Abstract
Aim
Cytotoxicity of root canal irrigants is important due to their close contact with host tissues. This study was to assess the possible impact of pH on cytotoxic effects of MTAD, 17% EDTA, and 2.6% NaOCl on the human gingival fibroblasts using MTT assay.
Materials and methods
Human gingival fibroblasts were exposed to the irrigants and their viability was assessed after 1, 6, and 12 h. The pH of the medium was measured in each interval. Light absorption values were measured for each culture medium using Elisa Reader device.
Results
NaOCl had significantly less cytotoxicity than EDTA and MTAD. Also irrigants cytotoxicity decreased in 12, 1, and 6 h, respectively.
Conclusion
It seems that variation of the pH resulted in variation in the cytotoxicity of solutions; i.e., it follows the pattern of the pH variation.
Keywords: Cytotoxicity, Ethylene diaminetetra acetic acid, MTAD, pH, Sodium hypochlorite
1. Introduction
Ideal irrigants should have a broad antimicrobial spectrum and high efficacy against anaerobic and facultative microorganisms organized in biofilms, dissolve necrotic pulp tissue remnants, inactivate endotoxin, and dissolve the smear layer (Zehnder, 2006). High biocompatibility is required for intracanal irrigants due to their close contact with periradicular tissue (Masillamoni et al., 1981). Root canal irrigants may be toxic (Spångberg et al., 1973) and induce inflammatory responses (Tanomaru Filho et al., 2002).
The antibacterial effectiveness, tissue dissolution capacity, and toxicity of sodium hypochlorite are the features of its concentration (Spångberg et al., 1973). The pH of the solution determines the amount of available chlorine. Above a pH of 7.6, the predominant form is hypochlorite, while below this value it is hypochlorous acid. Both forms are extremely reactive oxidizing agents (Smith and Martell, 1976). Hypochlorous acid is more bactericidal than hypochlorite (Bloomfield and Miles, 1979); thus, in order to increase the efficacy of hypochlorite solutions, lowering the pH is recommended. This is less toxic to vital tissues than nonbuffered counterparts (Dakin, 1915; Cotter et al., 1985; Siqueira et al., 1997). The caustic potential of hypochlorite solutions is affected by the available chlorine rather than by pH or osmolarity (Zehnder et al., 2002). The capacity for NaOCl to dissolve tissue is dependent on concentration, time, and pH. The more concentrated the NaOCl solution, the longer the solution is left in contact with host tissues, and higher pH levels all lead to greater tissue dissolution. Antibacterial ability increases as pH decreases; however, tissue dissolution decreases (Christensen et al., 2008).
Different pH is reported for MTAD (MTAD is a mixture of tetracycline isomer doxycycline, citric acid, and Tween 80 as a detergent) (Beltz et al., 2003; Tay et al., 2006). It has been stated that MTAD has minimal cytotoxicity against MC3T3-E1 and periodontal ligament cells compared with conventional irrigants (Yasuda et al., 2010).
It has also been reported that EDTA cytotoxicity is proportional to its concentration (Chan et al., 1999; Koulaouzidou et al., 1999). It has been shown that a concentration of 17% or 15% EDTA salt is toxic in vitro, whereas lower concentrations have reduced toxicity (Patterson, 1963; Koulaouzidou et al., 1999). Furthermore, it has been reported that the concentrations of 17% and 15% of EDTA, and 2.25% of NaOC1 were severely cytotoxic, whereas at 0.1% concentrations, the agents were moderately cytotoxic (Torneck, 1961; Patterson, 1963; Collet et al., 1981).
Therefore, many factors such as concentration, volume, time of exposure, temperature, pH, mechanical agitation, and tissue surface area can affect the characteristics of solutions (Moorer and Wesselink, 1982). This study assessed the possible impact of pH on cytotoxic effects of MTAD, 17% EDTA, and 2.6% NaOCl on human gingival fibroblasts (HGF) using MTT assay.
2. Materials and methods
This experimental in vitro study was conducted on human gingival fibroblasts obtained from Pasteur Institute, Tehran, Iran. HGF cells were maintained in modified Eagle’s medium (Life Technologies Inc., Gaithersburg, Md) supplemented with 1% nonessential amino acids and 10% charcoal-stripped fetal calf serum (Gemini Bio-Products, Calabasas, CA). Sabouraud (SAB) dextrose agar plates (Oxoid Ltd., Basingstoke, England) wereas used. The presence of microorganisms was then checked in two ways:
The addition of a few milliliters of Tripticase Soy Broth (BBL Microbiology Systems, Cockeysville, Md.) to the culture media. The culture media were placed in Bactec 9120 device (Becton–Dickinson, Franklin Lakes, NJ, USA) to detect probable bacterial growth using fluorescence.
The irrigants pH was measured with a Beckman Model G pH meter (Beckman Instruments Inc., Palo Alto, California, USA) using a Beckman 1190-90 glass electrode (Beckman Instruments Inc., Palo Alto, California, USA). The initial pH of the 2.6% NaOCl (Tianjin Ruifuxin Chemical Co., Ltd., Tianjin, China), 17% EDTA (MD cleanser, Meta Biomed co, Chong Ju City, Korea) and MTAD (BioPure MTAD, Dentsply, Tulsa, OK) was 5.31, 6.69, and 1.67, respectively. (Fig. 1)
Figure 1.
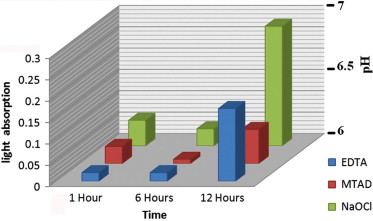
570 nm light absorption and pH alteration of different irrigants during different intervals.
During the growth process of fibroblasts, cell count was primarily assessed on the third day and then every day. Trypsin enzyme was first added to float fibroblasts; the solution was pulled and placed into a test tube. The test tube was then centrifuged for 10 min with 4 λ and 800–1000 rpm. The sediment was again centrifuged with 10 λ and the final solution was placed on a neobar slide and the cell count was checked under Nikon microscope (model E400; Nikon Inc., Melville, New York, USA). When the cell count was measured to be 1 × cell/ml in the veils of the culture media, cells were primarily washed with Hanks Balance Salt Solution (HBSS, Grand Island Biological Co., Grand Island, NY) and then suspended by the addition of trypsin (0.05% solution/0.5 mM EDTA). The final solution was divided into 96 cell plates (Nunc, Roskilde, Denmark). The total number of wells was 195 (three different time × (three test substances + two controls) × 13 wells in each). Test and control substances were added to each well. Phenol (pH 4.5) was included as the positive control and the culture medium served as negative control group (pH 7.4). Plates were incubated at 37 °C and 5% CO2 for 24 h. The cytotoxicity of irrigating solutions was assessed at 1, 6, and 12 h after incubation, evaluating the toxicity using the MTT colorimetric assay. This assay involves the ability of viable cells to convert a soluble tetrazolium salt, MTT [3-(4.5-dimethylthiazol-2-yl)-2-5-diphenylterazolum bromide], into a blue formazan end product by mitochondrial dehydrogenase enzymes (Mosmann, 1983). MTT (Merck, Darmstadt, Germany) was added (10 μl in each 100 μl) and the plates were incubated again for 3 h. Stop Mix solution (Visible Genetics, Inc., Toronto, Ontario, Canada) which includes N-dimethyformumide sodium dodecyl sulfate 20% was then added to the media (0.1 ml 50% solution for each well) and the plates were incubated for 1 h. Then the solution phase of the blue foramazan sediment was assured by pippetaging the wells. Finally, the light absorption in 570 nm was detected using spectrophotometer (Bio-Rad Benchmark Microplate Reader, Hercules, CA) and presented to the form of prints. Higher optical absorption reflects higher foramazan sediment formation, which in turn indicates higher viable cell counts.
Fibroblasts viability was assessed in 1, 6, and 12 h by the Elisa Reader (Argus 400 micro plate reader, Packard, Meriden, USA). Finally, a value would be assigned to each culture plate which reflects the light absorption in the given wavelength of Elisa Reader. This value shows the level of color production by the viable cells. Also, the pH of the media was measured in 1, 6, and 12 h.
2.1. Statistics
Comparisons were made on light absorption of different irrigants in given hours (one-way ANOVA; Post-Hoc test) and on light absorption of a given irrigant in different hours (General Linear Model). Data were classified into SPSS 13 (SPSS Inc., Chicago, Ill). The level of significance was set at 0.05.
3. Results
NaOCl had significantly less cytotoxicity than the other irrigants in all the studied intervals while MTAD had the most cytotoxicity at the 6 h interval. The cytotoxicity of all the irrigants was decreased in 6, 1, and 12 h respectively (Fig. 1). The pH values of the irrigants at 1, 6, and 12 h can be seen in Fig. 1.
One way ANOVA showed that light absorption differences were significant both for a given irrigant during different hours and for different irrigants at a given time (p < 0.05).
4. Discussion
The antimicrobial effectiveness and cytotoxicity of sodium hypochlorite is based on its high pH (hydroxyl ions action). The high pH of sodium hypochlorite interferes with cytoplasmic membrane integrity, with irreversible enzymatic inhibition, biosynthetic alterations in cell metabolism, and phospholipid destruction observed in lipidic peroxidation. The amino acid chloramination reaction forming chloramines interferes with cell metabolism (Estrela et al., 1995).
Previous studies have compared various root canal irrigants of different concentrations in terms of cytotoxicity. Zhang et al. (2003), using MTT assay, found that NaOCl, in concentrations less than 2.63%, is less cytotoxic against L929 cells followed by MTAD, REDTA, Peridex, 5.25% NaOCl, Ca(OH)2 paste, 3% H2O2, and eugenol. We evaluated the effect of pH on cytotoxicity of root canal irrigants and interestingly our results were consistent to the findings of Zhang et al. i.e., the least cytotoxicity was associated with 2.6% NaOCl. In the present study, the pH of the NaOCl added to the medium approached the neutral pH values in less time than did the other irrigants which may be due to NaOCl dispersal ability in aqueous medium. Lowering the pH of the root canal irrigant (e.g. sodium hypochlorite) has some advantages such as increased efficacy, lower toxicity to vital host tissues, increased antibacterial ability (Dakin, 1915; Bloomfield and Miles, 1979; Cotter et al., 1985; Siqueira et al., 1997; Christensen et al., 2008). As shown in most studies, low concentrations of NaOCl (2.6% and less) is associated with very low levels of cytotoxicity which is consistent to our findings.
Lowering the surface tension can result in more dispersion of the irrigant in the medium (Tasman et al., 2000; Giardino et al., 2006). Wettability of the irrigants correlates to their surface tension (Glantz and Hansson, 1972; Pecora et al., 1991). An irrigant antimicrobial activity can be increased by improving its wettability (Cameron, 1986). Adding a detergent to the root canal irrigant can increase wettability and antibacterial effect (Barbosa et al., 1994). In the present study, EDTA cytotoxicity followed the pattern of pH increase. It has no surfactant such as Tween 80, which resulted in lower cytotoxicity than MTAD. The cytotoxicity values of MTAD and EDTA were comparable except the 6 h interval. MTAD exhibited the most cytotoxicity in the 6 h interval which may be due to its acidic pH value and the presence of a detergent (Tween 80) in its composition and slower approach to neutral pH values in the medium. This may also help us explain the higher cytotoxicity of MTAD comparing to the other irrigants.
We used MTT assay due to its sensitivity in evaluating the cytotoxicity of dental materials. This method has the advantage of simplicity, rapidity, and repeatability, and it does not require radioisotopes (Mosmann, 1983).
In the present study, cytotoxicity was increased from 1 to 6 h postoperatively which is due to cell death in the medium. From 6 to 12 h, the level of light absorption was significantly increased which meant decrease in cytotoxicity. The viability of cells in the culture medium is dependant to on several factors including nutritional reserve, the cytotoxicity of different substances, dispersal ability of substances within the environment, and adaptation of the cells to the environment (Matsumoto et al., 1983; Freshney, 1992; Petronini et al., 1993; Kandror et al., 2002). A sudden increase can be seen in the amount of viable cells at 12 h interval which may be due to adaptation of the cells to the medium environment (cell renewal capacity) and approximation of the pH to the neutral values (Matsumoto et al., 1983; Freshney, 1992; Petronini et al., 1993; Kandror et al., 2002).
We only evaluated the effect of pH on cytotoxicity of some root canal irrigants. Further studies have to concentrate on other aspects besides pH.
5. Conclusion
It can be concluded that 2.6% NaOCl has the least cytotoxic effects on HGFs compared to other root canal irrigants. Variation of the pH resulted in variation in the cytotoxicity of solutions; i.e., it follows the pattern of the pH variation. Thus, use of root canal irrigants with initial neutral pH and high dispersal ability is recommended during endodontic treatment to avoid any possible negative effects on host tissues.
6. Conflict of interest
The authors deny any conflicts of interest related to this study.
Acknowledgments
We are indebted to Professor Houtan Aghili for the provision of laboratory facilities in the Department of Biomaterial Sciences, Kamal Asgar Research Center. Also special thanks to professors, Mohammad Saghiri, HajarAfsar Ladjvardi and Niloofar Bahramain for all of their contributions to this research.
References
- Barbosa S.V., Spangberg L.S., Almeida D. Low surface tension calcium hydroxide solution is an effective antiseptic. Int. Endod. J. 1994;27:6–10. doi: 10.1111/j.1365-2591.1994.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Beltz R.E., Torabinejad M., Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J. Endod. 2003;29:334–337. doi: 10.1097/00004770-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Bloomfield S.F., Miles G.A. The antibacterial properties of sodium dichloroisocyanurate and sodium hypochlorite formulations. J. Appl. Bacteriol. 1979;46:65–73. doi: 10.1111/j.1365-2672.1979.tb02582.x. [DOI] [PubMed] [Google Scholar]
- Cameron J.A. The effect of a fluorocarbon surfactant on the surface of the endodontic irrigant, sodium hypochlorite. A preliminary report. Aust. Dent. J. 1986;31:364–368. doi: 10.1111/j.1834-7819.1986.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Chan C.P., Jeng J.H., Hsieh C.C., Lin C.L., Lei D., Chang MC. Morphological alterations associated with the cytotoxic and cytostatic effects of citric acid on cultured human dental pulp cells. J. Endod. 1999;25:354–358. doi: 10.1016/S0099-2399(06)81171-4. [DOI] [PubMed] [Google Scholar]
- Christensen C.E., McNeal S.F., Eleazer P. Effect of lowering the pH of sodium hypochlorite on dissolving tissue in vitro. J. Endod. 2008;34:449–452. doi: 10.1016/j.joen.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Collet R., Massilamoni M., Kettering J., Torabinejad M. The biocompatibility of some root canal medicaments and irrigants. Int. Endod. J. 1981;14:115–120. doi: 10.1111/j.1365-2591.1981.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Cotter J.L., Fader R.C., Lilley C., Herndon D.N. Chemical parameters, antimicrobial activities, and tissue toxicity of 0.1% and 0.5% sodium hypochlorite solutions. Antimicrob. Agents Chemother. 1985;28:118–122. doi: 10.1128/aac.28.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin H.D. On the use of certain antiseptic substances in treatment of infected wounds. B.M.J. 1915;2:318–320. doi: 10.1136/bmj.2.2852.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrela C., Sydney G.B., Bammann L.L., Felippe O., Jr. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz. Dent. J. 1995;6:85–90. [PubMed] [Google Scholar]
- Freshney R.I. Oxford University Press; USA: 1992. Animal Cell Culture: A Practical Approach. [Google Scholar]
- Giardino L., Ambu E., Becce C., Rimondini L., Morra M. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J. Endod. 2006;32:1091–1093. doi: 10.1016/j.joen.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Glantz P.O., Hansson L. Wetting of dentine by some root canal medicaments. Odontol. Revy. 1972;23:205–210. [PubMed] [Google Scholar]
- Kandror O., DeLeon A., Goldberg A.L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA. 2002;99:9727–9732. doi: 10.1073/pnas.142314099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulaouzidou E.A., Margelos J., Beltes P., Kortsaris A.H. Cytotoxic effects of different concentrations of neutral and alkaline EDTA solutions used as root canal irrigants. J. Endod. 1999;25:21–23. doi: 10.1016/S0099-2399(99)80393-8. [DOI] [PubMed] [Google Scholar]
- Masillamoni C.R., Kettering J.D., Torabinejad M. The biocompatibility of some root canal medicaments and irrigants. Int. Endod. J. 1981;14:115–120. doi: 10.1111/j.1365-2591.1981.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Control of cell division in Saccharomyces cerevisiae mutants defective in adenylate cyclase and cAMP-dependent protein kinase. Exp. Cell. Res. 1983;146:151–161. doi: 10.1016/0014-4827(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moorer W.R., Wesselink P.R. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int. Endod. J. 1982;15:187–196. doi: 10.1111/j.1365-2591.1982.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Patterson S. In vivo and in vitro studies of the effect of the disodium salt of EDTA on human dentine. Oral. Surg. Oral. Med. Oral. Pathol. 1963;16:83–103. doi: 10.1016/0030-4220(63)90367-0. [DOI] [PubMed] [Google Scholar]
- Pecora J.D., Guimaraes L.F., Savioli R.N. Surface tension of several drugs used in endodontics. Braz. Dent. J. 1991;2:123–127. [PubMed] [Google Scholar]
- Petronini P.G., Alfieri R., De Angelis E., Campanini C., Borghetti A.F., Wheeler K.P. Different HSP70 expression and cell survival during adaptive responses of 3T3 and transformed 3T3 cells to osmotic stress. Br. J. Cancer. 1993;67:493–499. doi: 10.1038/bjc.1993.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira J.F., Jr., Machado A.G., Silveira R.M., Lopes H.P., de Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int. Endod. J. 1997;30:279–282. doi: 10.1046/j.1365-2591.1997.00096.x. [DOI] [PubMed] [Google Scholar]
- Smith R.M., Martell A.E. Plenum Press; New York: 1976. Critical Stability Constants. [Google Scholar]
- Spångberg L., Engström B., Langeland K. Biologic effects of dental materials. 3. Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral. Surg. Oral. Med. Oral. Pathol. 1973;36:856–871. doi: 10.1016/0030-4220(73)90338-1. [DOI] [PubMed] [Google Scholar]
- Tanomaru Filho M., Leonardo M.R., Silva L.A., Aníbal F.F., Faccioli L.H. Inflammatory response to different endodontic irrigating solutions. Int. Endod. J. 2002;35:735–739. doi: 10.1046/j.1365-2591.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- Tasman F., Çehreli Z.C., Ogan C., Etikan I. Surface tension of root canal irrigants. J. Endod. 2000;26:586–587. doi: 10.1097/00004770-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Tay F.R., Pashley D.H., Loushine R.J., Doyle M.D., Gillespie W.T., Weller R.N., King N.M. Ultrastructure of smear layer-covered intraradicular dentin after irrigation with BioPure MTAD. J. Endod. 2006;32:218–221. doi: 10.1016/j.joen.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Torneck C. Reaction of hamster tissue to drugs used in sterilization of the root canal. Oral. Surg. Med. Pathol. 1961;14:730–747. doi: 10.1016/0030-4220(61)90230-4. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Tatematsu Y., Fujii S., Maeda H., Akamine A., Torabinejad M., Saito T. Effect of MTAD on the differentiation of osteoblast-like cells. J. Endod. 2010;36:260–263. doi: 10.1016/j.joen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Zehnder M. Root canal irrigants. J. Endod. 2006;32:389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Zehnder M., Kosicki D., Luder H., Sener B., Waltimo T. Tissue-dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2002;94:756–762. doi: 10.1067/moe.2002.128961. [DOI] [PubMed] [Google Scholar]
- Zhang W., Torabinejad M., Li Y. Evaluation of cytotoxicity of MTAD using the MTT-tetrazolium method. J. Endod. 2003;29:654–657. doi: 10.1097/00004770-200310000-00010. [DOI] [PubMed] [Google Scholar]


