Abstract
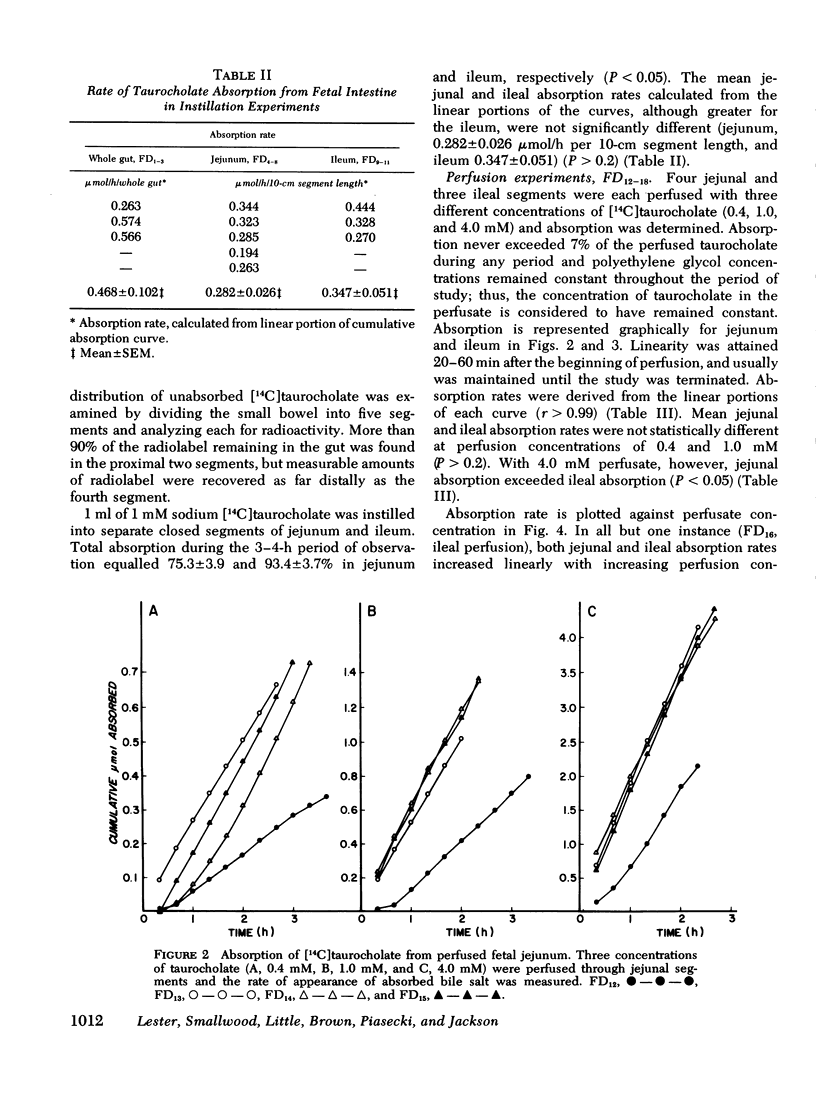
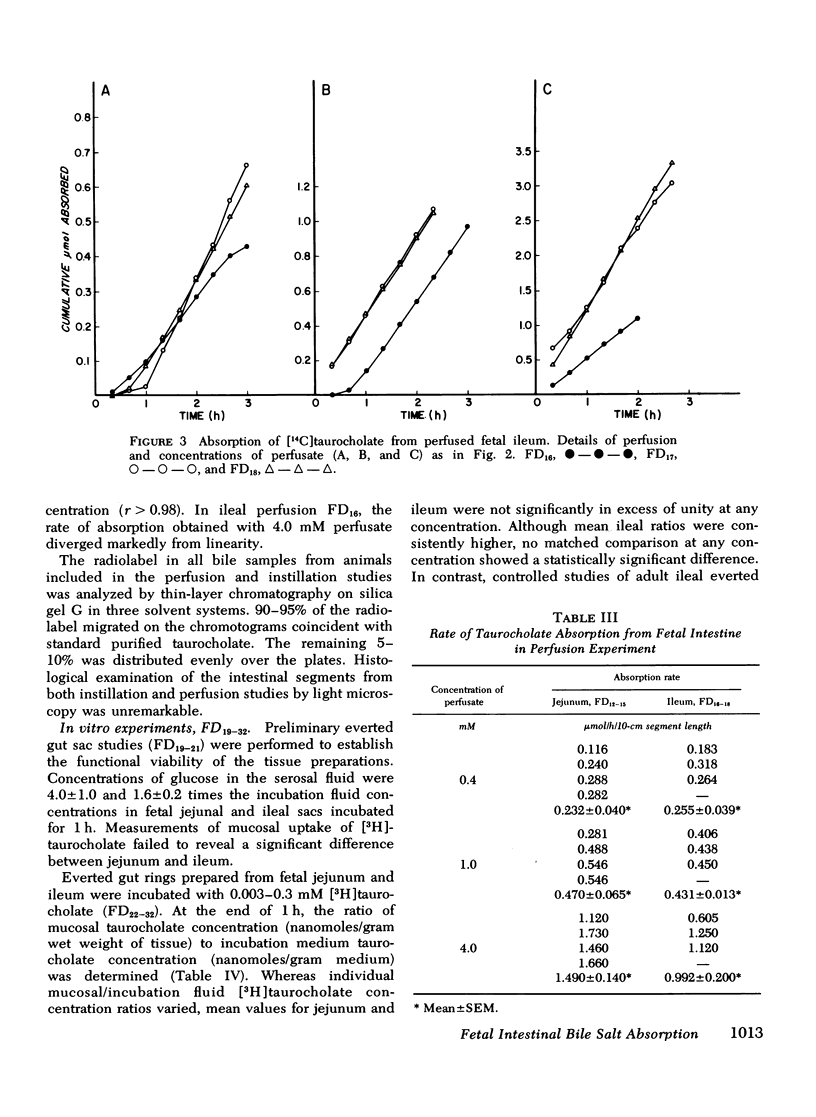
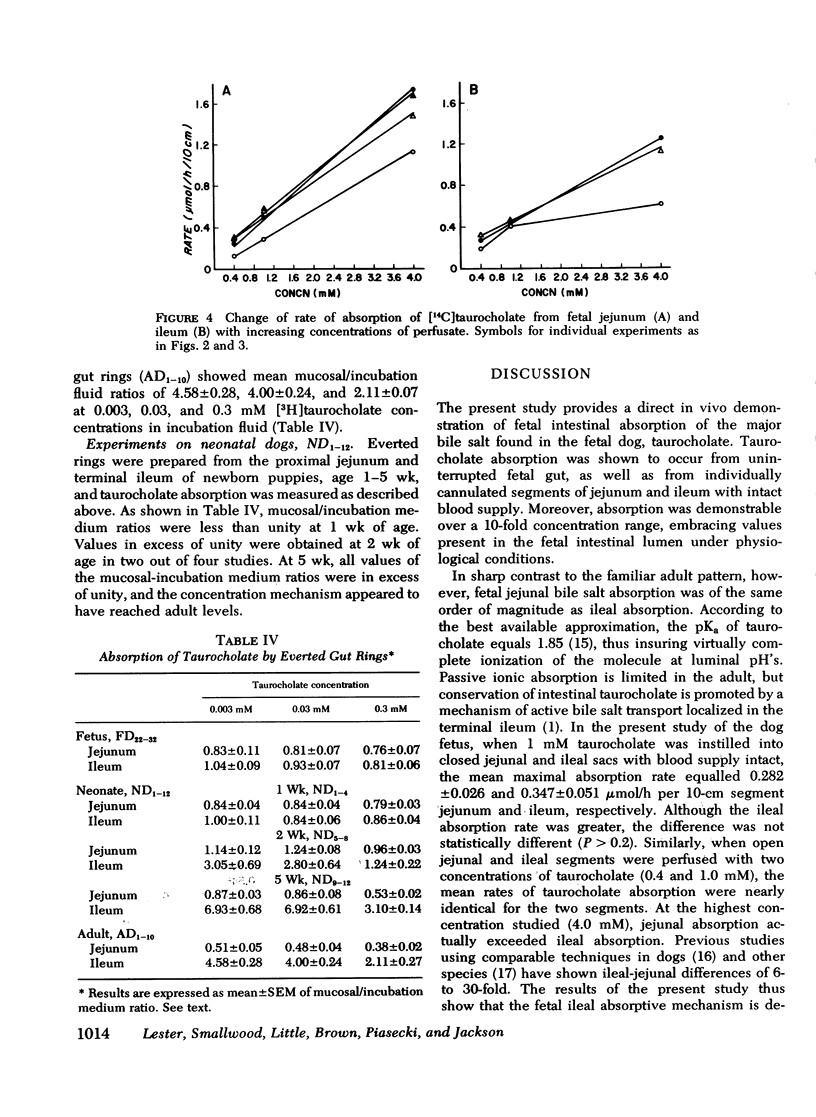
The intestinal absorption of sodium taurocholate was studied in the near-term fetal and neonatal dog. Absorption rates were measured in vivo in isolated loops of fetal jejunum and ileum. Absorption was also measured in vitro in everted sacs and rings of fetal and neonatal jejunum and ileum. The maximal rates of taurocholate absorption observed after instillation of 1 micronmol taurocholate into closed segments of fetal jejunum and ileum with intact blood supply were not significantly different (P less than 0.2), and equalled 0.282+/-0.026 (mean+/-SEM) and 0.347+/-0.051 micronmol/h per 10-cm segment length jejunum and ileum, respectively. Similarly, the rates of absorption from open segments of jejunum and ileum perfused with 0.4 and 1.0 mM taurocholate were nearly identical (0.232+/-0.040 and 0.255+/-0.039, respectively at 0.4 mM, and 0.470+/-0.065 and 0.431+/-0.013, respectively at 1.0 mm) (P greater than 0.2). At perfusate concentrations of 4.0 mM, moreoever, jejunal absorption exceeded ileal absorption (1.490+/-0.140 and 0.922+/-0.200, respectively (P less than 0.05). As expected, concentration of taurocholate by the mucosa was readily demonstrated in adult ileal, but not in adult jejunal everted rings. In contrast, there were no significant differences in mucosal uptake of taurocholate by fetal jejunal and ileal rings. Fetal ileal mucosal concentrations were not significantly above those in the incubation medium after 1-h exposure of the mucosa to 0.003, 0.03, and 0.3 mM taurocholate. Uptake was proportional to incubation medium concentration over the full range of values. This was also true of tissues from 1-wk-old neonates. However, by 2 wk of age, ileal mucosal concentration of taurocholate was evident and adult levels were attained by 5 wk of age. It is concluded that taurocholate is absorbed by the fetal gut and that ileal absorption is no more efficient than jejunal absorption. Although active glucose transport was demonstrable in both jejunum and ileum, it was not possible to demonstrate an ileal mechanism for active transport of taurocholate in the fetus. Active ileal transport was not demonstrable in the newborn until at least 2 wk after birth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORGSTROM B., DAHLQVIST A., LUNDH G., SJOVALL J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957 Oct;36(10):1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M. Difficulties in determining valid rate constants for transport and metabolic processes. Gastroenterology. 1970 Jun;58(6):863–874. [PubMed] [Google Scholar]
- Dietschy J. M. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968 May;9(3):297–309. [PubMed] [Google Scholar]
- Dowd S. R., Little J. M. Preparation of laurylsarcosyltaurine: a surface active constituent of crab gastric juice. J Lipid Res. 1976 Mar;17(2):154–155. [PubMed] [Google Scholar]
- Fomon S. J., Ziegler E. E., Thomas L. N., Jensen R. L., Filer L. J., Jr Excretion of fat by normal full-term infants fed various milks and formulas. Am J Clin Nutr. 1970 Oct;23(10):1299–1313. doi: 10.1093/ajcn/23.10.1299. [DOI] [PubMed] [Google Scholar]
- GLASSER J. E., WEINER I. M., LACK L. COMPARATIVE PHYSIOLOGY OF INTESTINAL TAUROCHOLATE TRANSPORT. Am J Physiol. 1965 Feb;208:359–362. doi: 10.1152/ajplegacy.1965.208.2.359. [DOI] [PubMed] [Google Scholar]
- JACKSON B. T., CLARKE J. P., EGDAHL R. H. Direct lead fetal electrocardiography with undisturbed fetal-maternal relationships. Surg Gynecol Obstet. 1960 Jun;110:687–692. [PubMed] [Google Scholar]
- JACKSON B. T., EGDAHL R. H. The performance of complex fetal operations in utero without amniotic fluid loss or other disturbances of fetal-maternal relationships. Surgery. 1960 Sep;48:564–570. [PubMed] [Google Scholar]
- Jackson B. T., Smallwood R. A., Piasecki G. J., Brown A. S., Rauschecker H. F., Lester R. Fetal bile salt metabolism. I. The metabolism of sodium cholate-14C in the fetal dog. J Clin Invest. 1971 Jun;50(6):1286–1294. doi: 10.1172/JCI106607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINGSLEY G. R., GETCHELL G. Direct ultramicro glucose oxidase method for determination of glucose in biologic fluids. Clin Chem. 1960 Oct;6:466–475. [PubMed] [Google Scholar]
- LACK L., WEINER I. M. In vitro absorption of bile salts by small intestine of rats and guinea pigs. Am J Physiol. 1961 Feb;200:313–317. doi: 10.1152/ajplegacy.1961.200.2.313. [DOI] [PubMed] [Google Scholar]
- LESTER R., SCHMID R. Intestinal absorption of bile pigments. I. The enterohepatic circulation of bilirubin in the rat. J Clin Invest. 1963 May;42:736–746. doi: 10.1172/JCI104766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R., Jackson B. T., Smallwood R. A. Fetal hepatic function. Birth Defects Orig Artic Ser. 1970 Jun;6(2):16–21. [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAYOUST M. R., ISSELBACHER K. J. STUDIES ON THE TRANSPORT AND METABOLISM OF CONJUGATED BILE SALTS BY INTESTINAL MUCOSA. J Clin Invest. 1964 Mar;43:467–476. doi: 10.1172/JCI104932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff E. R., Small N. C., Dietschy J. M. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972 Jun;51(6):1351–1362. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M., Dowling R. H., Redinger R. N. The enterohepatic circulation of bile salts. Arch Intern Med. 1972 Oct;130(4):552–573. [PubMed] [Google Scholar]
- Smallwood R. A., Lester R., Plasecki G. J., Klein P. D., Greco R., Jackson B. T. Fetal bile salt metabolism. II. Hepatic excretion of endogenous bile salt and of a taurocholate load. J Clin Invest. 1972 Jun;51(6):1388–1397. doi: 10.1172/JCI106934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. B., Bliss C. M., Donaldson R. M., Jr, Lester R. Characterization of newborn fecal lipid. Pediatrics. 1974 Apr;53(4):511–515. [PubMed] [Google Scholar]
- Watkins J. B., Ingall D., Szczepanik P., Klein P. D., Lester R. Bile-salt metabolism in the newborn. Measurement of pool size and synthesis by stable isotope technic. N Engl J Med. 1973 Mar 1;288(9):431–434. doi: 10.1056/NEJM197303012880902. [DOI] [PubMed] [Google Scholar]
- Watkins J. B., Szczepanik P., Gould J. B., Klein P., Lester R. Bile salt metabolism in the human premature infant. Preliminary observations of pool size and synthesis rate following prenatal administration of dexamethasone and phenobarbital. Gastroenterology. 1975 Sep;69(3):706–713. [PubMed] [Google Scholar]


