Abstract
Background and aim
Many risk factors that might contribute to the pathogenesis of atherosclerosis have been proposed, including chronic inflammation and infection. Furthermore, systemic inflammatory responses to periodontal bacteria have been suggested as a pathogenetic link between periodontal disease and atherosclerosis. The purpose of this study was to estimate the white blood cell (WBC) and platelet counts in chronic periodontitis patients.
Materials and methods
Fifty patients with chronic periodontitis and 50 patients with healthy periodontium were included in this study. Oral hygiene status, pocket probing depth (PPD) and clinical attachment level (CAL) were measured. During clinical evaluation, venous blood samples were taken to analyze the WBC and platelet counts. Statistical analysis was utilized to compare differences across groups.
Results
Periodontitis patients demonstrated a significantly higher WBC count (7.22 ± 1.42 × 109 cells/L) than that of control patients (5.64 ± 1.56 × 109 cells/L; P < 0.001). The platelet count of patients with chronic periodontitis (290.73 ± 56.56 × 109 cells/L) was also significantly higher compared to the healthy group (223.37 ± 50.27 × 109 cells/L; P < 0.001).
Conclusion
Levels of WBCs and platelets are elevated in periodontitis patients compared to healthy controls.
Keywords: WBC, RBC, Systematic disease, Thrombocyte, Periodontitis, Coronary heart disease, Oral conditions
1. Introduction
Atherosclerosis is a major cause of premature death, occurring because of a complex set of genetic and environmental factors. Hypertension, hypercholesterolemia, and cigarette smoking are classical risk factors for one half to one third of atherosclerosis cases (Folsom et al., 1997). Thus, it is likely that other, as yet unrecognized factors may contribute to the pathogenesis of atherosclerosis. Many other putative risk factors for atherosclerosis have been proposed, including traits related to obesity, inflammation, and infection (Hingorani and D’Aiuto, 2008). An elevated total white blood cell (WBC) count is a risk factor for atherosclerotic progression and has been found to be directly associated with an increased risk for coronary artery disease, stroke incidence, and mortality from cardiovascular disease (Danesh et al., 1999; Lee et al., 2001). Several prospective studies have shown a positive association between WBC count and coronary heart disease incidence. This association and the presence of mononuclear cells in atheromatous plaques suggest that atherosclerosis may be an inflammatory disease and be linked to local or distant infections or inflammatory conditions (Wheeler et al., 2004; Ross, 1999).
Chronic periodontitis is a common infection that manifests as local inflammation affecting the supporting tissues of the teeth (Monteiro et al., 2009). Studies have shown that periodontitis, aside from causing a local inflammatory reaction, can also exert a wide range of systemic effects. Recent research has suggested a possible association between periodontal disease and coronary artery disease (Monteiro et al., 2009; Lee et al., 2001). However, the mechanism linking periodontal disease to atherosclerosis and coronary artery disease is not yet clearly understood. It has been hypothesized that platelets and leukocytes may be more sensitive to stimulation by periodontal pathogens (Nicu et al., 2009) and that activated platelets and leukocytes might contribute to increased atherothrombotic activity (Monteiro et al., 2009; Buhlin et al., 2003 Sahingur et al., 2003; Papapanagiotou et al., 2009; D’Aiuto et al., 2004). Studies have shown that patients with periodontitis have elevated levels of WBCs and C-reactive protein compared to controls (Monteiro et al., 2009; Buhlin et al., 2003). Periodontitis has also been shown to be associated with an increase in plasma fibrinogen and an increase in platelet activation, which might contribute to a pro-coagulant state and thus an increased risk for atherosclerosis and cardiovascular disease (Sahingur et al., 2003; Papapanagiotou et al., 2009). D’Aiuto et al. (2004) showed that control of periodontitis significantly decreases serum mediators and markers of acute phase response, suggesting that the treatment of periodontitis may be beneficial in the control of atherosclerosis.
Although numerous studies have suggested an association between periodontitis and cardiovascular disease, the lack of reliable epidemiological and clinical data makes an assessment of risks between these 2 conditions difficult (Persson and Persson, 2008). A systematic review of the literature revealed a modest association between periodontitis and atherosclerosis (Scannapieco et al., 2003; Paraskevas et al., 2008). However, studies are still needed to determine how periodontitis contributes to atherosclerosis in susceptible subjects.
Therefore, the aim of our study was to determine whether plasma levels of WBCs and platelets were altered in patients with chronic periodontitis compared to healthy controls to help explain how periodontitis is linked to the development of atherosclerosis and cardiovascular disease.
2. Materials and methods
The study was initiated after the protocol had been approved by the Institutional Committee of Research Ethics at the College of Dentistry Research Center. Fifty subjects with chronic periodontitis (32 males and 18 females; mean age, 34.35 ± 7.82 years) were recruited from patients referred to the Periodontics Division at the College of Dentistry of King Saud University, Riyadh, Saudi Arabia. Subjects having more than 30% of their teeth with ⩾ 4 mm periodontal probing depth and ⩾ 2 mm attachment loss were included in the chronic periodontitis group. Fifty subjects (29 males and 21 females; mean age, 34 ± 6.20 years) with a clinically healthy periodontium were included in the control group.
All subjects in the study were systemically healthy and had no recent minor infection (as flu or sore throat) in the past 4–8 weeks requiring antibiotic therapy. Exclusion criteria were pregnancy or use of NSAIDs, antimicrobial drugs, mouthwashes, or vitamin supplements within the past 3 months. All subjects enrolled in the study were nonsmokers. After written informed consent was obtained from the study participants, they were requested to complete a health questionnaire.
The clinical periodontal examination was performed by single examiner. Calibration exercises for probing measurements were performed on 5 patients before initiating the actual study measurements. Intra-examiner agreement was good, with an 0.82 κ value. The clinical indexes, including plaque index (PI), bleeding on probing index (BPI), periodontal pocket depth (PPD), and clinical attachment level (CAL), were measured at the mesial, distal, buccal, and lingual aspects of each tooth (Silness and Löe, 1964; Löe and Silness, 1963; Ainamo and Bay, 1975).
Venous blood was collected into Vacutainer™ (Greiner, Bio-One Ltd, Stonehouse, UK) lithium heparin (17 IU/mL) tubes from the study patients between 9:00 and 10:00 am. The WBC and platelet counts were determined by using an automated particle counter within 24 h after vein puncture at the hematology laboratory of the medical school and university hospital.
3. Statistical analysis
All statistical analyses were performed using GraphPad InStat® software (GraphPad Software, San Diego CA, USA; www.graphpad.com), and the Student t-test was used to explore the differences between the two study groups. P < 0.05 was considered statistically significant.
4. Results
Table 1 shows the mean values of PI, BPI, PPD, and CAL. Mean values for the WBC count (×109 cells/L) and platelet count are graphically represented in Fig. 1. The mean WBC count was found to be higher in the periodontitis group (7.22 ± 1.42) compared to healthy controls (5.64 ± 1.56). The mean platelet count of subjects with chronic periodontitis (290.73 ± 56.56 × 109 cells/L) was also higher compared to the healthy group (223.37 ± 50.27 × 109 cells/L). The observed differences were found to be statistically significant (P < 0.001).
Table 1.
Mean values of plaque index (PI), bleeding on probing index (BPI), periodontal pocket depth (PPD), and clinical attachment loss (CAL) of the study subjects.
| Periodontitis, Mean ± SD | Healthy, Mean ± SD | |
|---|---|---|
| Age (years) | 34.35 ± 7.82 | 34.00 ± 6.20 |
| PI | 66.35 ± 15.09 | 10.39 ± 2.29 |
| BPI | 59.41 ± 16.30 | 8.22 ± 2.14 |
| PPD (mm) | 5.45 ± 0.40 | |
| CAL (mm) | 3.29 ± 0.57 |
Figure 1.
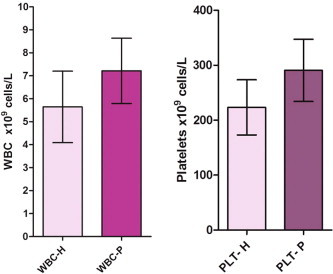
The WBC and platelet profile in periodontitis and healthy subjects.
5. Discussion
Periodontitis is associated with an increased risk of cardiovascular disease (CVD) (Nicu et al., 2009). The relationship between periodontitis and systemic disease, including cardiovascular disease, has been well recognized through epidemiological studies conducted during the past decade (Paraskevas et al., 2008). Modulation of cellular and protein components of the inflammatory process may be the basis of association between periodontitis and cardiovascular disease, but the mechanisms are not well understood. The present study aimed to compare the plasma levels of WBCs and platelets (risk factors in the development of CVD) between periodontal disease and periodontally healthy patients.
All clinical periodontal examinations were performed by a single, experienced periodontist to minimize interexaminer variation, although a calibration exercise in probing measurements was performed on five patients before initiation of the actual study measurements.
Results of the present study indicate that individuals with periodontal infection have a higher WBC count (7.22 ± 1.42 × 109 cells/L) and platelet count compared to healthy controls (5.64 ± 1.56 × 109 cells/L), which is presumably related to the chronic inflammatory nature of the disease. This is in agreement with what has been reported before that peripheral polymorphonuclear leukocytes from periodontitis patients show more twice the stimulation than healthy controls (Gustafsson and Asman, 1996). In addition, earlier studies have demonstrated elevation of total leukocyte count in periodontitis (Monteiro et al., 2009; Wakai et al., 1999; Shi et al., 2008) and lowering of leukocyte levels following periodontal therapy (Christan et al., 2002). Such an increased leukocyte count in periodontitis may carry a high risk of coronary heart disease, because there are several plausible mechanisms by which the WBCs can promote atherosclerosis, thrombosis, and myocardial ischemia (Mattila et al., 1989; Nicu et al., 2009; Persson et al., 2005). In addition, studies have shown that elevated WBC count is a risk factor for coronary artery disease (Danesh et al., 1999; Lee et al., 2001). Hyper-reactive white blood cells can be induced in patients with infections such as periodontal disease and promote atherosclerosis at distant sites, particularly in areas of disturbed blood flow (Johnstone et al., 2007). This hyper-reactivity might be due to their circulating through the periodontal lesions or might be a constitutive feature of patients with periodontal disease. Irrespective of the responsible mechanisms, these cells may be capable of facilitating atheroma formation (Meurman et al., 2004).
Granulocytes and monocytes are involved in the pathogenesis of atherosclerosis. Normal endothelium does not allow for the attachment of leukocytes, but monocyte-derived macrophages produce oxidants that can induce endothelial injury and subsequent thrombus formation (Fuster and Lewis, 1994). Activated WBCs also reflect the inflammatory activity of atherosclerosis that perpetuates vascular injury and ischemia, according to Ernst et al. (1987), who outlined 3 mechanisms by which leukocytes might contribute to microvascular injury and promote atherosclerosis: pressure-dependent plugging of microvessels, rheologically altering deformability and forming aggregates when provoked by a variety of stimuli, and releasing activated substances including oxygen-free radicals, proteolytic enzymes, and arachidonic acid metabolites.
Leukocytes have a wide range of biological effects, some of which protect against vascular disease while some are damaging (Danesh et al., 1999). Hence, it is important to determine the associations between specific components of the leukocyte count and coronary artery disease, for which some studies have suggested a particular role for granulocytes and neutrophils (Wheeler et al., 2004; Helgadottir et al., 2004). Also, an increase in percentage of neutrophils has been demonstrated in aggressive (Shi et al., 2008) as well as chronic periodontitis (Monteiro et al., 2009).
The results of our study indicate that the group with chronic periodontal infection has higher mean platelet counts (290.73 ± 56.56 × 109 cells/L) compared to the healthy group (223.37 ± 50.27 × 109 cells/L). The observed differences were found to be statistically significant (P < 0.001). This was in agreement with previous studies that have shown an increase in platelet levels and activation in periodontitis patients (Papapanagiotou et al., 2009; Wakai et al., 1999) and that periodontal treatment results in a lowering of platelet levels (Christan et al., 2002). It has been demonstrated that dental plaque bacteria, including the periodontal pathogen Porphyromonas gingivalis, induces platelet activation and aggregation (Lourbakos et al., 2001).
Platelets and leukocytes activated during bacteremia can go onto excite other cells, enhancing the likelihood of atheroma plaque formation. Activated platelets release pro-inflammatory mediators, exposing pro-inflammatory receptors and resulting in the platelets’ binding to leukocytes and endothelial cells (Kamath et al., 2001). It has been proposed that activated platelets regulate chemokine release by the monocytes in inflammatory lesions (Weyrich et al., 1996). These functions make platelets important components of the thrombotic and inflammatory processes, and platelet activation has been implicated in atherosclerosis and coronary artery disease (Kamath et al., 2001).
6. Conclusions
From the results of the present study, we conclude that chronic periodontitis may elevate WBC and platelet counts compared to healthy control patients and might be considered one of the mechanisms that explain how periodontitis is linked to the development of atherosclerosis and cardiovascular disease.
Acknowledgments
The author thanks Prof. Anil Sukumaran and Dr. Hamdan Alghamdi for their technical assistance in this study. Deepest appreciation goes to Dr. Youssef Al Jabbari for his valuable advice and editing comments during the preparation of this manuscript.
References
- Ainamo J., Bay I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975;25(4):229–235. [PubMed] [Google Scholar]
- Buhlin K., Gustafsson A., Pockley A.G., Frostegard J., Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur. Heart J. 2003;24(23):2099–2107. doi: 10.1016/j.ehj.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Christan C., Dietrich T., Hägewald S., Kage A., Bernimoulin J. White blood cell count in generalized aggressive periodontitis after non-surgical therapy. J. Clin. Periodontol. 2002;29(3):201–206. doi: 10.1034/j.1600-051x.2002.290303.x. [DOI] [PubMed] [Google Scholar]
- D’Aiuto F., Parkar M., Andreou G., Suvan J., Brett P.M., Ready D., Tonetti M.S. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J. Dent. Res. 2004;83(2):156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- Danesh J., Muir J., Wong Y., Ward M., Gallimore J., Pepys M. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur. Heart J. 1999;20(13):954–959. doi: 10.1053/euhj.1998.1309. [DOI] [PubMed] [Google Scholar]
- Ernst E., Hammerschmidt D.E., Bagge U., Matrai A., Dormandy J.A. Leukocytes and the risk of ischemic diseases. J. Am. Med. Assoc. 1987;257(17):2318–2324. [PubMed] [Google Scholar]
- Folsom A.R., Wu K.K., Rosamond W.D., Sharrett A.R., Chambless L.E. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96(4):1102–1108. doi: 10.1161/01.cir.96.4.1102. [DOI] [PubMed] [Google Scholar]
- Fuster V., Lewis A. Conner Memorial Lecture. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation. 1994;90(4):2126–2146. doi: 10.1161/01.cir.90.4.2126. [DOI] [PubMed] [Google Scholar]
- Gustafsson A., Asman B. Increased release of free oxygen radicals from peripheral neutrophils in adult periodontitis after Fc delta-receptor stimulation. J. Clin. Periodontol. 1996;23(1):38–44. doi: 10.1111/j.1600-051x.1996.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Helgadottir A., Manolescu A., Thorleifsson G., Gretarsdottir S., Jonsdottir H., Thorsteinsdottir U., Samani N.J., Gudmundsson G., Grant S.F., Thorgeirsson G., Sveinbjornsdottir S., Valdimarsson E.M., Matthiasson S.E., Johannsson H., Gudmundsdottir O., Gurney M.E., Sainz J., Thorhallsdottir M., Andresdottir M., Frigge M.L., Topol E.J., Kong A., Gudnason V., Hakonarson H., Gulcher J.R., Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nature Genet. 2004;36(3):233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- Hingorani A.D., D’Aiuto F. Chronic inflammation, periodontitis and cardiovascular diseases. Oral Dis. 2008;14(2):102–104. doi: 10.1111/j.1601-0825.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- Johnstone A.M., Koh A., Goldberg M.B., Glogauer M. A hyperactive neutrophil phenotype in patients with refractory periodontitis. J. Periodontol. 2007;78(9):1788–1794. doi: 10.1902/jop.2007.070107. [DOI] [PubMed] [Google Scholar]
- Kamath S., Blann A., Lip G. Platelet activation: assessment and quantification. Eur. Heart J. 2001;22(17):1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- Lee C., Folsom A., Nieto F., Chambless L., Shahar E., Wolfe D. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am. J. Epidemiol. 2001;154(8):758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- Löe H., Silness J. Periodontal disease in pregnancy I Prevalence and severity. Acta Odontol. Scand. 1963;21(6):533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Lourbakos A., Yuan Y., Jenkins A., Travis J., Andrade-Gordon P., Santulli R., Potempa J., Pike R.N. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood. 2001;97(12):3790–3797. doi: 10.1182/blood.v97.12.3790. [DOI] [PubMed] [Google Scholar]
- Mattila K.J., Nieminen M.S., Valtonen V.V., Rasi V.P., Kesäniemi Y.A., Syrjälä S.L., Jungell P.S., Isoluoma M., Hietaniemi K., Jokinen M.J. Association between dental health and acute myocardial infarction. B.M.J. 1989;298(6676):779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurman J.H., Sanz M., Janket S.J. Oral health, atherosclerosis, and cardiovascular disease. Crit. Rev. Oral Biol. Med. 2004;15(6):403–413. doi: 10.1177/154411130401500606. [DOI] [PubMed] [Google Scholar]
- Monteiro A.M., Jardini M.A., Alves S., Giampaoli V., Aubin E.C., Neto A.M., Gidlund M. Cardiovascular disease parameters in periodontitis. J. Periodontol. 2009;80(3):378–388. doi: 10.1902/jop.2009.080431. [DOI] [PubMed] [Google Scholar]
- Nicu E.A., Van der Velden U., Nieuwland R., Everts V., Loos B.G. Elevated platelet and leukocyte response to oral bacteria in periodontitis. J. Thromb. Haemost. 2009;7(1):162–170. doi: 10.1111/j.1538-7836.2008.03219.x. [DOI] [PubMed] [Google Scholar]
- Papapanagiotou D., Nicu E., Bizzarro S., Gerdes V., Meijers J., Nieuwland R., van der Velden U., Loos B.G. Periodontitis is associated with platelet activation. Atherosclerosis. 2009;202(2):605–611. doi: 10.1016/j.atherosclerosis.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Paraskevas S., Huizinga J.D., Loos B.G. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 2008;35(4):277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- Persson G.R., Persson R.E. Cardiovascular disease and periodontitis: an update on the associations and risk. J. Clin. Periodontol. 2008;35(Suppl 8):362–379. doi: 10.1111/j.1600-051X.2008.01281.x. [DOI] [PubMed] [Google Scholar]
- Persson G.R., Pettersson T., Ohlsson O., Renvert S. High-sensitivity serum C-reactive protein levels in subjects with or without myocardial infarction or periodontitis. J. Clin. Periodontol. 2005;32(3):219–224. doi: 10.1111/j.1600-051X.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340(2)::115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sahingur S., Sharma A., Genco R., De Nardin E. Association of increased levels of fibrinogen and the-455G/A fibrinogen gene polymorphism with chronic periodontitis. J. Periodontol. 2003;74(3):329–337. doi: 10.1902/jop.2003.74.3.329. [DOI] [PubMed] [Google Scholar]
- Scannapieco F.A., Bush R.B., Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann. Periodontol. 2003;8(1):38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- Shi D., Meng H., Xu L., Zhang L., Chen Z., Feng X., Lu R., Sun X., Ren X. Systemic inflammation markers in patients with aggressive periodontitis: a pilot study. J. Periodontol. 2008;79(12):2340–2346. doi: 10.1902/jop.2008.080192. [DOI] [PubMed] [Google Scholar]
- Silness J., Löe H. Periodontal disease in pregnancy II Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964;22(1):121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Wakai K., Kawamura T., Umemura O., Hara Y., Machida J., Anno T., Ichihara Y., Mizuno Y., Tamakoshi A., Lin Y., Nakayama T., Ohno Y. Associations of medical status and physical fitness with periodontal disease. J. Clin. Periodontol. 1999;26(10):664–672. doi: 10.1034/j.1600-051x.1999.261006.x. [DOI] [PubMed] [Google Scholar]
- Weyrich A.S., Elstad M.R., McEver R.P., McIntyre T.M., Moore K.L., Morrissey J.H., Prescott S.M., Zimmerman G.A. Activated platelets signal chemokine synthesis by human monocytes. J. Clin. Invest. 1996;97(6):1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J.G., Mussolino M.E., Gillum R.F., Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur. Heart J. 2004;25(15):1287–1292. doi: 10.1016/j.ehj.2004.05.002. [DOI] [PubMed] [Google Scholar]


