Abstract
Previous animal and laboratory studies have shown the possible cariostatic effect of iron. The purpose of this study was to in vitro study the effect of different concentrations of four iron supplements on the initiation of dental caries.
Materials and methods: Four products of iron supplements were used namely fre-in-sol, ferotonic, feromin and ferose. Two hundred extracted teeth were distributed randomly into 10 groups. Eight groups were from the four iron products in two concentrations (100% and 50%) in addition to the positive and negative control groups. Mutans streptococci bacteria (6715) grown in Todd Hewitt Broth were used. Assessment of decalcification and cavitation was done daily for 60 days.
Results: It showed that different iron – supplement products play cariostatic effect in the initiation of the dental caries. With the exception of 100% and 50% ferose, both 100% and 50% concentrations of all supplements have cariostatic effect. The mean dates for decalcification varied with lowest for the positive control (12.2 days) and the highest was for 50% feromin. Cavitation was seen in the positive control and 100% ferose groups with mean on the first day of cavitation of 57 days.
Conclusion: It was concluded that iron may have cariostatic effect on the in vitro development of dental caries in human teeth.
1. Introduction
Dental caries is a very common disease affecting humans. It is caused by Mutans streptococci (MS) bacteria which was present in the oral cavity and have been identified as the principal bacteria causing dental caries in human (Hamada and Slade, 1980). Dental caries ranks among the most significant human disease. In Saudi Arabia, the prevalence of dental caries in children has been reported in different studies (Al-Shammery et al., 1990; Al-Tamimi and Peterson, 1998; Wyne, 2008) A recent study Wyne (2008) reported the prevalence of dental caries in Saudi children was 74% because caries can continue throughout life and preventive measures must be part of everyone’s lifestyle.
Preventive methods include fluoride, fissure sealants, dietary advice and patient education, as well as recall visits (Duckworth and Bebington, 1993). It is known that fluoride is effective in reducing dental caries (Bawden et al., 1978; Bjarnason and Finnbogason, 1991). Another possible caries preventive method is by using minerals (Lonnerdal et al., 1983).
It has been found that iron decreases the caries development in desalivated rats (Rosalen et al., 1996; Miguel et al., 1997). Results of previous experiments suggest that iron (Fe) added to a cariogenic diet can reduce the incidence of dental caries in animals and humans (Opperman and Rolla, 1980; Davey and Embery, 1992; Larsson et al., 1992). Devulapalle and Mooser (2001) have shown that iron ions are strong inhibitors of the glucosetransferase enzyme (GTF). Gierat-Kucharzewska and Karasinski (2006) and Clarke et al. (2006) have shown that iron plays an important role in the development of dental caries. Furthermore, Berlutti et al. (2004) concluded that iron could have a key role in the protection of the oral cavity from Streptococcus mutans pathogenicity.
Our previous study investigated the effect of one iron supplement (Fre-in-sol) on the initiation of dental caries (Al-Shalan and Al-Askar, 2006). Results have shown that iron plays a cariostatic role in the development of dental caries. Knowing the fact that pediatricians prescribe different iron supplements for children suffering from anemia, it might be interesting to study the effect of other iron supplements products on the initiation of dental caries. Therefore, the aim of this study was to in vitro study the effect of different concentrations of four iron supplements on the initiation of dental caries.
2. Materials and methods
2.1. Teeth preparation
Two hundred extracted caries and restoration-free human permanent premolar teeth were used. These teeth were collected, cleaned and stored using thymol at room temperature until used. Teeth were prepared as described previously (Al-Shalan and Al-Askar, 2006). In brief, teeth were mounted using a cold cure acrylic resin covering the tooth to cemento-enamel-junction. Selected area on the buccal surface of the teeth was covered with a drop of colored nail polish. Then the whole coronal part was covered with transparent nail polish. After the varnish dries, the colored parts were removed to leave one exposed enamel surface of approximately 0.5 cm2. Teeth were randomly distributed to ten groups (Table 1).
Table 1.
Distribution of teeth to experimental and control groups (N = 200).
| Group number | Group name (N = 20/group) |
Media (μl) |
||
|---|---|---|---|---|
| Bacteria | 10% Sucrose | Iron | ||
| First | Positive control | 100 | 100 | 000a |
| Second | Negative control | 000a | 100 | 000a |
| Third | 100% fre-in-sol | 100 | 100 | 100 |
| Fourth | 50% fre-in-sol | 100 | 100 | 100 |
| Fifth | 100% ferotonic | 100 | 100 | 100 |
| Sixth | 50% ferotonic | 100 | 100 | 100 |
| Seventh | 100% feromin | 100 | 100 | 100 |
| Eighth | 50% feromin | 100 | 100 | 100 |
| Ninth | 100% ferose | 100 | 100 | 100 |
| Tenth | 50% ferose | 100 | 100 | 100 |
100 ml dH2O.
2.2. Iron sources
Four products of iron supplements were used. These products were: (1) Fre-in-sol (Bristol Myers Squibb Company, New Jersey, USA), (2) Ferotonic (Ram Pharmaceutical, Amman, Jordan), (3) Feromin (Riyadh Pharma, Riyadh, KSA) and (4) Ferose (Spimaco Al Qassim Pharmaceutical Plant, Saudi Arabia). As supplied, two concentrations (100% and 50%) were used per group. The 100% concentration means that the content of the bottle was used directly without dilution. Whereas, 50% concentration was prepared by adding 50% of the product to 50% distilled water (dH2o) in sterilized containers. A total of eight experimental groups were made.
2.3. Artificial caries experiment
Ten 24-well ELISA plates (2.0 ml volume/well) were used. Each plate was assigned to one group. The mounted teeth were placed in a ELISA well containing immersed solution media of 1.00 ml of THB containing MS bacteria strain (6715), 100 μl of 10% sucrose and 100 μl iron in different concentrations/products. For the positive control group, no iron was used. No bacteria were used for the negative control group. For both control groups, 100 μl dH2o was used. The plates were incubated in an anaerobic chamber at 37 °C.
2.4. Caries progress evaluation
The progress of dental caries initiation and progression was evaluated by visual examination (decalcification) and tactile examination (cavitations). The decalcification and caries progression were recorded daily for 60 days. The evaluations were done independently by two examiners and the lower value was taken.
2.5. Statistical analysis
Data was entered and analyzed using descriptive statistics to show the general behavior of the data. Chi-square test was used to determine the relationship between the groups. If the chi-square was not valid due to the small number of outcome, Fisher exact test was used. The significant level was fixed at 0.05 probabilities.
3. Results
3.1. Visual examination
Fig. 1 shows that all teeth in group one and ten (positive control and 50% ferose, respectively) developed decalcification. Other groups (except the negative control) showed decalcification in some teeth. Table 2 summarizes the descriptive data of the visual examination. The means of the first day of decalcification was 12.2 days for group one and 17.5 days for group 10. Additionally, group three (100% fre-in-sol) and group eight (50% feromin) showed only two teeth decalcified. In these two groups, the means of the initial day of decalcification was 29 days for group three (100% fre-in-sol) and 50 days for group eight (50% feromin). Furthermore, group four (50% fre-in-sol), five (100% ferotonic), six (50% ferotonic) and seven (100% feromin) showed only three teeth decalcified. The means of the first day of decalcification was 36 days for group three and 45 days for the other three groups. In group nine (100% ferose), 16 teeth showed decalcification and the means of initial day of decalcification was 35.12 days.
Figure 1.
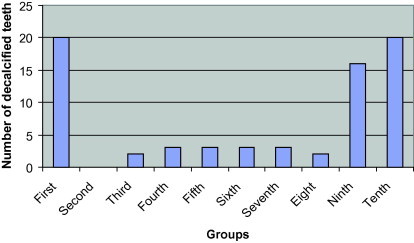
Number of decalcified teeth per group after 60 days.
Table 2.
The descriptive statistics (number and mean) of decalcification date for all groups using visual examination.⁎
| Group number | Group name | Number of decalcified teeth | Means of the decalcified dates |
|---|---|---|---|
| First | Positive control | 20 | 12.2 |
| Second | Negative control | 0 | 0 |
| Third | 100% fre-in-sol | 2 | 29 |
| Fourth | 50% fre-in-sol | 3 | 36 |
| Fifth | 100% ferotonic | 3 | 45.3 |
| Sixth | 50% ferotonic | 3 | 45.3 |
| Seventh | 100% feromin | 3 | 45.3 |
| Eighth | 50% feromin | 2 | 50 |
| Ninth | 100% ferose | 16 | 35.12 |
| Tenth | 50% ferose | 20 | 17.45 |
After 60 days.
The number (%) of sound teeth group at the end of the study using visual examination is shown in Table 3. All teeth in positive control group and 50% ferose group developed decalcification. In group nine (100% ferose), 4 teeth (out of 20) did not show decalcification. Group four (50% fre-in-sol), five (100% ferotonic), six (50% ferotonic) and seven (100% feromin) showed 85% of teeth were sound (N = 17). Additionally, Table 3 shows that group three (100% fre-in-sol) and group eight (50% feromin) showed 90% of the teeth were sound (N = 18). At the end of the experiment, 72 teeth were decalcified and 128 teeth were sound.
Table 3.
Number and percentage of sound teeth per group at the end of the study using visual examination.
| Group | Name | Number of decalcified teeth | Number of sound teeth | Percent of sound teeth |
|---|---|---|---|---|
| First | Positive control | 20 | 0 | 0 |
| Second | Negative control | 0 | 20 | 100 |
| Third | 100% fre-in-sol | 2 | 18 | 90 |
| Fourth | 50% fre-in-sol | 3 | 17 | 85 |
| Fifth | 100% ferotonic | 3 | 17 | 85 |
| Sixth | 50% ferotonic | 3 | 17 | 85 |
| Seventh | 100% feromin | 3 | 17 | 85 |
| Eighth | 50% feromin | 2 | 18 | 90 |
| Ninth | 100% ferose | 16 | 4 | 20 |
| Tenth | 50% ferose | 20 | 0 | 0 |
| Overall | 72 | 128 | 64 | |
Multiple comparisons between groups (number of teeth decalcified) diagnosed by visual examination using chi-square test is shown in Table 4. Statistical analysis between different groups showed significant differences (P < 0.05) between the control and other groups except group ten. Significant difference (P = 0.0001) was found between group one (positive control) and all other groups except group nine which showed marginal significant difference (P = 0.053). Other significant difference was found between group nine and ten (100% ferose, 50% ferose, respectively) and all other groups (P = 0.000).
Table 4.
Multiple comparisons between groups (number of teeth decalcified) diagnosed by visual examination using chi-square test.
| Group | Second | Third | Fourth | Fifth | Sixth | Seventh | Eighth | Ninth | Tenth |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.053 | 1.000 |
| Group 2 | – | 0.244 | 0.115 | 0.115 | 0.115 | 0.115 | 0.244 | 0.000 | 0.000 |
| Group 3 | – | – | 0.500 | 0.500 | 0.500 | 0.500 | 0.698 | 0.000 | 0.000 |
| Group 4 | – | – | – | 0.698 | 0.698 | 0.698 | 0.500 | 0.000 | 0.000 |
| Group 5 | – | – | – | – | 0.669 | 0.669 | 0.500 | 0.000 | 0.000 |
| Group 6 | – | – | – | – | – | 0.669 | 0.500 | 0.000 | 0.000 |
| Group 7 | – | – | – | – | – | – | 0.500 | 0.000 | 0.000 |
| Group 8 | – | – | – | – | – | – | – | 0.000 | 0.000 |
| Group 9 | – | – | – | – | – | – | – | – | 0.530 |
a If number of cells had expected frequency were more than 20%, the exact fisher test was used.
3.2. Tactile examination
Table 5 summarizes the descriptive data of the tactile examination used for the diagnosis of cavitations. It shows that only five teeth developed cavitation. Four teeth were from group one (positive control) and one tooth from group nine (100% ferose). The means on the first day of cavitations was 57 days. Furthermore, data shows that 80% of group one (positive control), 95% from group nine (100% ferose) and all other groups were sound. Statistical analysis between different groups is shown in Table 6. Significant difference between group one (positive control) and all other groups (P = 0.024) except group nine (100% ferose) which showed no significant difference (P = 0.091).
Table 5.
The descriptive statistic (number and means) on the first day of cavitation and number and percentage of sound teeth using tactile examination. (∗Experimental period = 60 days).
| Group | Name | Number of carious teeth | Means of the first day of cavitations | % Of sound teeth |
|---|---|---|---|---|
| First | Positive control | 4 | 57 | 80 |
| Second | Negative control | 0 | 0 | 100 |
| Third | 100% fre-in-sol | 0 | 0 | 100 |
| Fourth | 50% fre-in-sol | 0 | 0 | 100 |
| Fifth | 100% ferotonic | 0 | 0 | 100 |
| Sixth | 50% ferotonic | 0 | 0 | 100 |
| Seventh | 100% feromin | 0 | 0 | 100 |
| Eighth | 50% feromin | 0 | 0 | 100 |
| Ninth | 100% ferose | 1 | 57 | 95 |
| Tenth | 50% ferose | 0 | 0 | 100 |
Table 6.
Multiple comparisons between group’s means of number of cavitations by using chi-square test by tactile examination (If number of cells had expected frequency more than 20%, the exact fisher test was used.)
| Group | Second | Third | Fourth | Fifth | Sixth | Seventh | Eighth | Ninth | Tenth |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | 0.024 | 0.024 | 0.024 | 0.024 | 0.024 | 0.024 | 0.024 | 0.091 | 0.024 |
| Group 2 | – | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.500 | 1.000 |
| Group 3 | – | – | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.500 | 1.000 |
| Group 4 | – | – | – | 1.000 | 1.000 | 1.000 | 1.000 | 0.500 | 1.000 |
| Group 5 | – | – | – | – | 1.000 | 1.000 | 1.000 | 0.500 | 1.000 |
| Group 6 | – | – | – | – | – | 1.000 | 1.000 | 0.500 | 1.000 |
| Group 7 | – | – | – | – | – | – | 1.000 | 0.500 | 1.000 |
| Group 8 | – | – | – | – | – | – | – | 0.500 | 1.000 |
| Group 9 | – | – | – | – | – | – | – | – | 0.500 |
4. Discussion
The objective of this study was to investigate the effect of different iron – supplement products on the initiation of dental caries using an in vitro model. An in vitro caries model was used for ease of control of different factors (e.g. saliva and host factors) that may play a role in the initiation and progression of dental caries. An in vitro model was used previously (Al-Shalan and Al-Askar, 2006; Lammers, 1990; Kotsanos, 1991; Featherstone, 1996; Hall, 1997). In addition, extracted teeth were collected from young patients where the teeth were extracted for orthodontics reason. The results from the in vitro models might be regarded as guidance for future in vivo studies. Such studies are important before human trials are conducted.
The iron products used in this study are used widely for the treatment of anemia (Oski, 1993). Additional products are available and future studies might investigate their effect on the initiation of dental caries. In this study, 100% of the products were supplied and the 50% dilution was used. It is possible that the iron concentration in each product is different. Therefore, a better control will be to standardize the iron concentration per product which may be investigated in future studies.
In addition to the experimental groups, positive and negative control groups were used in this study. The objective of using positive control was to ensure that the model we used is effective in producing decalcification and/or cavitations. The negative control group was used to rule out the possibility of contamination that might occur. The results showed that both control groups served their purposes.
The diagnosis of dental caries is fundamental to the practice of dentistry. Early stage of dental caries is difficult to diagnose (Dodds, 1993; Rosen et al., 1996). Caries is diagnosed by examination of teeth including visual inspection, radiograph and tactile examination by dental explorer (Dodds, 1993).
4.1. Visual examination
In this study, visual examination was used for the diagnosis of decalcification. Results showed that the teeth in the positive control group developed decalcification on all the teeth. In contrast, the teeth in the negative control group did not develop any decalcification. This showed the good controls used. If a contamination took place, negative control might have developed decalcification in some teeth. This may ensure to a certain limit, the elimination of contamination possibility in our study.
Results showed that teeth in groups containing fre-in-sol, ferotonic and feromin developed less decalcification as compared to ferose groups. This might be attributed to different iron product used. Fre-in-sol, ferotonic and feromin contain ferrous sulphate and ferose contain iron (III) – hydroxide polymaltose complex. Torell (1988) reported that application of different iron product in term of content have different cariostatic effect. For ferrous sulphate groups, it was found to have cariostatic effect on experimental hamster studies when applied topically to the teeth as when added to the drinking water or the diet (Dodds, 1993; Emilson and Krasse, 1972). Rosalen et al. (1996) and Miguel et al. (1997) had demonstrated that the iron sources containing ferrous sulphate reduced the development of dental caries in intact and desalivated rat.
For the ferose group, ferose contain iron III – hydroxide polymaltose complex. There is no cariostatic effect compared to other ferrous sulphate groups. This might be attributed to the hydrolysis of maltose that produced two molecules of glucose. Another possible effect is the formation of complex between hydroxide plymaltose and iron that may lead to have very few iron to react with the dental enamel (Torell, 1988).
4.2. Tactile examination
Dental explorer was used for diagnosis of cavitation (Tactile examination). This examination was used in different studies (Penning et al., 1992; El-Housseiny and Jamjoum, 2001; Lussi et al., 2001). This technique has been criticized due to the possibility of transferring microorganism from one side to another. In addition, a sharp explorer can actually produce irreversible traumatic defect in the early demineralized area (Ekstrand et al., 1987; Penning et al., 1992). All teeth in group 1 and group 10, and 16 teeth from group 9 developed decalcification. The result showed that only five teeth developed cavitation. Four teeth were from group 1 (positive control) and one tooth from group 9 (100% ferose). Significant difference was found between group 1 (positive control) and all other groups except group 9 (100% ferose). The findings were confirmed by visual examination. As the experiment extended for 60 days, there is a need to extend the time as all teeth diagnosed as cavitations by tactile examination occurred in the last three days.
4.3. Effect of Iron on dental caries
Our results confirmed those of the previous studies that showed a cariostatic effect of ferrous sulphate (Miguel et al., 1997; Devulapalle and Mooser, 2001). Iron is able to cover the enamel surface with protecting layers. Acid protective layer effect has been demonstrated in vitro. (Al-Shalan and Al-Askar, 2006; Clarkson et al., 1984). It can be assumed that these protective layers will be firmly bound to the organic parts of the enamel as hydrous iron oxides and clay materials have great affinity to organic materials (Craig, 1979). This affinity has been utilized in the clinical work to increase the adhesion between composite resins and dentine by mordanting with ferric chloride (Jedrychowski et al., 1981).
Gels and crystal of hydrous iron oxides are capable of adsorbing various ions and of nucleating various crystalline substances. From a dental point of view, the adsorption of calcium and phosphate ions is of special interest as it indicates that iron may have a repairing function (Torell, 1988). This can be by nucleating the precipitation of salivary calcium and phosphate ions as apatite or other phosphates on the enamel surface. Therefore, the iron may have the effect of replacing minerals which can have been dissolved during the acid phases of the caries process. In addition, owing to the above-mentioned affinity to organic materials iron ought to be able to mediate the fixation of remineralized particles to the organic parts of the enamel (Torell, 1988).
The concept of a possible role of iron in the remineralization of human enamel is supported by several studies. Bachra and van Harskamp (1970) showed that extremely low concentrations of iron could initiate precipitation of calcium apatite from meta stable calcifying buffer systems. Moreover, remineralized caries lesion was reported to contain more iron than intact enamel surface layers (Torell, 1988).
5. Conclusion
Based on the results of this in vitro study, we conclude Different iron supplement products used in this study play cariostatic effect in the development of the dental caries. With the exception of 100% ferose, both 100% and 50% concentrations of all remaining products have cariostatic effect.
References
- Al-Shalan T.A., Al-Askar A. In vitro effect of different concentrations of iron on the initiation of dental caries: pilot study. Saudi Dent. J. 2006;18:86–90. [Google Scholar]
- Al-Shammery A.R., Guile E.E., El-Backly M. Prevalence of caries in primary school children in Saudi Arabia. Community Dent. Oral Epidemiol. 1990;18:320–321. doi: 10.1111/j.1600-0528.1990.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Al-Tamimi S., Peterson P.E. Oral health situation of school children, mothers and school teachers in Saudi Arabia. Int. Dent. J. 1998;48:180–186. doi: 10.1111/j.1875-595x.1998.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Bachra B.N., van Harskamp G.A. The effect of polyvalent metal ions on the stability of a buffer system for calcification in vitro. Calcified Tissue Res. 1970;4:359–365. doi: 10.1007/BF02279138. [DOI] [PubMed] [Google Scholar]
- Bawden J.W., Wennberg A., Hammarström L. In vivo and in vitro study of Fe uptake in developing rat molars. Acta Odontol. Scand. 1978;36:271–277. doi: 10.3109/00016357809029077. [DOI] [PubMed] [Google Scholar]
- Berlutti F., Ajello M., Bosso P., Morea C., Petrucca A., Antonini G., Valenti P. Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. Biometals. 2004;17:271–278. doi: 10.1023/b:biom.0000027704.53859.d3. [DOI] [PubMed] [Google Scholar]
- Bjarnason S., Finnbogason S.Y. Effect of different fluoride levels in dentifrices on the development of a proximal caries. Caries Res. 1991;25:207–212. doi: 10.1159/000261369. [DOI] [PubMed] [Google Scholar]
- Clarke M., Locker D., Berall G., Pencharz P., Kenny D.J., Judd P. Malnourishment in a population of young children with severe early childhood caries. Pediatr. Dent. 2006;28:254–259. [PubMed] [Google Scholar]
- Clarkson B.H., Wefel J.S., Miller I. The effect of polyvalent metal ion mordanting on caries-like lesion progression in enamel. J. Dent. Res. 1984;63:13–18. doi: 10.1177/00220345840630010101. [DOI] [PubMed] [Google Scholar]
- Craig G.G. Effect of FeF2 and FeF3 on the dissolution rate of bovine enamel. J. Dent. Res. 1979;58:16–54. doi: 10.1177/00220345790580061701. [DOI] [PubMed] [Google Scholar]
- Davey H., Embery G. Metal ion in oral hygiene products. In: Embery G., Rolla G., editors. Clinical and Biological Aspects of Dentifrices. Oxford University Press; Oxford: 1992. pp. 165–172. [Google Scholar]
- Devulapalle K.S., Mooser G. Glucosyltransferase inactivation reduces dental caries. J. Dent. Res. 2001;80:466–469. doi: 10.1177/00220345010800021301. [DOI] [PubMed] [Google Scholar]
- Dodds M. Dilemmas in caries diagnosis – applications to current practice and need for research. J. Dent. Educ. 1993;57:433–438. [PubMed] [Google Scholar]
- Duckworth R.M., Bebington U.K. The science behind caries prevention. Int. Dent. J. 1993;43:529–539. [PubMed] [Google Scholar]
- Ekstrand K., Qvist V., Thylstrup A. Light microscope study of the effect of probing in occlusal surfaces. Caries Res. 1987;21:368–374. doi: 10.1159/000261041. [DOI] [PubMed] [Google Scholar]
- El-Housseiny A.A., Jamjoum I.I. Evaluation of visual, explorer and a laser device for detection of early occlusal caries. J. Clin. Pediatr. Dent. 2001;26:41–48. doi: 10.17796/jcpd.26.1.ch28322k5837j772. [DOI] [PubMed] [Google Scholar]
- Emilson C.G., Krasse B. The effect of iron salts on experimental dental caries in the hamster. Arch. Oral Biol. 1972;17:1439–1443. doi: 10.1016/0003-9969(72)90103-3. [DOI] [PubMed] [Google Scholar]
- Featherstone J.D. Modeling the caries-inhibitory effects of dental materials. Dent. Mater. 1996;12:194–197. doi: 10.1016/s0109-5641(96)80021-2. [DOI] [PubMed] [Google Scholar]
- Gierat-Kucharzewska B., Karasinski A. Influence of chosen elements on the dynamics of cariogenic process. Biol. Trace Elem. Res. 2006;111:53–62. doi: 10.1385/BTER:111:1:53. [DOI] [PubMed] [Google Scholar]
- Hall A.F. Determination of plaque pH changes within the trough of an in situ appliance used to study mineral changes in early carious lesions. Caries Res. 1997;31:50–54. doi: 10.1159/000262374. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H.D. Biology, immunology and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski J.R., Caputo A.A., Prola J. Influence of a ferric chloride mordant solution on resin-dentin retention. J. Dent. Res. 1981;60:134–138. doi: 10.1177/00220345810600020701. [DOI] [PubMed] [Google Scholar]
- Kotsanos N. Influence of post-eruptive age of enamel on its susceptibility to artificial caries. Caries Res. 1991:241–250. doi: 10.1159/000261371. [DOI] [PubMed] [Google Scholar]
- Lammers P.C. Influence of fluoride on in vitro remineralization of artificial subsurface lesions determined with a sandwich technique. Caries Res. 1990:81–85. doi: 10.1159/000261244. [DOI] [PubMed] [Google Scholar]
- Larsson B., Johansson I., Ericson T. Prevalence of caries in adolescents in relation to diet. Community Dent. Oral Epidemiol. 1992;20:133–137. doi: 10.1111/j.1600-0528.1992.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B., Keen C.L., Ohtake M., Tamua T. Iron, zinc, copper and manganese in infant formulas. Am. J. Dis. Child. 1983;137:433–437. doi: 10.1001/archpedi.1983.02140310015003. [DOI] [PubMed] [Google Scholar]
- Lussi A., Megert B., Longbottom C., Reieh F., Francecut P. Clinical performance of a laser fluorescence device for detection of occlusal caries lesions. Eur. J. Oral Sci. 2001;109:14–19. doi: 10.1034/j.1600-0722.2001.109001014.x. [DOI] [PubMed] [Google Scholar]
- Miguel J.C., Bowen W.H., Pearson S.K. Influence of iron alone or with fluoride on caries development in desalivated and intact rats. Caries Res. 1997;31:244–248. doi: 10.1159/000262407. [DOI] [PubMed] [Google Scholar]
- Opperman R.V., Rolla G. Effect of some polyvalent cations on the acidogenicity of dental plaque in vivo. Caries Res. 1980;14:422–427. doi: 10.1159/000260485. [DOI] [PubMed] [Google Scholar]
- Oski F.A. Iron deficiency in infancy and childhood. New Engl. J. Med. 1993;329:190–193. doi: 10.1056/NEJM199307153290308. [DOI] [PubMed] [Google Scholar]
- Penning C., van Amerongen J.P., Seef R.E., ten Cate J.M. Validity of probing for fissure caries diagnosis. Caries Res. 1992;26:445–449. doi: 10.1159/000261485. [DOI] [PubMed] [Google Scholar]
- Rosalen P.L., Bowen W.H., Pearson S.K. Effect of copper co-crystallized with sugar on caries development in desalivated rats. Caries Res. 1996;30:367–372. doi: 10.1159/000262344. [DOI] [PubMed] [Google Scholar]
- Rosen B., Birkhed D., Nilsson K., Olavi G., Egelberg J. Reproducibility of clinical caries diagnoses on coronal and root surfaces. Caries Res. 1996;30:1–7. doi: 10.1159/000262129. [DOI] [PubMed] [Google Scholar]
- Torell P. Iron and dental caries. Swed Dent. J. 1988;12:113–124. [PubMed] [Google Scholar]
- Wyne A.H. Caries prevalence, severity, and pattern in preschool children. J. Contemp. Dent. Pract. 2008;9:24–31. [PubMed] [Google Scholar]


