Abstract
Importance
Homer proteins are a family of scaffolding proteins of the postsynaptic density. Homer-3 colocalizes and modulates the activity of group I metabotropic glutamate receptors (mGluR1 and mGluR5). Cerebellitis has been reported in association with antibodies to mGluR1. We describe the second patient with cerebellitis and Homer-3 antibodies and report a novel, highly specific immunoblot assay.
Observations
A 38-year-old man had acute onset of headache, nausea, vomiting, and confusion. He developed a pancerebellar syndrome during the ensuing week. Extensive studies did not reveal any tumor. Cerebrospinal fluid analysis showed a white blood cell count of 60/µL (to convert to ×109 per liter, multiply by 0.001). Brain magnetic resonance imaging findings were normal. For 2 years, the patient was treated with intravenous immunoglobulins and steroids, with partial improvement of the cerebellar ataxia. The patient was negative for onconeural (Hu, Yo, Ri, CV2, Tr, amphiphysin, and Ma2), glutamic acid decarboxylase, and mGluR1 antibodies. Immunohistochemistry on rat brain revealed immunostaining of the cerebellar molecular layer. Homer-3 antibodies were demonstrated by immunoblot of recombinant Homer-3. The clinical features of this patient and a previously described patient with Homer-3 antibodies are similar to those of patients with mGluR1 antibodies.
Conclusions and Relevance
We report the second case of autoimmune cerebellar ataxia associated with Homer-3 antibodies. The presence of Homer-3 autoantibodies should be considered in the differential diagnosis of patients with subacute cerebellar ataxia of unknown cause.
Cerebellar ataxia of subacute onset and cerebrospinal fluid (CSF) pleocytosis typically occur in children and young adults following a viral infection or a self-limited respiratory or gastrointestinal febrile episode.1 In older patients, subacute cerebellar ataxia may have a paraneoplastic origin.2 In this setting, onconeural antibodies are excellent markers to identify the cerebellar syndrome as paraneoplastic.2 Systematic screening for autoantibodies in patients with nonparaneoplastic cerebellitis has identified a limited number of relevant antibodies: glutamic acid decarboxylase,3 metabotropic glutamate receptor type 1 (mGluR1),4 and contactin-associated protein 2.5 However, there is a subset of patients with acute cerebellar degeneration in whom onconeural or autoimmune antibodies are not identified despite a suspected autoimmune cause. Recently, antibodies against Homer-3 were identified in a 65-year-old woman with cerebellitis.6 The Homer-3 isoform is almost exclusively found in the cerebellum at the postsynaptic density.7 Metabotropic glutamate receptor type 1 is a G-protein–coupled cell surface receptor that mediates slow cation conductance and is highly expressed at the perisynaptic site of Purkinje cell dendritic spines.4 The intracellular domain of mGluR1 interacts with Homer-3, enabling multimerization and clustering of the receptor, which are critical for neuroadaptation.7 We report a second case of cerebellitis with Homer-3 antibodies and compare the clinical profile with patients with mGluR1 antibodies.
REPORT OF A CASE
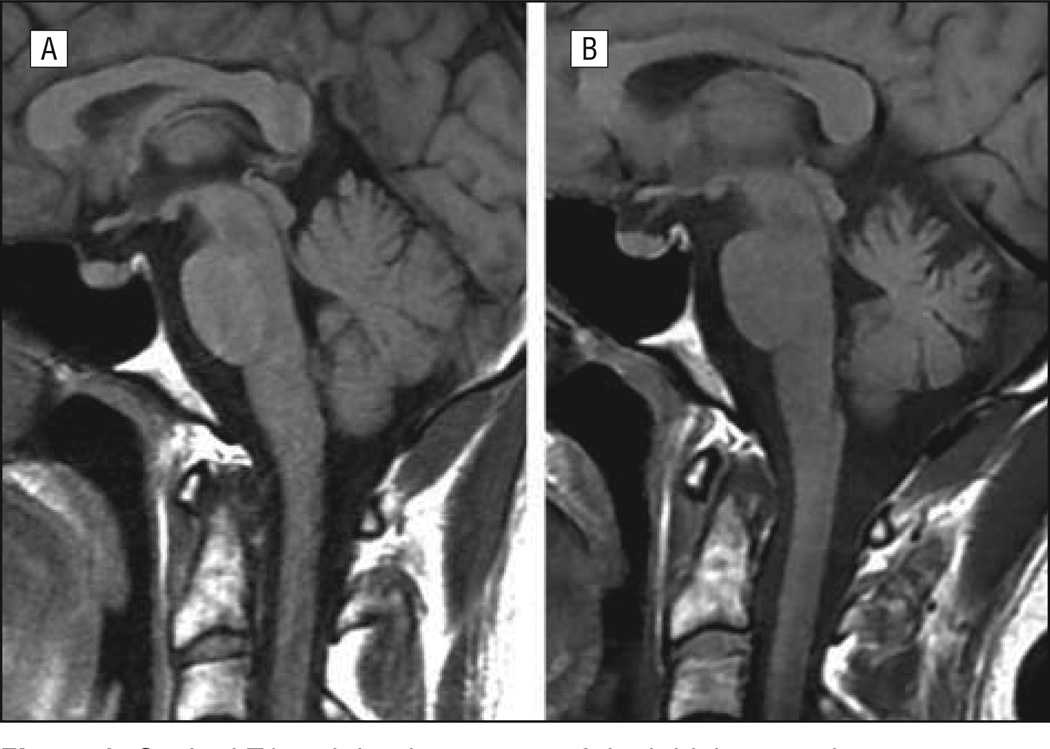
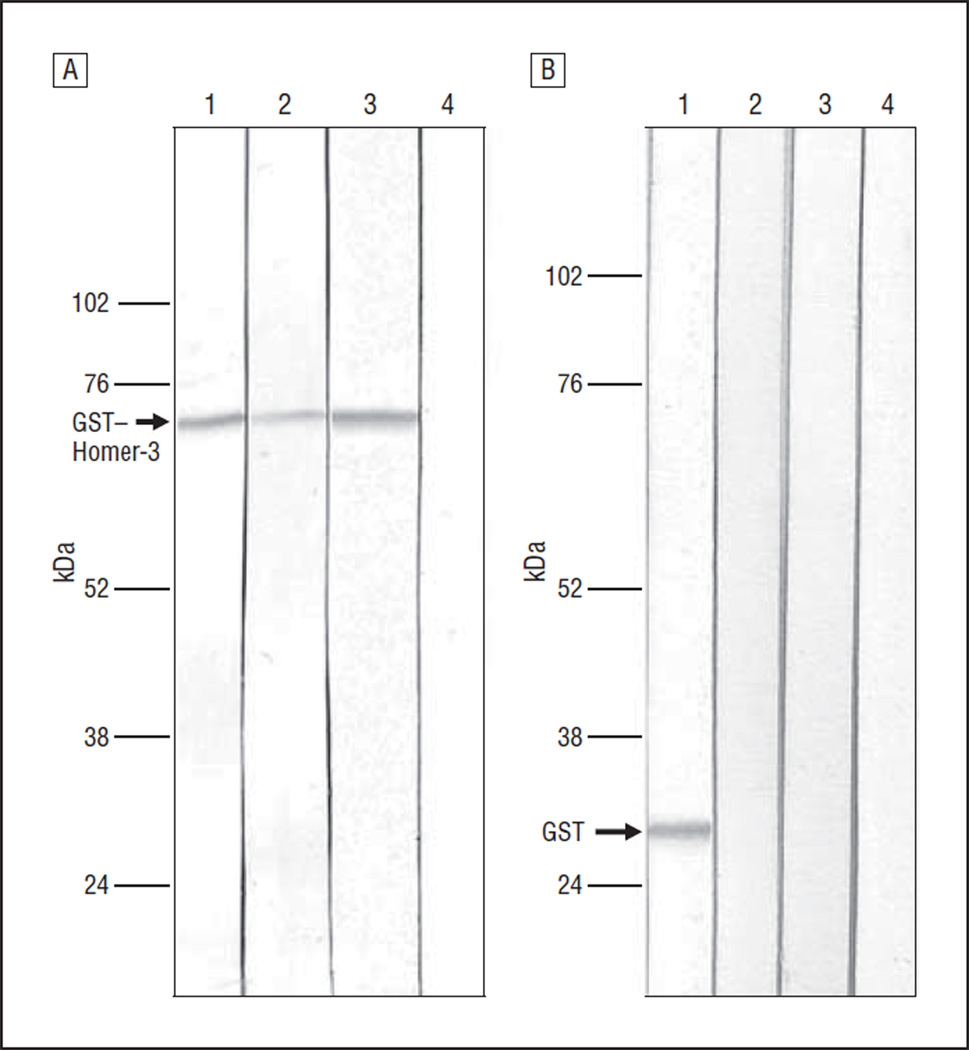
A 38-year-old man was admitted for acute onset of headache, nausea, vomiting, and confusion. He rapidly developed a pancerebellar syndrome and complex partial seizures with secondary generalization. Neurological examination demonstrated drowsiness, dysarthria, bilateral horizontal nystagmus, moderate limb dysmetria, and gait ataxia. Optic fundus examination disclosed bilateral papilledema. Lumbar puncture revealed elevated opening pressure (36 cm H2O), mild pleocytosis (white blood cell count, 60/µL [to convert to ×109 per liter, multiply by 0.001], 78% lymphocytes), elevated protein level (0.111 g/dL; to convert to grams per liter, multiply by 10), negative oligoclonal bands, and normal IgG index. Results on brain magnetic resonance imaging (MRI) and screening for antigliadin antibodies, viral and bacterial infections, tumor, and systemic, metabolic, and thyroid diseases were negative. The patient was treated with cycles of intravenous immunoglobulins and steroids with partial improvement. Later, he received oral steroids for 24 months. Currently, the patient is on a daily dose of 7.5 mg of prednisone. He has mild dysarthria and gait ataxia, but he is able to walk without help and can independently carry out basic daily activities. Repeated screening for neoplasia has been negative, and the last brain MRI showed mild atrophy of the vermis and cerebellar hemispheres (Figure 1). Results of screening the patient’s serum (CSF was not available) for antibodies to neuronal cell surface antigens (N-methyl-d-aspartate receptor [NMDAR], α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor [AMPAR], γ-aminobutyric acid B receptor [GABABR], mGluR1, mGluR5, leucine-rich glioma inactivated 1 [LGI1], and contactin-associated protein 2 [Caspr2]), onconeural antigens (Hu, Yo, Ri, CV2, Tr, amphiphysin, and Ma2), and glutamic acid decarboxylase were negative. Indirect immunohistochemistry on rat brain sections showed an intense immunostaining of the molecular layer of the cerebellum and weaker reactivity with the cytoplasm of Purkinje cells. This pattern of immunostaining was identical to that seen in a previous patient with Homer-3 antibodies.6 To confirm the presence of these antibodies, we developed an immunoblot with purified Homer-3 protein fused with a glutathione S-transferase tag. Briefly, the complementary DNA fragment encoding the Homer-3 was subcloned to the pGEX-4T3 plasmid (GE Healthcare Life Sciences) that contains a glutathione S-transferase tag. Glutathione sepharose 4B (GE Healthcare Life Sciences) was used to purify the expressed protein. Immunoblot with purified Homer-3 showed that the patient’s serum identified a protein band of 73 kDa corresponding to Homer-3 (Figure 2A). A similar band was identified using serum of the previously described patient with Homer-3 and a commercial Homer-3 antibody (Santa Cruz Biotechnology, Inc) but was not identified with 45 control serum samples, including samples from patients with ataxia (23 samples) and healthy subjects (22 samples). Positive serum samples did not recognize the glutathione S-transferase tag (Figure 2B).
Figure 1.
Sagittal T1-weighted sequence of the initial magnetic resonance image showed no cerebellar abnormalities (A), whereas the last brain magnetic resonance image disclosed mild cerebellar atrophy (B).
Figure 2.
Immunoblots. A, Immunoblot of glutathione S-transferase (GST)–tagged Homer-3. Lanes are positive control6 (1), patient’s serum (2), Homer-3 commercial antibody (3), and normal serum (4). B, Immunoblot of GST tag. Lanes are GST commercial antibody (1), patient’s serum (2), normal serum (3), and positive control6 (4).
Clinical features of patients with Homer-3 and mGluR1 antibodies are summarized in the Table. Most patients were female and middle-aged except one who was 19 years old. Two of the 4 patients with mGluR1 had a remote history of Hodgkin lymphoma. No other patients developed an underlying tumor or additional autoimmune disorder. The onset of disease was always subacute, the initial MRI findings were normal, and all presented with CSF pleocytosis. Cerebellar symptoms improved, at least partially, in half of the patients.
Table.
Clinical Features of Cerebellitis Associated With Homer-3 or mGluR1 Antibodies
| Patient No./ Age/Sex |
Source | Antibody | AI or Tumor |
Subacute Onset |
Extracerebellar Features |
Initial/Follow-up MRI Results (Time Since Diagnosis, mo) |
WBC Count in CSF, /µL |
Treatment | Outcome (Follow-up, mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1/M/38 | Current study | Homer-3 | No | Yes | Initial encephalitis | Normal/mild cerebellar atrophy (10) | 60 | Prednisone, IVIg | Partial recovery (24) |
| 2/F/65 | Zuliani et al,6 2007 | Homer-3 | No | Yes | No | Normal/NA | 27 | Prednisone | Severe ataxia (72) |
| 3/F/19 | Sillevis Smitt et al,4 2000 | mGluR1 | Hodgkin lymphomaa | Yes | No | Normal/NA | 28 | Prednisone, IVIg, plasma exchange | Full recovery (7) |
| 4/F/49 | Sillevis Smitt et al,4 2000 | mGluR1 | Hodgkin lymphomab | Yes | Short-term memory loss | Normal/normal (6) | NA | Plasma exchange | Severe ataxia (12) |
| 5/F/50 | Marignier et al,8 2010 | mGluR1 | No | Yes | Transient initial headache | Normal/mild cerebellar atrophy (40) | 190 | Prednisone, IVIg, mycophenolate mofetil | Partial recovery (40) |
| 6/M/69 | Lancaster et al,9 2011 | mGluR1 | No | Yes | No | Normal/cerebellar atrophy (12) | 8 | Prednisone | Severe ataxia (48) |
Abbreviations: AI, autoimmune disease; CSF, cerebrospinal fluid; IVIg, intravenous immunoglobulins; mGluR1, metabotropic glutamate receptor type 1; MRI, magnetic resonance imaging; NA, not available; WBC, white blood cell. SI conversion factor: To convert WBC count to ×109 per liter, multiply by 0.001.
Hodgkin lymphoma diagnosed 2 years earlier.
Hodgkin lymphoma diagnosed 9 years earlier.
COMMENT
We describe a second patient with cerebellitis associated with Homer-3 antibodies, suggesting that these autoantibodies are relevant in the diagnosis of primary autoimmune cerebellitis. To confirm the presence of Homer-3 antibodies, we developed an immunoblot test using a glutathione S-transferase–tagged purified fusion protein that provides unambiguous results. Antibodies against Homer-3 were previously identified in a 65-year-old woman with a similar clinical picture of subacute onset of ataxia, CSF pleocytosis, and normal findings on brain MRI.6 Repeated tumor screenings failed to show an underlying tumor in both patients, suggesting that Homer-3 antibodies associate with nonparaneoplastic cerebellitis. Presently, only a few antibodies may be useful to identify nonparaneoplastic cerebellar ataxia. The targeted antigens are synaptic cell surface proteins such as mGluR1,8 whereas glutamic acid decarboxylase and Homer-3 are intracellular. The cerebellar ataxia associated with glutamic acid decarboxylase antibodies usually has a more chronic evolution and pleocytosis is unusual.10 A recent study identified Caspr2 antibodies in 9 of 88 patients (10%) considered to have idiopathic ataxia.5 Seven of these patients had additional disorders (limbic encephalitis, sarcoidosis, other antibodies) or limited clinical information (none with pleocytosis) and the serum samples of the remaining 2 patients did not react with cerebellum, suggesting that other mechanisms were likely involved.5
Homer-3 and mGluR1 are expressed predominantly in the dendritic spines of the Purkinje cells of the cerebellum. Homer-3 specifically binds to the mGluR1 C-terminus, thus regulating the trafficking and clustering of mGluRs and modulating their functional activity.7 Homer-3 also binds to inositol 1,4,5-triphosphate receptors in the smooth endoplasmic reticulum. The physical linkage of mGluR1 to inositol 1,4,5-triphosphate receptors via Homer-3 complexes is physiologically important for postsynaptic calcium responses to mGluR1 stimulation.7
In line with the close relationship between mGluR1 and Homer-3, the clinical symptoms of our patient and the previously published Homer-3 case6 are very similar to the symptoms of 4 described patients with mGluR1 antibodies.4,8,9 Our patient and one with mGluR1 antibodies (Table) initially presented with additional symptoms of encephalitis, a relatively common finding in patients with cerebellitis.1 Both antibody specificities associate with subacute onset of cerebellar ataxia, normal findings on brain MRI, CSF inflammatory changes, and partial response to immunotherapy. The latter is probably explained by the observation of Purkinje cell loss at the autopsy of one of the patients with mGluR1 antibodies (patient 4, Table).11 It is unclear whether the suboptimal response to first-line immunotherapies is due to the fact that Purkinje cell loss occurs earlier in the clinical course and whether more intense immunotherapy up front could prevent irreversible damage of the Purkinje cells.
The detection of well-characterized autoantibodies is important in the diagnostic workup of patients with cerebellar ataxia to establish a probable paraneoplastic or autoimmune cause. We have now developed a highly reliable immunoblot test for the detection of Homer-3 antibodies. Our report confirms the association of Homer-3 antibodies with primary autoimmune cerebellitis. These antibodies as well as those targeting the closely related receptor mGluR1 should be included in the differential diagnosis of middle-aged patients with subacute-onset cerebellitis.
Acknowledgments
Funding/Support: This study was supported in part by grants PS09/0193 and 11/01780 (Dr Dalmau) from the Fondo de Investigaciones Sanitarias, grants R01 NS077851 and R01 CA89054 from the National Institutes of Health (Dr Dalmau), a McKnight Neuroscience of Brain Disorders award (Dr Dalmau), and a grant from the Fundació la Marató de TV3 (Dr Dalmau). Dr Höftberger was supported by Project J3230 from the Fonds zur Förderung der wissenschaftlichen Forschung.
Footnotes
Author Contributions: Study concept and design: Höftberger, Dalmau, and Graus. Acquisition of data: Höftberger, Sabater, Ortega, and Graus. Analysis and interpretation of data: Höftberger, Sabater, and Graus. Drafting of the manuscript: Höftberger, Sabater, and Dalmau. Critical revision of the manuscript for important intellectual content: Höftberger, Sabater, Ortega, and Graus. Obtained funding: Höftberger, Dalmau, and Graus. Administrative, technical, and material support: Höftberger and Sabater. Study supervision: Dalmau and Graus.
Conflict of Interest Disclosures: Dr Dalmau receives royalties from Athena Diagnostics for a patent for the use of Ma2 as an autoantibody test and receives licensing fees from Euroimmun for a patent for the use of NMDAR as an autoantibody test.
Additional Contributions: Mercè Alba, BS, provided technical assistance.
REFERENCES
- 1.Klockgether T, Döller G, Wüllner U, Petersen D, Dichgans J. Cerebellar encephalitis in adults. J Neurol. 1993;240(1):17–20. doi: 10.1007/BF00838440. [DOI] [PubMed] [Google Scholar]
- 2.Shams’ili S, Grefkens J, de Leeuw B, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126(pt 6):1409–1418. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 3.Honnorat J, Saiz A, Giometto B, et al. Cerebellar ataxia with anti-glutamic acid decarboxylase antibodies: study of 14 patients. Arch Neurol. 2001;58(2):225–230. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- 4.Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21–27. doi: 10.1056/NEJM200001063420104. [DOI] [PubMed] [Google Scholar]
- 5.Becker EB, Zuliani L, Pettingill R, et al. Contactin-associated protein-2 antibodies in non-paraneoplastic cerebellar ataxia. J Neurol Neurosurg Psychiatry. 2012;83(4):437–440. doi: 10.1136/jnnp-2011-301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuliani L, Sabater L, Saiz A, Baiges JJ, Giometto B, Graus F. Homer 3 autoimmunity in subacute idiopathic cerebellar ataxia. Neurology. 2007;68(3):239–240. doi: 10.1212/01.wnl.0000251308.79366.f9. [DOI] [PubMed] [Google Scholar]
- 7.Xiao B, Tu JC, Petralia RS, et al. Homer regulates the association of group 1metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21(4):707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 8.Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627–630. doi: 10.1001/archneurol.2010.51. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77(18):1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(pt 10):2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 11.Coesmans M, Smitt PA, Linden DJ, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol. 2003;53(3):325–336. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]