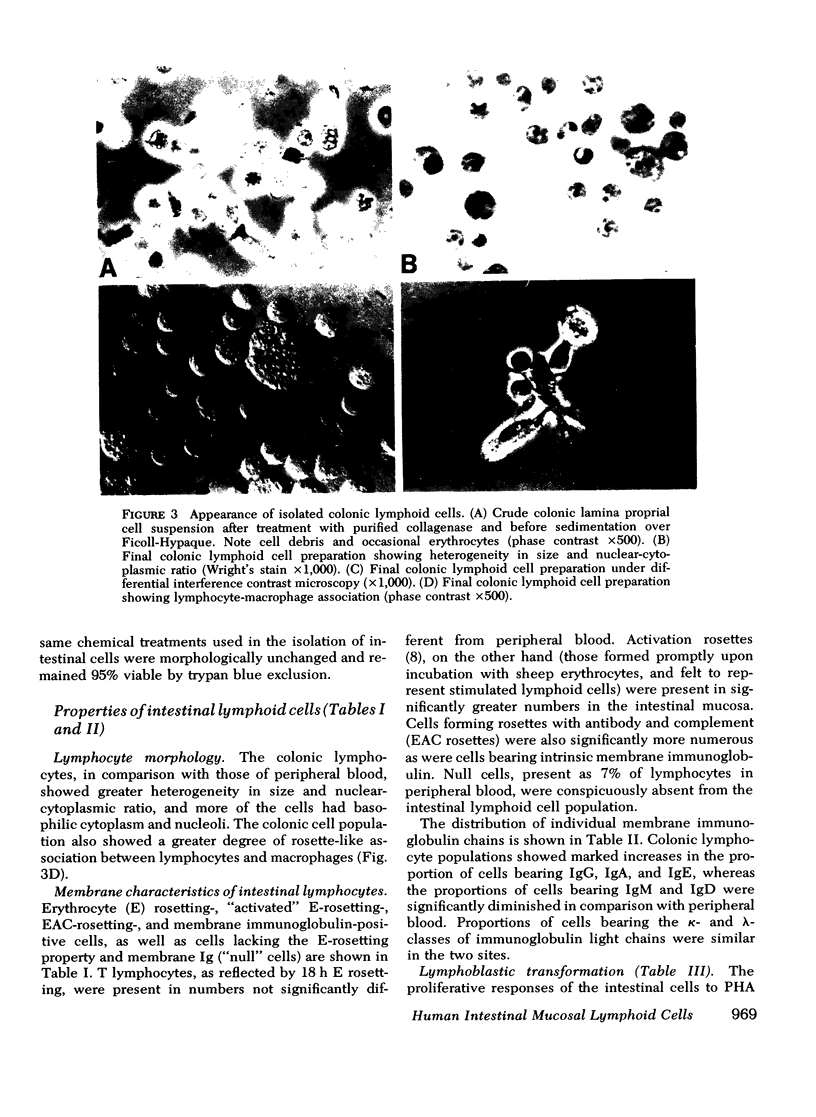
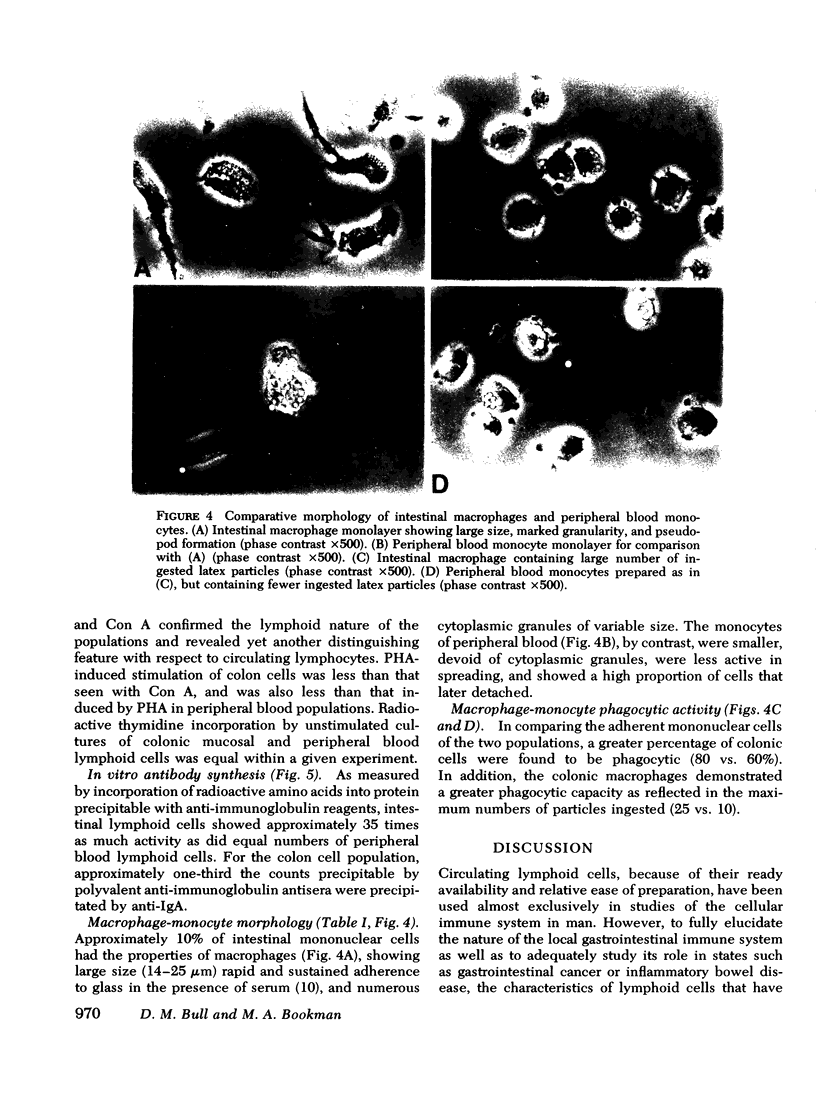
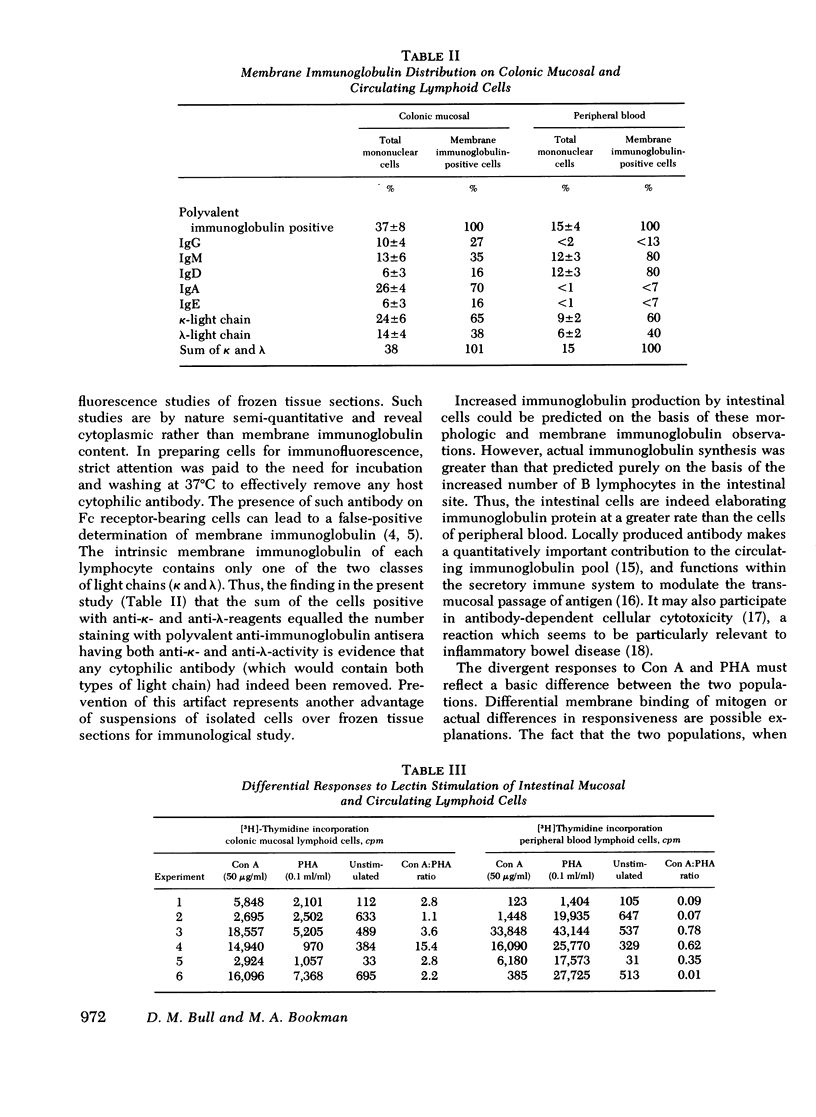
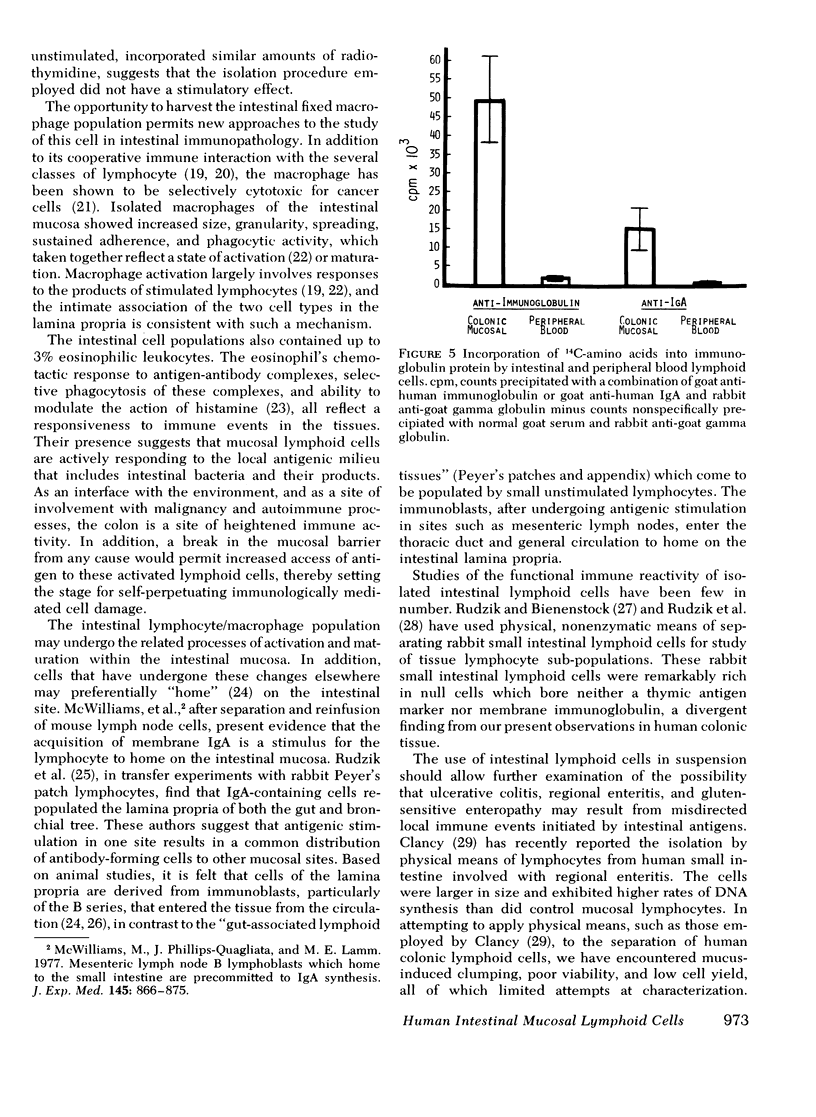
Viable suspensions of human colonic mucosal lymphoid cells have been prepared by sequential treatment of tissue with dithiothreitol, EDTA in calcium- and magnesium-free salt solutions, and purified collagenase. The intestinal lymphocyte population, in comparison with that of peripheral blood, had greater numbers of bone marrow-derived cells, particularly cells bearing membrane IgA; showed spontaneous association with macrophages; underwent rapid rosette formation with sheep erythrocytes; and demonstrated increased in vitro synthesis of immunoglobulin. Total thymus-derived cells were equal in the two populations. Decreases were found in "null" cell numbers, in cells bearing membrane IgD and IgM, and in responsiveness to phytohemagglutinin. Macrophage/monocytes in the intestinal population were increased in size, granularity, motility, sustained glass adherence, and phagocytic activity. Human intestinal lymphoid cells appear to constitute a cell population that is more "mature" and/or "activated", in comparison with the lymphoid cells of peripheral blood. The method of preparation should lend itself to the study of inflammatory bowel disease, gastrointestinal cancer, and the intestinal secretory immune system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bull D. M., Bienenstock J., Tomasi T. B., Jr Studies on human intestinal immunoglobulin A. Gastroenterology. 1971 Mar;60(3):370–380. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chess L., Levine H., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. VI. Further characterization of the surface Ig negative, E rosette negative (null cell) subset. J Immunol. 1975 Dec;115(6):1483–1487. [PubMed] [Google Scholar]
- Clancy R. Isolation and kinetic characteristics of mucosal lymphocytes in Crohn's Disease. Gastroenterology. 1976 Feb;70(2):177–180. [PubMed] [Google Scholar]
- David J. R. Macrophage activation by lymphocyte mediators. Fed Proc. 1975 Jul;34(8):1730–1736. [PubMed] [Google Scholar]
- Ehlenberger A. G., McWilliams M., Phillips-Quagliata J. M., Lamm M. E., Nussenzweig V. Immunoglobulin-bearing and complement-receptor lymphocytes constitute the same population in human peripheral blood. J Clin Invest. 1976 Jan;57(1):53–56. doi: 10.1172/JCI108268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller C. A., King G. W., Hurtubise P. E., Sagone A. L., LoBuglio A. F. Characterization of glass adherent human mononuclear cells. J Immunol. 1973 Nov;111(5):1610–1612. [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975 Jan 1;141(1):138–154. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- MacLennan I. C., Loewi G., Howard A. A human serum immunoglobulin with specificity for certain homologous target cells, which induces target cell damage by normal human lymphocytes. Immunology. 1969 Dec;17(6):897–910. [PMC free article] [PubMed] [Google Scholar]
- Mavligit G. M., Jubert A. V., Gutterman J. U., McBride C. M., Hersh E. M. Immune reactivity of lymphoid tissues adjacent to carcinoma of the ascending colon. Surg Gynecol Obstet. 1974 Sep;139(3):409–412. [PubMed] [Google Scholar]
- McWilliams M., Phillips-Quagliata J. M., Lamm M. E. Mesenteric lymph node B lymphoblasts which home to the small intestine are precommitted to IgA synthesis. J Exp Med. 1977 Apr 1;145(4):866–875. doi: 10.1084/jem.145.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney J. J., Waksman B. H. Activation of normal rabbit macrophage monolayers by supernatants of antigen-stimulated lymphocytes. J Immunol. 1970 Nov;105(5):1138–1145. [PubMed] [Google Scholar]
- Parrott D. M., Ferguson A. Selective migration of lymphocytes within the mouse small intestine. Immunology. 1974 Mar;26(3):571–588. [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulins as cell receptors. Ann N Y Acad Sci. 1971 Dec 31;190:420–431. doi: 10.1111/j.1749-6632.1971.tb13552.x. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., Churchill W. H., Jr, David Macrophages activated in vitro with lymphocyte mediators kill neoplastic but not normal cells. J Immunol. 1975 Jan;114(1 Pt 2):293–299. [PubMed] [Google Scholar]
- Rudzik O., Bienenstock J. Isolation and characteristics of gut mucosal lymphocytes. Lab Invest. 1974 Mar;30(3):260–266. [PubMed] [Google Scholar]
- Rudzik O., Clancy R. L., Perey D. Y., Bienenstock J., Singal D. P. The distribution of a rabbit thymic antigen and membrane immunoglobulins in lymphoid tissue, with special reference to mucosal lymphocytes. J Immunol. 1975 Jan;114(1 Pt 1):1–4. [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Stern B. K., Jensen W. E. Active transport of glucose by suspensions of isolated rat intestinal epithelial cells. Nature. 1966 Feb 19;209(5025):789–790. doi: 10.1038/209789a0. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Tomasi T. B., Huizenga K. A., Spencer R. J., Shorter R. G. In vitro studies of inflammatory bowel disease. Surface receptors of the mononuclear cell required to lyse allogeneic colonic epithelial cells. Gastroenterology. 1976 Feb;70(2):171–176. [PubMed] [Google Scholar]
- Strom T. B., Tilney N. L., Carpenter C. B., Busch G. J. Identity and cytotoxic capacity of cells infiltrating renal allografts. N Engl J Med. 1975 Jun 12;292(24):1257–1263. doi: 10.1056/NEJM197506122922402. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Calderon J. Evaluation of the role of macrophages in immune induction. Fed Proc. 1975 Jul;34(8):1737–1742. [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Immunoglobulin-receptors revisited. Science. 1975 Sep 19;189(4207):964–969. doi: 10.1126/science.1083069. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974 Sep;67(3):531–550. [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]