Epithelial-mesenchymal interactions by morphogenetic signaling molecules dictate patterning of the gastrointestinal tract during development and maintain homeostasis of the adult intestinal epithelium.1 The development of an intestinal adenoma can be viewed as a morphogenetic patterning event.2 During this process of adenomagenesis, the epithelial stem cell compartment is clonally expanded by genetic mutations that activate the Wnt signaling pathway. Despite the fact that these activating mutations act cell autonomously, the relevance of epithelial-mesenchymal interactions for morphogenetic events suggests that mesenchymal genes may still be required for adenomagenesis to occur. In this issue of Gastroenterology, Nik et al., now demonstrate the critical role of mesenchymal Foxf2 expression in adenoma initiation and growth.3
Negative feedback loops control intestinal epithelial homeostasis
The epithelial cells that cover the intestinal mucosa are continuously replenished from a pool of rapidly cycling cells. The fate of these proliferating cells depends on Wnt signaling which drives nuclear accumulation of β-catenin. In the nucleus β-catenin complexes with DNA-bound Tcf transcription factors and activates a transcriptional program specific for both intestinal stem cells and transit amplifying cells.4 Careful expression analyses of Wnt target genes has established that a subset of these genes such as Lgr5 and Ascl2marks the intestinal stem cells5 but the precise mechanisms that differentiate stem cells from transit amplifying cells have not been identified.
Homeostatic systems such as the rapidly renewing intestinal epithelium are self regulating systems that depend on negative feedback loops. The output (differentiated cells) produces a signal that restricts the input (rate of proliferation).This results in a tight restriction of epithelial proliferation which is dependent on the integrity of the surface epithelium. Wounding will result in loss of the negative feedback signal allowing the proliferating cells to rapidly increase the production of differentiated cells which restores the production of the feedback signal and restores homeostasis. The negative feedback loops that control intestinal epithelial homeostasis have only partially been resolved but appear to include both epithelial and mesenchymal factors.6, 7 One of the factors that plays a key role is Indian Hedgehog. Indian Hedgehog is a morphogen produced by differentiated enterocytes of the adult small intestine8 and colon.9 Conditional activation of Hedgehog signaling in the adult gut leads to reduced Wnt signaling and depletion of proliferating cells in the crypt whereas conditional loss of Indian Hedgehog signaling results in activation of Wnt signaling and increased epithelial proliferation. This is sufficient to result in features of epithelial repair such as crypt lengthening and fissioning.8, 10 Effects of Hedgehog signaling on the proliferating cells are indirect and exclusively mediated via the mesenchyme. During development Foxf1 and Foxf2 are mesodermally expressed transcription factors that are regulated by Hedgehog signaling. The relevance of Foxf expression to mediate effects of Hedgehog signaling during development of the gut is underscored by two previous findings of the Carlsson lab. The first is that Foxf1 heterozygous mice have a foregut and airway phenotype that is identical to that of Sonic Hedgehog mutant mice.11, 12 The second is the developing intestine of Foxf1 and Foxf2 mutant mice shows ectopic activation of Wnt signaling on the villi and expansion of epithelial precursor cells,13 a phenotype similar to mice in which Hedgehog is specifically inhibited in the intestine during development.14–16 In their publication in this issue of Gastroenterology the group shows for the first time that Foxf2 is a mesenchymally expressed negative regulator of the epithelial precursor cells in the adult intestine. Nik et al. examine a Foxf2+/− mutant mouse that was previously generated by their group. In addition they generated a novel BAC transgenic mouse in which extra copies of Foxf2 and surrounding genomic region were introduced and Foxf2 expression is therefore driven from its own regulatory sequences. This technique therefore allows overexpression of genes in a spatiotemporal manner that approaches the regulation of expression of the endogenous gene. Using these tools to modulate expression of Foxf2, the authors find that the gene dose of Foxf2 negatively correlates with the rate of epithelial proliferation and number of Lgr5 positive intestinal epithelial stem cells (Figure 1).
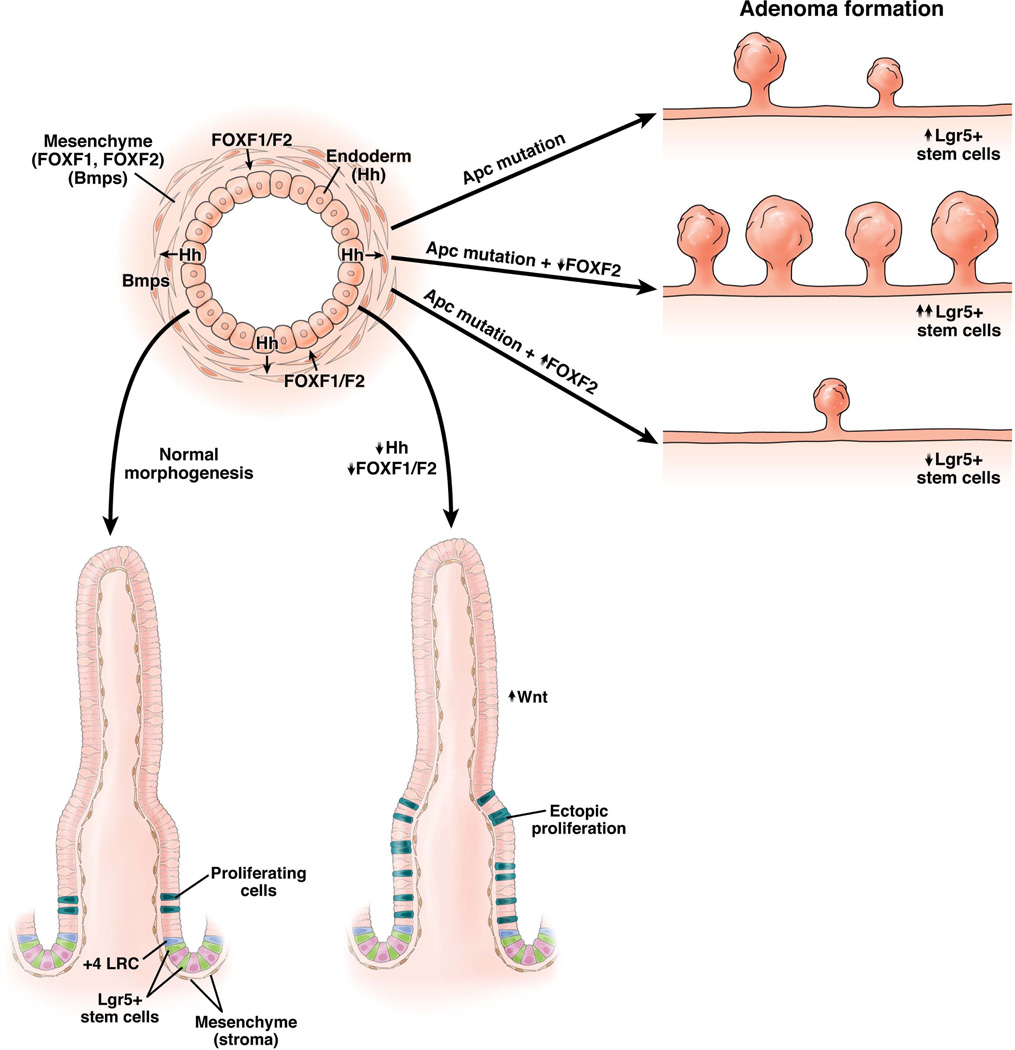
Fig. 1.
Mesenchymal FoxF2 regulates intestinal epithelial proliferation and modulates adenoma initiation and growth in Apcmin/+ mice. Epithelial-mesenchymal cross-talk plays a critical role in crypt-villus morphogenesis. Hedgehog signals to mesenchyme. Bmps and FoxF1/F2 are key mesenchymal factors that crosstalk to epithelium. Reduced FoxF1/F2 expression results in increased proliferation and increased Wnt signaling during villus morphogenesis (bottom left), and FoxF2 gene dosage of regulates adenoma initiation and growth (top right).
So what is the nature of the mesenchymal negative feedback signal that is regulated by Hedgehog signaling and Foxf2? This is likely not a linear pathway, multiple mechanisms may exist that are most likely not completely overlapping between Hedgehog signaling and Foxf2. One of the proteins that have been examined as a potential Hedgehog/Foxf2 regulated factor is Bone Morphogenetic Protein (Bmp)-4. During development Bmp4 is expressed exclusively in the mesenchyme,15 regulated by Hedgehog signaling,15 and completely absent in Foxf2−/− mice.13 However in contrast to the remarkable intestinal developmental phenotype of Hedgehog mutant and Foxf2 mutant mice, two independent groups have shown that mice that overexpress the Bmp antagonist Noggin in the intestine do not have a phenotype until after the suckling to weaning transition around postnatal day 21.15, 17 Noggin overexpressing and Bmpr1a mutant mice develop polyps in adult mice.17, 18 However these polyps are hamartomas that have a remarkable mesenchymal expansion rather than the epithelial expansion that is typical for adenomas (for discussion see 19) and that is not observed in Hedgehog or Foxf mutant mice. In their current publication, Nik et al. perform an extensive analysis of potential modulators of Wnt signaling that could be controlled by Foxf2. They find no change in Bmp4 expression but find that Foxf2 controls the expression of Sfrp1 a soluble antagonist of Wnt signaling in the intestinal mesenchyme. This suggests that Sfrp1 may mediate at least some of the negative regulation by Foxf2 on the epithelial stem cells and rate of proliferation. However, as the authors point out, Foxf2 is a transcriptional regulator that will more broadly affect the behavior of the mesenchymal cells that express it and other mechanisms are therefore likely to exist.
In conclusion, Indian Hedgehog and Foxf2 seem to be components of an epithelial-mesenchymal negative feedback loop that controls intestinal epithelial homeostasis. Although Foxf2 may control the epithelial precursor cell compartments via multiple mechanisms, Sfrp1 may be an important epithelial to mesenchymal signal in this loop.
Mesenchymal modulation of adenoma initiation and growth
Most sporadically occurring intestinal adenomas form by mutations that activate the Wnt signaling pathway. Since Wnt signaling drives stem cell and transit amplifying cell fate, such mutations abnormally expand the size of the epithelial precursor cell compartment.4 The most frequent mutations that activate the Wnt pathway are mutations in APC. Germ line mutations in APC are also the cause of the rare Familiar Adenomatous Polyposis syndrome in humans, and cause a similar syndrome in the Apcmin/+ mouse mutant that was used in the studies of Nik et al. Even though mutations in APC expand the precursor cell compartment this expansion is not a linear phenomenon. Adenomas are very slow growing and can in fact regress. A study in which adenomas of <10 mm in size where left in situ to be removed at a follow up endoscopy 3 years later showed that there was no significant increase in adenoma size over the three year observation period.20 This indicates that despite the increased Wnt signaling that results from an APC mutation, negative regulatory mechanisms are still in place. Some may even be specifically activated by the increased size of proliferating cells. We know very little about such barriers to adenoma progression that have to be overcome during the adenoma to carcinoma sequence. The work by Nik et al. now suggests that at some of these factors are mesenchymally derived and transcriptionally regulated by Foxf2 (Figure 1).
In conclusion, negative feedback loops act to maintain homeostasis in the normal intestinal epithelium. Such negative regulatory mechanisms also affect epithelial cells that have already acquired the genetic mutations that initiate adenoma growth. They are a likely explanation of the slow progression of adenomas and the observation that adenomas can even regress. Clearly some of the restraints on normal epithelial proliferation and the adenoma to carcinoma progression are imposed by epithelial mesenchymal cross talk. Nik et al have now identified Foxf2 as a key mesenchymal transcriptional regulator in this interaction. The further characterization of such negative regulatory mechanisms will be a key area of research to understand the adenoma to carcinoma sequence and may point to novel avenues in chemoprevention and chemotherapy.
Acknowledgments
Funding: GvdB is supported by European Research Council (European Community’s Seventh Framework Program 241344 and VIDI grant from Netherlands Organizaton for Scientific Research.
DR is supported by NIH grants R01 DK46122, R01 DK61216 and P3052574
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: neither of the authors reports a conflict of interest.
Reference List
- 1.Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res. 2010;156:180–187. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Brink GR, Offerhaus GJ. The morphogenetic code and colon cancer development. Cancer Cell. 2007;11:109–117. doi: 10.1016/j.ccr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Nik AM, Reyahi A, Ponten F, Carlsson P. Foxf2 in intestinal fibroblasts reduces Lgr5+ stem cell number and adenoma formation by Wnt pathway inhibition. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 5.van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De PM, Anti M, Van Gijn ME, Suijkerbuijk S, van de Wetering M, Marra G, Clevers H. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104:15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Wang L, Iordanov H, Swietlicki EA, Zheng Q, Jiang S, Tang Y, Levin MS, Rubin DC. Epimorphin(−/−) mice have increased intestinal growth, decreased susceptibility to dextran sodium sulfate colitis, and impaired spermatogenesis. J Clin Invest. 2006;116:1535–1546. doi: 10.1172/JCI25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dop WA, Heijmans J, Buller NV, Snoek SA, Rosekrans SL, Wassenberg EA, van den Bergh Weerman MA, Lanske B, Clarke AR, Winton DJ, Wijgerde M, Offerhaus GJ, Hommes DW, Hardwick JC, de Jonge WJ, Biemond I, van den Brink GR. Loss of Indian Hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology. 2010;139:1665–1676. 1676. doi: 10.1053/j.gastro.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 9.van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, Nielsen C, Gaffield W, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36:277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 10.Zacharias WJ, Madison BB, Kretovich KE, Walton KD, Richards N, Udager AM, Li X, Gumucio DL. Hedgehog signaling controls homeostasis of adult intestinal smooth muscle. Dev Biol. 2011;355:152–162. doi: 10.1016/j.ydbio.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 12.Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- 13.Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- 14.Kosinski C, Stange DE, Xu C, Chan AS, Ho C, Yuen ST, Mifflin RC, Powell DW, Clevers H, Leung SY, Chen X. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 16.Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, Crowell T, Olson D, Davidson NO, Burkly LC. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–482. doi: 10.1053/gast.2002.31102. [DOI] [PubMed] [Google Scholar]
- 17.Haramis AP, Begthel H, van den Born M, van EJ, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 18.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 19.Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008;8:806–812. doi: 10.1038/nrc2467. [DOI] [PubMed] [Google Scholar]
- 20.Almendingen K, Hofstad B, Vatn MH. Does a family history of cancer increase the risk of occurrence, growth, and recurrence of colorectal adenomas? Gut. 2003;52:747–751. doi: 10.1136/gut.52.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]