Abstract
Background
Fetal alcohol spectrum disorders (FASD) result from heavy prenatal alcohol exposure, and are characterized, in some cases, by CNS anomalies and cognitive impairment. Regional patterns of neuroanatomical abnormalities suggest that alcohol exerts selective damage on the developing fetal brain. This study assessed brain-behavior relationships in a sample of youth with histories of heavy prenatal alcohol exposure. The aim was to characterize how structural brain alterations observed in our previous studies relate to cognitive deficits commonly reported in individuals with histories of heavy prenatal alcohol exposure.
Methods
Twenty-one youth (mean age 13 years) with histories of heavy prenatal alcohol exposure and seven non-exposed healthy comparison subjects underwent structural magnetic resonance imaging (MRI) and neurobehavioral testing. Regional brain volumes within the alcohol-exposed group were correlated with neuropsychological measures of cognitive control and verbal learning/recall, as these aspects of cognition have previously been shown to be vulnerable to alcohol teratogenesis.
Results
Between-group effect sizes revealed moderate to large cognitive performance and brain volume decrements in alcohol-exposed subjects, compared to typically developing peers. Within the alcohol-exposed group, volume of the caudate nuclei was the most consistent predictor of neuropsychological performance, after controlling for potentially confounding variables including total brain volume, IQ, and age.
Conclusions
These data are consistent with previous research associating gestational alcohol exposure with structural and functional changes of the caudate nucleus. Our findings extend this previous work by demonstrating that volume reductions of the caudate have behavioral relevance for this population, in relation to cognitive control and verbal learning and recall abilities.
Keywords: fetal alcohol spectrum disorders (FASD), fetal alcohol syndrome (FAS), brain-behavior correlations, verbal learning/recall, cognitive control
Introduction
Fetal alcohol syndrome (FAS), defined by growth deficiency, craniofacial signs, and central nervous system (CNS) anomalies, was first described in the medical literature over 35 years ago (Jones and Smith, 1973, Lemoine et al., 1968). Since the earliest formal descriptions of FAS, it has become increasingly apparent that prenatal alcohol exposure can be associated with cognitive and behavioral deficits, even in the absence of the characteristic facial features and growth retardation required for a diagnosis of FAS (e.g., Mattson and Riley, 1998, Mattson et al., 1997). In light of the continuum of effects resulting from prenatal alcohol exposure, the term fetal alcohol spectrum disorders (FASD) is now used to describe the range of outcomes attributable to gestational alcohol exposure, from classically defined cases of FAS to more phenotypically subtle cases (Bertrand et al., 2005). In the U.S., incidence estimates range from about 1–2 cases of FAS per 1000 births (May and Gossage, 2001, May et al., 2009), with the rate of affected individuals who do not meet diagnostic criteria for FAS likely several-fold higher (Sampson et al., 1997). In recognition of the teratogenic capacity of alcohol, the U.S. Surgeon General currently recommends that pregnant women completely abstain from consuming alcohol (US Department of Health and Human Services, 2005). FASD is associated with a wide range of cognitive deficits, which may include impaired general intelligence, problems with attention, learning and memory, executive functions, visuospatial and motor skills (for review, see Kodituwakku, 2007, Mattson et al., 2011). IQ scores are wide-ranging, and though the majority of individuals with FASD do not meet diagnostic criteria for intellectual deficiency, gestational alcohol exposure is thought to be a leading cause of intellectual deficits (Pulsifer, 1996). Individuals with FASD are at increased risk for developing mental illness (Barr et al., 2006, Fryer et al., 2007a, O’Connor et al., 2002), low academic achievement, legal sequelae, and poor interpersonal functioning (Streissguth et al., 2004).
In vivo neuroimaging studies of clinical samples have characterized the structural brain changes associated with FASD (for review, see Norman et al., 2009, Lebel et al., 2011). In addition to overall reductions in brain volume, specific vulnerability of certain brain regions has been reported, including the corpus callosum (Bookstein et al., 2002, Riley et al., 1995, Autti-Rämö et al., 2002), cerebellum (Sowell et al., 1996, Autti-Rämö et al., 2002), and caudate nucleus (Archibald et al., 2001, Mattson et al., 1996b, Astley et al., 2009). To date, fewer studies have characterized anomalies in brain structure in relation to cognitive performance in youth with FASD, though meaningful brain structure-function relationships have been described in this population (cf. Sowell et al., 2008, Li et al., 2008, Coles et al., 2011). The identification of specific neurobehavioral phenotypes associated with fetal alcohol-related brain abnormalities remains a research priority in the field and could be useful in improving identification and diagnosis across the fetal alcohol spectrum (Stratton et al., 1996, Riley et al., 2003, Mattson et al., 2010, Mattson and Riley, 2011). In this study, we relate structural brain alterations to neurocognitive deficits commonly associated with FASD. Specifically, we examined the extent to which a set of a priori brain volumes of interest could predict cognitive performance within a sample of youth with histories of heavy prenatal alcohol exposure on measures of cognitive control and verbal learning, two aspects of cognition that have been shown to be sensitive to prenatal alcohol exposure based on neuropsychological assessment.
Deficits in cognitive control, the set of abilities that guide cognition and behavior in the service of internal goals, are frequently described in FASD, including deficits in response inhibition, an aspect of cognitive control. For example, task component analysis of the Delis-Kaplan Executive Function Scale (D-KEFS) Color-Word Interference Test, a modified Stroop test, revealed that decreased performance of children with histories of heavy prenatal alcohol exposure was best accounted for by an inhibitory control deficit, rather than by deficits in lower-order task components (Mattson et al., 1999). Commission errors on a vigilance task were sensitive to prenatal alcohol exposure in children at 7 years of age (Streissguth et al., 1986), and these inhibition difficulties persisted when these children were followed-up at 14 years of age (Streissguth et al., 1994). Similar findings have been described in adults, both with FAS and non-dysmorphic FASD; Stroop performance was among the variables within an executive functioning test battery most strongly associated with prenatal alcohol exposure, independent of IQ (Connor et al., 2000). While many studies support inhibitory control difficulties in individuals with FASD, conflicting reports do exist (Kodituwakku et al., 1995). However, in general, results converge across existing research to suggest that for individuals with prenatal alcohol exposure, poor response inhibition, in addition to sustained attention deficits, may contribute to impaired attention, which is a hallmark feature of FASD.
As with inhibitory control functions, neuropsychological research indicates that verbal learning/recall abilities are also compromised in FASD. Verbal memory deficits do not appear to be global, but rather may be specific to explicit aspects of memory. Implicit verbal memory, assessed via lexical priming, was intact in a group of children with histories of heavy prenatal alcohol exposure (Mattson and Riley, 1999), while several other studies have reported explicit verbal learning and recall deficits (Mattson et al., 1996a, Mattson and Roebuck, 2002, Willford et al., 2004, Crocker et al., 2011). Importantly, these studies are consistent in reporting relatively greater performance deficits in immediate recall versus retention of verbal information, suggesting that learning and recall performance deficits in FASD are likely driven by impairment in acquisition of verbal information, more so than by impairment of retrieval processes.
In addition to the neurpsychological literature, functional neuroimaging findings showing fMRI response alterations in task-specific brain areas in the context of both response inhibition (Fryer et al., 2007b) and verbal learning (Sowell et al., 2007) support the hypothesis that CNS alterations underlie these compromised cognitive functions in FASD. In this study, we selected brain regions of interest (bilateral frontal, parietal, and temporal lobes, cerebellum, caudate nuclei, and hippocampi) based on findings from previous volumetric MRI studies of FASD (Archibald et al., 2001, Astley et al., 2009). The goals of the study were to evaluate the predictive power of these regional volumes to account for cognitive control and verbal learning deficits within a sample of individuals with histories of heavy prenatal alcohol exposure.
Materials and Methods
Subjects
Study participants included youth (ages 9–21 years) with histories of heavy prenatal alcohol exposure (ALC; n=21) and typically developing peers (CON; n=7). ALC subjects were selected from a retrospective cohort of individuals with heavy prenatal alcohol exposure who are enrolled in an ongoing study at the Center for Behavioral Teratology (CBT), San Diego State University (SDSU). Prenatal alcohol exposure was determined via maternal report to be minimal in CON subjects (defined as less than one drink per week on average and never more than 2 drinks on any one occasion during pregnancy) (Mattson et al., 2010). In contrast, all alcohol-exposed subjects had documented histories of heavy prenatal alcohol exposure and were evaluated by a dysmorphologist with expertise in alcohol teratogenesis (KLJ). The multimodal medical examination includes physical growth measurements, craniofacial structure analysis, alcohol exposure history, and record review. Under this retrospective recruitment protocol, exact dose and timing of maternal alcohol consumption were not always available. However, documentation of an abusive pattern of alcohol consumption, typically compatible with a maternal alcohol use disorder, was demonstrated for all cases in the ALC group. Furthermore, heavy prenatal alcohol exposure is defined in our cohort as maternal consumption of at least 4 drinks per occasion at least once per week, or 14 drinks per week during pregnancy. Twelve of the 21 individuals in the ALC group met criteria for FAS (Jones and Smith, 1973, Hoyme et al., 2005), while the remaining nine individuals did not meet these criteria. For the subgroup that did not have FAS, the modal reported maternal alcohol consumption was 6–8 drinks per day, throughout pregnancy duration. Clinical characteristics of the ALC group were as follows: mean height (FAS=17.4 percentile, non-FAS=58.9 percentile); mean weight (FAS=24.8 percentile, non-FAS=69.5 percentile). Height and weight measurements were unavailable for 2 FAS and 1 non-FAS subjects. Mean philtrum and horizontal palpebral fissure lengths were 1.63 cm and 2.12 cm, respectively for the individuals diagnosed with FAS, and 1.43 cm and 2.64 cm, respectively for the alcohol-exposed individuals without FAS. Dysmorphology measurements were unavailable for some subjects who were previously diagnosed with FAS (2 FAS subjects did not have palpebral fissure measurements and 5 did not have philtrum lengths); dysmorphology measurements were available for all non-FAS alcohol-exposed subjects.
Subjects in the ALC group were typically referred to the CBT by medical providers, other health care or social service professionals, or were self-referred. CON subjects were recruited via community outreach or advertisements. Intelligence testing was conducted with an age appropriate version of the Wechsler Intelligence Scale (Wechsler, 1991). Written informed parental consent and subject assent were obtained via protocols approved by the Institutional Review Boards at SDSU and the University of California, San Diego (UCSD). Subjects received a financial incentive for participation in the study. Exclusionary criteria included a history of head trauma, contraindication for MRI scanning (e.g., metallic implants in the body), claustrophobia, or serious medical conditions (e.g., epilepsy).
Neuropsychological Test Selection and Assessment Procedures
Neuropsychological variables were selected to represent two key cognitive abilities thought to be affected by prenatal alcohol exposure: 1) cognitive control and 2) verbal learning and recall. Accordingly, subjects were tested with the Wisconsin Card Sorting Test (WCST, Heaton et al., 1993) and the California Verbal Learning Test-Child Version (CVLT-C, Delis et al., 1994). The cognitive control domain was measured by WCST perseverative responses and CVLT-C false positive errors. The verbal learning/recall domain was measured by CVLT-C List A total score, reflecting verbal learning, and CVLT-C long delay free (LDF) recall, reflecting verbal recall. These specific measures were selected from a larger concurrently-administered 2 hour test battery covering a broad range of cognitive abilitites. From this same battery, we also selected two control variables in order to test the specificity of hypothesized findings. For this purpose, we included performance on a receptive language measure, the Peabody Picture Vocabulary Test—Revised version (PPVT-R, Dunn and Dunn, 1997) and the Arithmetic subtest of the Wide Range Achievement Test – 3rd edition (WRAT-3, Wilkinson, 1993). Subjects underwent neuropsychological testing by a trained, reliable psychometrist using standardized adminstration procedures. Responses were scored and scaled to published norms. These procedures were double-checked by a seperate trained psychometrist and reviewed by a clinical neuropsychologist (S.N.M).
Neuroimaging Acquisition and Analysis
Anatomical brain scans were collected for each subject with a 1.5T magnet (Signa: General Electric, Milwaukee) at the Scripps Clinic Green Hospital in La Jolla, California. The acquisition protocol was a gradient-echo (SPGR) T1-weighted series with TR = 24ms, TE = 5ms, NEX = 2, flip angle = 45 degrees, 24 cm field of view, in-plane resolution 0.9375 by 0.9375 mm, with contiguous 1.2mm sections, for 124 slices. In addition, two fast spin-echo (FSE) series were collected for co-registration with the SPGR protocol. Scan parameters for the separate FSE image sets were: 1) a proton density-weighted sequence with TR = 3000 ms, TE = 17 ms, and 2) a T2-weighted sequence with TR = 3800 ms, TE = 102 ms. The field of view for the FSE series was 24 cm, with contiguous 4mm sections.
Segmentation and parcellation of brain images was achieved through previously described, reliable procedures (Jernigan et al., 1990, Jernigan et al., 2001). Briefly, skull-stripping techniques were used to remove extracranial tissue, after which images were subjected to inhomogeneity correction, co-registraion of all series, interpolation to isotropic voxel dimensions and standard AC-PC orientation, tissue segmentation (gray, white, cerebrospinal fluid (CSF)), and brain region parcellation. These semi-automated segmentation procedures were undertaken by blinded, trained analysts who achieved excellent reliability as reported by Archibald and colleagues, ranging from 0.88 to 0.99 for the different regions of interest, with the majority >0.95 (Archibald et al., 2001).
Based on previous structural neuroimaging findings in FASD, we selected six brain regions for correlation with neuropsychological test performance: bilateral frontal, parietal, and temporal lobes, cerebellum, caudate nuclei, and hippocampi. These regions were circumscribed on the standardized tissue-segmented images using a standard rule set. The frontal lobe was defined as the region anterior to the central sulcus and superior to the sylvian fissure, including the cingulate. Parietal lobe was defined as the region posterior to the central sulcus and anterior to the sylvian fissure. Temporal lobe was defined as inferior to the sylvian fissure. Posterior boundaries of both temporal and parietal lobes extended to the occiptial notch. Hippocampus was defined as extending from the posterior pulvinar to just posterior of the long columns of the fornix and inferiorly through the bend of the parasubiculum. Caudate nuclei were separated from the nucleus accumbens using the inferior extent of the lateral ventricles. See Figure 1.
Figure 1.
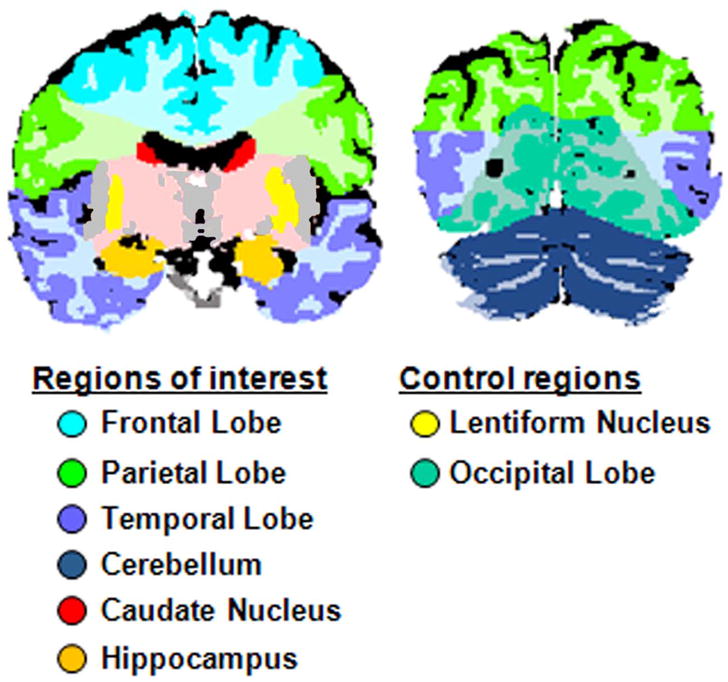
Two representative coronal sections depicting neuroanatomical definitions for the regions of interest examined.
In order to better evaluate the validity of hypothesized regional effects, lenticular nuclei and occipital lobe volumes were included in this study, as controls to the regions of interest, as these regions were not expected to correlate with the neurobehavioral outcome measures. For most neuroanatomical regions of interest (ROIs) volume estimates were combined across both grey and white matter bilaterally, except for the subcortical nuclei (lenticular and caudate) and hippocampal ROIs which were comprised solely of grey matter. CSF volumes were not examined in this study.
Statistical Analysis Overview
After ensuring appropriateness of using parametric models, statistical analyses were conducted in IBM’s SPSS, version 16 for the MacIntosh. The analytic strategy included within-group (ALC) regression analyses to determine: 1) if brain volumes of interest (entered as an independent variable) accounted for significant variance in neuropsychological test performance (entered as a dependent variable) and 2) if significant relationships were observed, whether these regional volumetric predictors were robust in the presence of potentially confounding factors.
Below, we first describe pairwise group differences between alcohol-exposed and comparison youth, in order to establish neurocognitive impairment and regional volumetric brain reductions in our alcohol-exposed sample, compared to typically developing individuals. Given the small number of controls with both neuroanatomical and neuropsychological data available for analysis (n=7), analyses of brain-behavior relationships within the control group would have limited clinical significance and would be statistically underpowered. Therefore, further analyses were not pursued with the control group, and the analytic focus of our study was on evaluating the relationship between regional brain volumes and cognitive performance, within the alcohol-exposed group. Accordingly, in order to evaluate hypotheses concerning the extent to which a priori regional volumes were predictive of cognitive control and verbal learning in subjects with histories of prenatal alcohol exposure, we conducted two sets of hierarchical regressions:
The first set of regression models determined whether each of six a priori ROI volumes and two control volumes independently predicted cognitive outcomes of interest. Because microcephaly is a common result of prenatal alcohol exposure, regional volume effects were evaluated in consideration of total volume of the cerebral cranium (CCV). However, two of the ROIs (frontal and temporal lobe volumes) were highly correlated with CCV (r >.85). This is not unexpected, given the large size of these regions. Therefore, analyses of frontal and temporal volumes did not include CCV in the analytical model as the reliability of multiple regression is compromised by highly correlated independent variables. Analyses involving all other regions were corrected for CCV, by entering CCV into the first step of a hierarchical regression model, and the ROI (or control region) into the second step of the regression model, as the threshold set for multicollinearity among independent variables was not exceeded (r <.85).
The second set of hierarchical regressions were follow-up analyses undertaken to determine whether significant ROI predictors (based on the first set of analyses) remained significant after statistically controlling for other factors that might be expected to influence neurobehavioral outcome. Additional factors examined were: IQ, age, presence of a FAS diagnosis, and other brain regions anatomically and/or functionally associated with the neurobehavioral outcome under examination.
WCST data for two of the 21 ALC subjects were unavailable due to incomplete testing and two different young adult ALC subjects did not receive the CVLT-C because of their age. Thus, each set of regression analyses included 19 alcohol-exposed subjects, with the exception of regression models using the hippocampus or temporal lobe volumes which included 18 alcohol-exposed subjects due to exclusion of hippocampal and temporal lobe volumes from one individual, due to localized artifcat resulting in poor image quality in these regions.
Results
Between-group findings
Table 1 displays mean sample demographics, neuropsychological test performance, and regional brain volume estimates, along with p value and effect sizes (Cohen’s d) for between-group comparisons of neuropsychological test performance and regional brain volumes. The groups did not differ on age, sex, race, SES, or handedness. They did differ, as expected, on FSIQ. We report brain volume and neuropsychological data from a small group of demographically matched control subjects in order to provide a basis for comparison to our alcohol-exposure sample (see Table 1). However, we emphasize that between-group brain volume differences in this cohort are previously reported (Archibald et al., 2001), and that hypotheses regarding regression of cognitive performance on brain volume within the ALC group are the focus of the current study.
Table 1.
Sample demographics, neuropsychological test performance, and brain volume estimates for subjects in the alcohol-exposed (ALC) and non-exposed control (CON) groups (n.s., p <.05).
| ALC [M (SD)] | CON [M (SD)] | p | Cohen’s d | |
|---|---|---|---|---|
| N | 21 | 7 | ||
| Age | 13.06 (3.77) | 13.20 (2.51) | n.s. | - |
| Sex [n (%) female] | 9 (42.9) | 3 (42.9) | n.s. | - |
| Race [n (%)Caucasian] | 16 (76.2) | 5 (71.4) | n.s. | - |
| Handedness [n (%)right handed] | 19 (90.5) | 7 (100.0) | n.s. | - |
| SES | 41.64 (14.05) | 49.28 (12.13) | n.s. | - |
| FSIQ | 83.33 (13.48) | 105.74 (10.45) | <.001 | 1.86 |
| WCST Perseverative Responses (Standard score) | 90.79 (15.77) | 107.71 (6.58) | .012 | 1.40 |
| CVLT-C List A Learning (T-score) | 44.95 (14.82) | 57.86 (7.80) | .040 | 1.09 |
| CVLT-C Long Delay Free Recall (Z-score) | −0.70 (1.62) | 0.29 (0.95) | .147 | .75 |
| CVLT-C False Positives (Z-score) | 0.10 (1.51) | −0.57 (0.35) | .261 | .61 |
| PPVT-R (Standard score) | 84.05 (15.53) | 105.71 (17.44) | .005 | 1.31 |
| WRAT-3 Arithmetic (Standard score) | 83.00 (16.56)_ | 104 (13.98) | .006 | 1.37 |
| Cerebral Cranial Volume (cc) | 1124.18 (144.55) | 1321.45 (87.4) | .002 | 1.65 |
| Bilateral Cerebellum Volume (cc) | 144.78 (21.35) | 166.97 (10.21) | .014 | 1.33 |
| Bilateral Caudate Nucleus Volume (cc) | 7.11 (1.33) | 8.88 (0.90) | .003 | 1.55 |
| Bilateral Lenticular Nucleus Volume (cc) | 13.69 (1.79) | 15.55 (1.41) | .019 | 1.16 |
| Bilateral Hippocampus Volume (cc) | 11.08 (1.58) | 11.26 (1.08) | .803 | 0.13 |
| Bilateral Parietal Lobe Volume (cc) | 234.20 (39.23) | 283.61 (20.20) | .004 | 1.58 |
| Bilateral Frontal Lobe Volume (cc) | 439.87 (58.30) | 499.90 (35.94) | .017 | 1.24 |
| Bilateral Temporal Lobe Volume (cc) | 201.73(27.65) | 239.62 (29.71) | .009 | 1.32 |
| Bilateral Occipital Lobe Volume (cc) | 147.63 (24.91) | 178.32 (29.97) | .012 | 1.11 |
A Priori Brain Region of Interest (ROI) Hierarchical Regression Models Within the Alcohol-Exposed Group
Neither hippocampal nor parietal lobe volumes predicted any of the neuropsychological performance outcomes, after accounting for CCV (p > .05). Additionally, temporal lobe volume, entered as a single predictor, did not account for significant variance in neuropsychological performance (p > .05). Also, as hypothesized, control regions of interest (occipital and lenticular nuclei volumes) were not significant predictors (p > .05). Therefore, further analyses were not pursued with temporal, parietal, hippocampal, or control volumes.
Frontal lobe volume, entered as a single predictor, accounted for significant variance in CVLT-C List A performance (F (1, 17) = 6.45; p < .05; Adjusted R2 = .23), but did not predict other neuropsychological performance outcomes (p > .05). After accounting for CCV, cerebellar volume predicted CVLT-C false positive errors, (p = .02), indicating that the two-step model should be retained (F (2, 16) = 5.92; p < .05; Adjusted R2 = 0.35; ΔR2 = 0.23). Cerebellar volume was not a significant predictor for either of the learning/recall measures or WCST performance, after accounting for CCV variance (p > .05).
Lastly, caudate nucleus volume emerged as the most consistent predictor of cognitive performance within the ALC group, accounting for significant variance, above and beyond CCV, across all of the neuropsychological outcomes that were examined (.001 < p < .03); see Figure 2. Regression model statistics corresponding to the two-step model were as follows, for WCST preservative responses: (F (2, 16) = 5.98; p < .05; Adjusted R2 = 0.36; ΔR2 = 0.20); for CVLT-C false positive errors: (F (2, 16) = 13.62; p < .05; Adjusted R2 = 0.58; ΔR2 = 0.44); for CVLT-C List A total: (F (2, 16) = 9.41; p < .05; Adjusted R2 = 0.48; ΔR2 = 0.26), and for CVLT-C LDF: (F (2, 16) = 6.24; p < .05; Adjusted R2 = 0.37; ΔR2 = 0.33).
Figure 2.
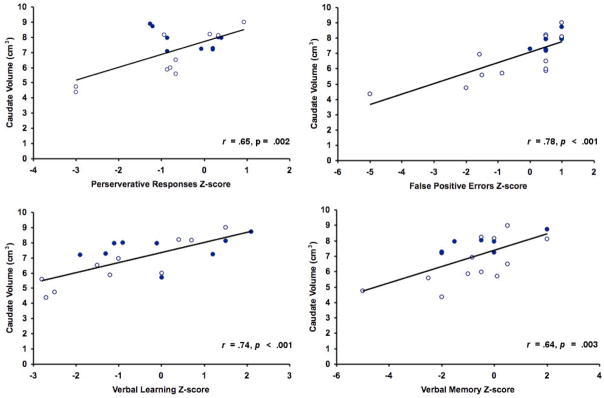
Scatterplots showing relationship between raw caudate volume and neuropsychological test performance, within alcohol-exposed youth (n=19). Neuropsychological test scores were inverted†, if necessary, so that for all neuropsychological variables, higher scores reflect better performance. Open circles depict values of individuals meeting FAS criteria, while closed circles depict values of individuals with histories of prenatal alcohol exposure who did not meet FAS criteria. † The CVLT-C false positives variable was inverted (by multiplying the attained Z-score by -1) so that higher scores indicated better performance. The other variables did not requre inversion, as they were already scaled such that higher scores reflected better performance.
Follow-Up Analyses for Regions That Predicted Neurobehavioral Outcome Within the Alcohol-Exposed Group
Cerebellum
Cerebellar volume predicted CVLT-C false positive errors, after entering IQ, age, or FAS diagnosis into the first step of a hierarchical regression model. Corresponding p values for the cerebellar independent variable, entered as a second model step, ranged from < .001 to .04. However, volume of the cerebellum (p > .05) did not predict CVLT-C false positive errors above and beyond the caudate, indicating that cerebellar volume did not explain additional variance in CVLT-C false positive error scores, after taking into consideration caudate volume. Next, tissue type (i.e., grey vs. white matter) was examined to determine whether the observed effect associating cerebellar volume and CVLT-C false positives was differentially explained by tissue type. The CVLT-C false positive score was regressed on total cerebellar grey matter volume entered as a first step, and total cerebellar lobe white matter volume entered as a second step. Results indicated that for the first step of the model, total cerebellar grey matter was a significant predictor (p < .05) of CVLT-C false positives performance, but that total cerebellar white matter did not predict any additional variance in the dependent variable, above grey matter [2-step model: (F Change (2, 16) = 2.78; p = .11; Adjusted R2 = 0.38; ΔR2 = 0.10]. A similar pattern of results was yielded when CVLT-C false positives was regressed on total cerebellar white matter volume entered as a first step, and total cerebellar grey matter volume entered as a second step. The first step of the model indicated that frontal lobe white matter was a significant predictor of false positives performance (p < .05), but total grey matter did not predict additional variance, beyond white matter [2-step model: (F change (2, 16) = 1.93; p = .18; Adjusted R2 = 0.45; ΔR2 = .06].
Frontal Lobes
With regard to the relationship between frontal volume and CVLT-C List-A scores, the frontal ROI independent variable remained a significant predictor after first entering age (p = .02), or FAS diagnosis (p = .02), and was marginally predictive (p = .05), when IQ was entered first into the regression model. Frontal volume (p > .05) did not predict neurobehavioral performance above and beyond the caudate, indicating that this ROI could not account for additional variance in CVLT-C List-A performance after taking caudate volume into consideration. Next, tissue type (i.e., grey vs. white matter) was examined to determine whether the observed effect associating frontal lobe volume and CVLT-C List A Total was differentially explained by tissue type. The CVLT-C List A Total score was regressed on total frontal lobe grey matter volume entered as a first step, and total frontal lobe white matter volume entered as a second step. Results indicated that for the first step of the model, total frontal grey matter was a marginally significant predictor (p = .05) of CVLT-C List A performance, but that total frontal white matter did not predict any additional variance in the dependent variable, above grey matter [2-step model: (F Change (2, 16) = 1.26; p = .28; Adjusted R2 = 0.17; ΔR2 = 0.06]. A similar pattern of results was yielded when CVLT-C List A was regressed on total frontal lobe white matter volume entered as a first step, and total frontal lobe grey matter volume entered as a second step. The first step of the model indicated that frontal lobe white matter was a significant predictor of CVLT-C List A performance (p < .05), but total grey matter did not predict additional variance, beyond white matter [2-step model: (F change (2, 16) = 0.18; p = .67; Adjusted R2 = 0.17; ΔR2 < 0.01].
Caudate Nuclei
Bilateral volume of the caudate nuclei remained a significant predictor across all 4 behavioral outcome measures examined, after accounting for other potential sources of variance, including CCV, other regional brain volumes, IQ score, age, or FAS diagnosis. See Table 2 for regression model statistics.
Table 2.
Results of hierarchical regression analyses using caudate volume to predict neuropsychological performance in the ALC group (n=19). Values presented are p values corresponding to the caudate predictor and adjusted R2 and ΔR2 corresponding to the final regression equation for each analysis.
| Model IVs | WCST PR | CVLT-C FP | CVLT-C A Total | CVLT-C LDF | |
|---|---|---|---|---|---|
| Step 1 | Step 2 | p-caudate predictor (2-Step model adjusted R2; ΔR2) | p-caudate predictor (2-Step model adjusted R2; ΔR2) | p-caudate predictor (2-Step model adjusted R2; ΔR2) | p-caudate predictor (2-Step model adjusted R2; ΔR2) |
| Caudate | __ | .002 (0.39) | <0.001 (0.58) | <0.001 (0.51) | 0.003 (0.37) |
| Total Brain | Caudate | 0.033 (0.36; 0.20) | 0.001 (0.58; 0.44) | 0.009 (0.48; 0.26) | 0.007 (0.37; 0.33) |
| Frontal Lobe | Caudate | 0.012 (0.36; 0.28) | 0.001 (0.57; 0.43) | 0.008 (0.49; 0.27) | 0.005 (0.37; 0.36) |
| Lenticular Nucleus | Caudate | 0.039 (0.38; 0.18) | 0.002 (0.56; 0.32) | 0.004 (0.48; 0.33) | 0.051 (0.36; 0.16) |
| Cerebellum | Caudate | 0.070 (0.36; 0.13) | 0.006 (0.58; 0.23) | 0.020 (0.51; .020) | 0.030 (0.34; 0.20) |
| FSIQ | Caudate | 0.004 (0.36; 0.40) | <0.001 (0.67; 0.51) | 0.001 (0.54; 0.47) | 0.005 (0.36; 0.37) |
| Age | Caudate | 0.002 (0.57; 0.32) | <0.001 (0.73; 0.49) | 0.001 (0.49; 0.50) | 0.005 (0.39; 0.35) |
| FAS Diagnosis | Caudate | 0.005 (0.36; 0.37) | 0.001 (0.56; 0.46) | <.001 (0.54; 0.58) | 0.002 (0.40; 0.46) |
Additional Follow-up Results
Across all follow-up analyses, the presence of a FAS diagnosis was not a significant predictor of cognitive performance within this sample of alcohol-exposed youth (p > .05). Lastly, in order to further test the specificity of the relationship of caudate volume to the cognitive functions examined, language and mathematical tasks were selected as “control” neuropsychological outcomes, and regression analyses were conducted to determine whether caudate volume predicted variance in receptive vocabulary performance (PPVT-R) or arithmetic academic achievement (WRAT-3). Results indicated that caudate volume was not predictive of PPVT-R performance or WRAT-3 arithmetic performance (p > .05).
Discussion
Our results show differential capability of regional brain volumes to predict performance in two key aspects of cognition that are sensitive to prenatal alcohol exposure: cognitive control and verbal learning/recall. Volumes of the hippocampi, temporal, and parietal lobes were not predictive of cognitive control or verbal learning/recall performance. In addition, consistent with our hypotheses regarding our a priori defined control regions of interest, occipital lobe and lenticular nuclei volumes were not related to the neurobehavioral outcomes evaluated. The cerebellum and frontal volumes were each related to one of the four cognitive outcomes examined, indicating that these regions have some predictive capability, though not above caudate volume. Additionally, results from follow-up analyses of parcellated frontal and cerebellar white and grey matter did not support differential predictive capability of frontal or cerebellar ROIs, by tissue type. This suggests that the effects observed based on the combined tissue types are best explained by contributions from both regional grey and white matter to explaining variance in the cognitive outcomes examined. Of the brain regions examined, caudate volume alone accounted for significant variance across all four of the neurobehavioral outcomes examined, suggesting that caudate volume is a powerful predictor of both cognitive control and verbal learning/recall abilities in youth with histories of heavy prenatal alcohol exposure. The brain-behavior relationships involving the caudate remained significant after controlling for several factors that might be expected to account for variance in cognitive performance including CCV, IQ, FAS diagnosis, age, and other regional brain volumes of functional and/or anatomical interest. As hypothesized, caudate volume was not predictive of receptive language performance or arithmetic achievement, further underscoring the explicitness of the observed relationships. Thus, within alcohol-exposed youth, caudate volume was, by far, the most consistent predictor of two neuropsychological outcomes of relevance to this population, cognitive control and verbal learning/recall.
The present findings are supported by previous work across several neuroimaging modalities associating altered structure and function of the caudate nucleus with FASD. Disproportionate reduction of caudate volume in children with heavy prenatal alcohol exposure was reported over a decade ago (Mattson et al., 1994), and has since been replicated in larger samples both by our research group (Archibald et al., 2001) and independently (Astley et al., 2009). Consistent with these observations, a recent study reported disproportionate reductions of several subcortical grey matter structures in a sample of children with FASD, with the caudate and the globus pallidus showing the largest tissue reductions of the structures examined (Nardelli et al., 2011). Functional neuroimaging techniques have revealed decreased glucose metabolism in the caudate and other subcortical structures (Clark et al., 2000) and decreased fMRI response in the caudate, during behavioral inhibition task performance (Fryer et al., 2007b). Spectroscopy has shown increases in the brain metabolite NAA within the caudate, though this finding is difficult to interpret as NAA decreases are typically expected with pathology; this preliminary investigation also found a dose-dependent relationship between prospectively reported maternal alcohol consumption and caudate volume decreases (Cortese et al., 2006). Possible mechanisms underlying caudate volume reductions include neurodegeneration during fetal development via ethanol-triggered apoptosis, as the caudate nucleus and fronto-parietal regions have been identified as having a particular vulnerability to ethanol-associated neuroapoptosis (Guerri et al., 2009).
The basal ganglia, which include the caudate, are involved in governing aspects of attentional control, learning (reinforcement-guided and explicit), and determining reward salience (Saint-Cyr, 2003), which may help to explain our findings of reduced cognitive control and verbal learning performance in relation to caudate volume decreases in the brains of youth with prenatal alcohol exposure. In particular, the caudate’s pivotal role in mediating cognitive control is increasingly appreciated. Theoretical conceptualizations of attention-deficit/hyperactivity disorder (ADHD), which, like FASD, is associated with poor inhibitory control, disrupted behavior and adaptive dysfunction, have emphasized the role of pathology in prefrontal-subcortical, prefrontal-parietal, and prefrontal-cerebellar circuits that guide the development of efficient self-regulation of behavior (Nigg and Casey, 2005). Thus, for youth with FASD, it is possible that both focal damage to the caudate nucleus and compromised connectivity with associated structures contribute to characteristic cognitive control deficits and learning impairments.
Though the present study’s findings strongly implicate caudate volume reductions in relation to behavioral deficits, our findings were not limited to the caudate, consistent with research indicating many other brain structures appear to be vulnerable to alcohol teratogenesis (Lebel et al., 2011, Norman et al., 2009). We observed that cerebellar volume predicted false positive errors on a list-learning task, independent of IQ, age, and FAS diagnosis. This finding is supported by recent research on cerebellar contributions to non-motor functions (Desmond, 2010), including a functional role for the cerebellum in aspects of executive control (Stoodley and Schmahmann, 2009). In addition, frontal lobe volume was positively related to verbal learning ability, independent of age and FAS diagnosis. Thus, within our alcohol-exposed sample, verbal learning (i.e., encoding), but not verbal recall was predicted by frontal lobe volume. In a separate study, cortical thinning (i.e., reduced cortical thickness) in right dorsal frontal regions correlated with poorer verbal recall (Sowell et al., 2008) although verbal learning was not examined. Taken together, these results suggest that structural changes in frontal regions may be particularly important for understanding verbal learning and memory deficits in this population. Neither the cerebellar nor the frontal volume relationship remained significant after first accounting for caudate volume, reflecting shared variance in cognitive performance between the caudate and these other structures. Though speculative, this shared variance may reflect a joint contribution of these regions to the cognitive tasks performed. While beyond the scope of the current study, the prospect of alterations to functional and/or anatomical connections among these regions, and the impact of potential dysconnectivity on cognitive control and verbal learning/recall could be evaluated in future studies using connectivity-focused imaging modalities.
Notably, we did not detect brain-behavior relationships in the parietal or temporal regions, despite previous findings that posterior cortical regions are a target of alcohol teratogenesis (Sowell et al., 2002). It may be that the large, bilateral ROIs employed in this study lacked the anatomical specificity to detect meaningful brain-behavior relationships in the posterior cerebrum that finer-grain analyses of brain structure may have revealed. Also, the neuropsychological measures used as dependent variables in the study likely reflect multiple aspects of complex problem solving (WCST) and verbal learning (CVLT-C), and thus limit our ability to make inferences about discrete cognitive processes. This study is limited by several additional factors. Foremost, though the observed predictive relationships involving the caudate were robust to several potentially confounding variables, other factors that might account for variance in cognitive performance were not assessed (e.g., additional prenatal drug exposures, early childhood environment). In addition, due to the retrospective recruitment protocol, we are unable to examine quantitiative dose-response relationships of maternal alcohol consumption. Small sample sizes limited our abilitiy to stratify analyses by alcohol exposure status, and future analyses comparing control subjects to alcohol-exposed youth with and without FAS would be a useful extension of this work. Association between severity of dysmorphic features and memory deficits in FASD has been shown to be mediated by hippocampal volume (Coles et al., 2011), underscoring the relevance of examining dysmorphic severity in relation to brain-behavior relationships in FASD.
Despite these limitations, these data demonstrate the behavioral relevance of caudate reductions in youth with prenatal alcohol expoure in relation to several outcomes tapping cognitive control and verbal learning/recall, which are important cognitive functions for adaptive functioning and scholastic success. Executive functions are predicitive of social skills in children with prenatal alcohol exposure (Schonfeld et al., 2006), emphasizing the clinical significance of investigating neurocognitive impairment in this population. In conclusion, our results: i) converge with previous literature that links prenatal alcohol exposure to structural and functional changes in the caudate nuclei, and ii) relate caudate damage to neurobehavioral consequences in affected offspring. Continued distillation of the relationships governing brain alterations and behavioral phenotype in individuals with FASD is central to improving our understanding of the complex pathogenesis arising from prenatal alcohol exposure.
Acknowledgments
The authors thank the families who graciously participate in our studies and the members of the Center for Behavioral Teratology for ongoing assistance and support. Olivia Bjorkquist provided assistance preparing data for analysis. Jessica O’Brien assisted in preparing the manuscript. Research funded by grants R01 AA10417, R01 AA019605, T32 MH089920 and U01 AA014834.
References
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti-Rämö I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Developmental Medicine and Child Neurology. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Barr HM, Bookstein FL, O’Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy as a predictor of psychiatric disorders on the structured clinical interview for DSM-IV in young adult offspring. American Journal of Psychiatry. 2006;163:1061–1065. doi: 10.1176/ajp.2006.163.6.1061. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. Morbidity and Mortality Weekly Report Recommendations and Reports. 2005;54:1–14. [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anatomical Record Part B: New Anatomist. 2002;269:162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- Clark CM, Li D, Conry J, Conry R, Loock C. Structural and functional brain integrity of fetal alcohol syndrome in nonretarded cases. Pediatrics. 2000;105:1096–1099. doi: 10.1542/peds.105.5.1096. [DOI] [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain and Cognition. 2011;75:67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Developmental Neuropsychology. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Cortese BM, Moore GJ, Bailey BA, Jacobson SW, Delaney-Black V, Hannigan JH. Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: Preliminary findings in the caudate nucleus. Neurotoxicology and Teratology. 2006;28:597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2011;35:1114–1121. doi: 10.1111/j.1530-0277.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test - Children’s Version. San Antonio: The Psychological Corporation; 1994. [Google Scholar]
- Desmond JE. Trends in cerebellar research. Behavioural Neurology. 2010;23:1–2. doi: 10.3233/BEN-2010-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Examiner’s Manual for the Peabody Picture Vocabulary Test. Circle Pines, Minnesota: American Guidance Service; 1997. [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007a;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism: Clinical and Experimental Research. 2007b;31:1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol and Alcoholism. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1993. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Ostergaard AL, Fennema-Notestine C. Mesial temporal, diencephalic, and striatal contributions to deficits in single word reading, word priming, and recognition memory. Journal of the International Neuropsychological Society. 2001;7:63–78. doi: 10.1017/s1355617701711071. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Press GA, Hesselink JR. Methods for measuring brain morphologic features on magnetic resonance images. Validation and normal aging. Archives of Neurology. 1990;47:27–32. doi: 10.1001/archneur.1990.00530010035015. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neuroscience and Biobehavioral Reviews. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcoholism: Clinical and Experimental Research. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychology Review. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru J-P, Menuet J-C. Les enfants de parents alcooliques. Anomalies observees. A propos de 127 cas [Children of alcoholic parents. Abnormalities observed in 127 cases] Ouest Medical. 1968;21:476–482. [Google Scholar]
- Li Z, Ma X, Peltier S, Hu X, Coles CD, Lynch ME. Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imaging and Behavior. 2008;2:39–48. doi: 10.1007/s11682-007-9013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. Journal of the International Neuropsychological Society. 1999;5:462–471. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Research and Health. 2011;34:51–55. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996a;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. Journal of Pediatrics. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, Jones KL. A decrease in the size of the basal ganglia following prenatal alcohol exposure: A preliminary report. Neurotoxicology and Teratology. 1994;16:283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996b;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2002;26:875–882. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP CIFASD . Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2010;34:1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Research and Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2011;35:1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J. Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. The American Journal of Drug and Alcohol Abuse. 2002;28:743–754. doi: 10.1081/ada-120015880. [DOI] [PubMed] [Google Scholar]
- Pulsifer MB. The neuropsychology of mental retardation. Journal of the International Neuropsychological Society. 1996;2:159–176. doi: 10.1017/s1355617700001016. [DOI] [PubMed] [Google Scholar]
- Riley EP, Guerri C, Calhoun F, Charness ME, Foroud TM, Li T-K, Mattson SN, May PA, Warren KR. Prenatal alcohol exposure: Advancing knowledge through international collaborations. Alcoholism: Clinical and Experimental Research. 2003;27:118–135. doi: 10.1097/01.ALC.0000047351.03586.A3. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA. Frontal-striatal circuit functions: Context, sequence, and consequence. Journal of the International Neuropsychological Society. 2003;9:103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychology. 2006;12:439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reduction in lobules I-V. Alcoholism: Clinical and Experimental Research. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. NeuroReport. 2007;18:635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cerebral Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cerebral Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. The National Academies Press; Washington D.C: 1996. p. 213. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Parrish-Johnson JC, Kirchner GL, Martin DC. Attention, distraction and reaction time at age 7 years and prenatal alcohol exposure. Neurobehavioral Toxicology and Teratology. 1986;8:717–725. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF. Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring -- a longitudinal prospective study. Alcoholism: Clinical and Experimental Research. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. US Surgeon General releases advisory on alcohol use in pregnancy. US Department of Health and Human Services; Washington, DC: 2005. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Wilkinson GS. Manual for The Wide Range Achievement Test. Wilmington: Delaware, Wide Range, Inc; 1993. [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2004;28:497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]


