Abstract
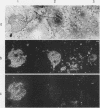
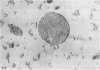
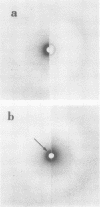
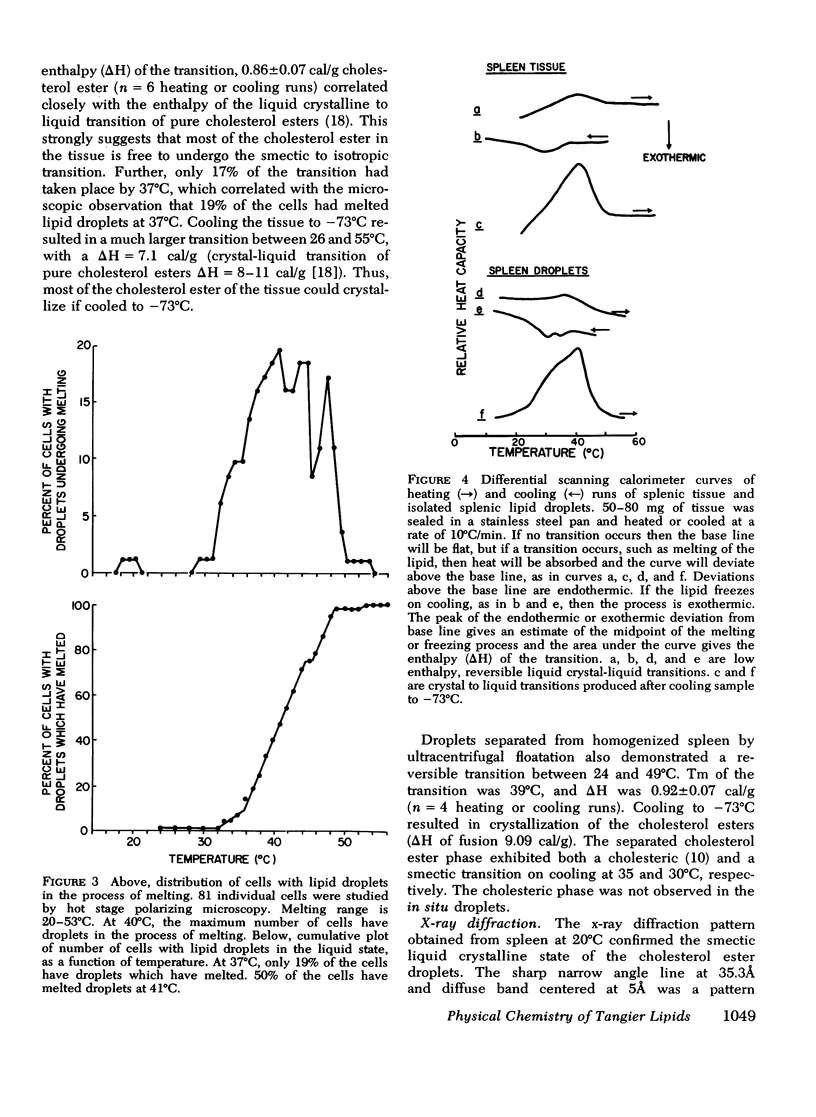
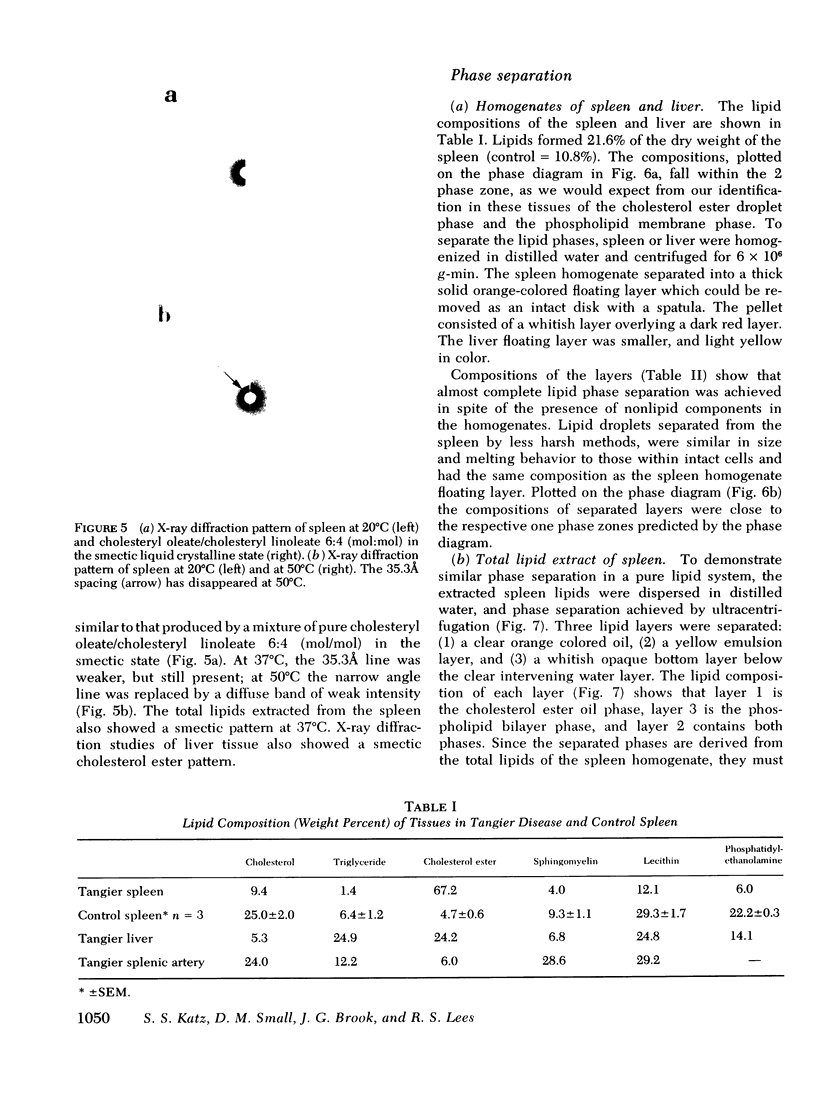
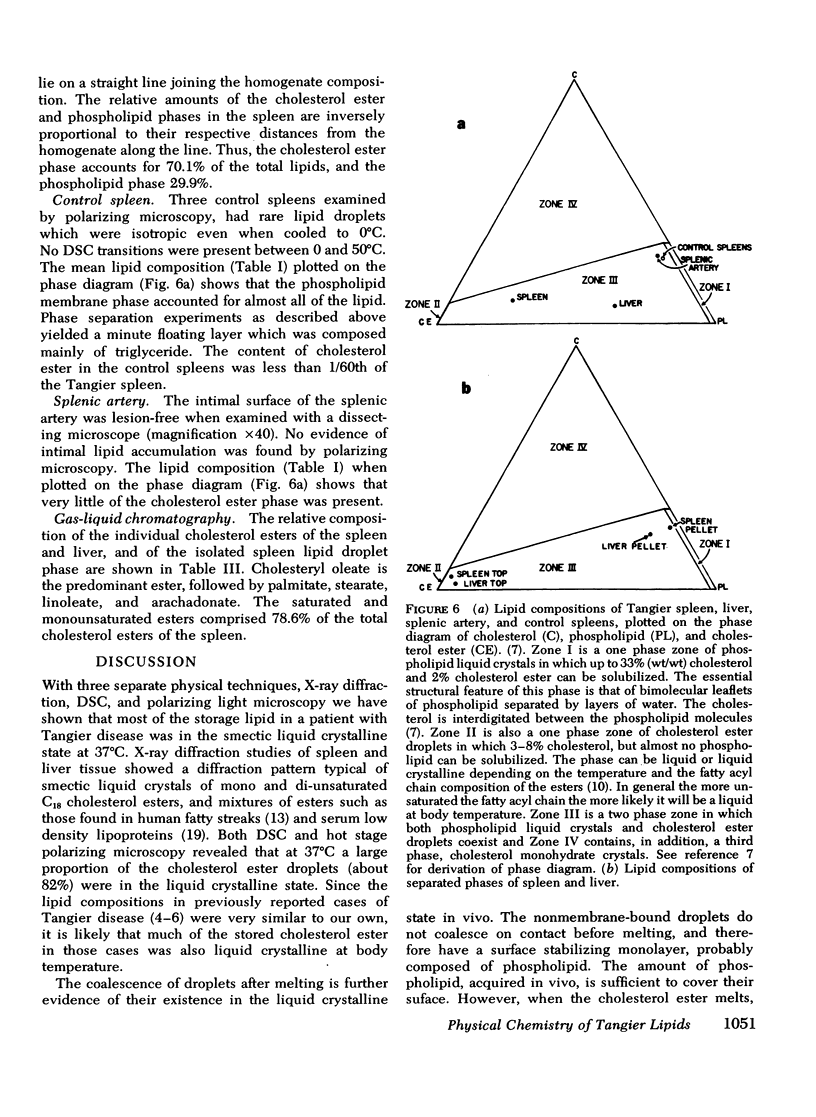
The physical states and phase behavior of the lipids of the spleen, liver, and splenic artery from a 38-yr-old man with Tangier disease were studied. Many intracellular lipid droplets in the smectic liquid crystalline state were identified by polarizing microscopy in macrophages in both the spleen and liver, but not in the splenic artery. The droplets within individual cells melted sharply over a narrow temperature range, indicating a uniform lipid composition of the droplets of each cell. However different cells melted over a wide range, 20-53°C indicating heterogeneity of lipid droplet composition between cells. Furthermore, most of the cells (81%) had droplets in the liquid crystalline state at 37°C. X-ray diffraction studies of splenic tissue at 37°C revealed a diffraction pattern typical of cholesterol esters in the smectic liquid crystalline state. Differential scanning calorimetry of spleen showed a broad reversible transition from 29-52°C, with a maximum mean transition temperature at 42°C, correlating closely with the polarizing microscopy observations. The enthalpy of the transition, 0.86±0.07 cal/g of cholesterol ester, was quantitatively similar to that of the liquid crystalline to liquid transition of pure cholesterol esters indicating that nearly all of the cholesterol esters in the tissue were free to undergo the smectic-isotropic phase transition.
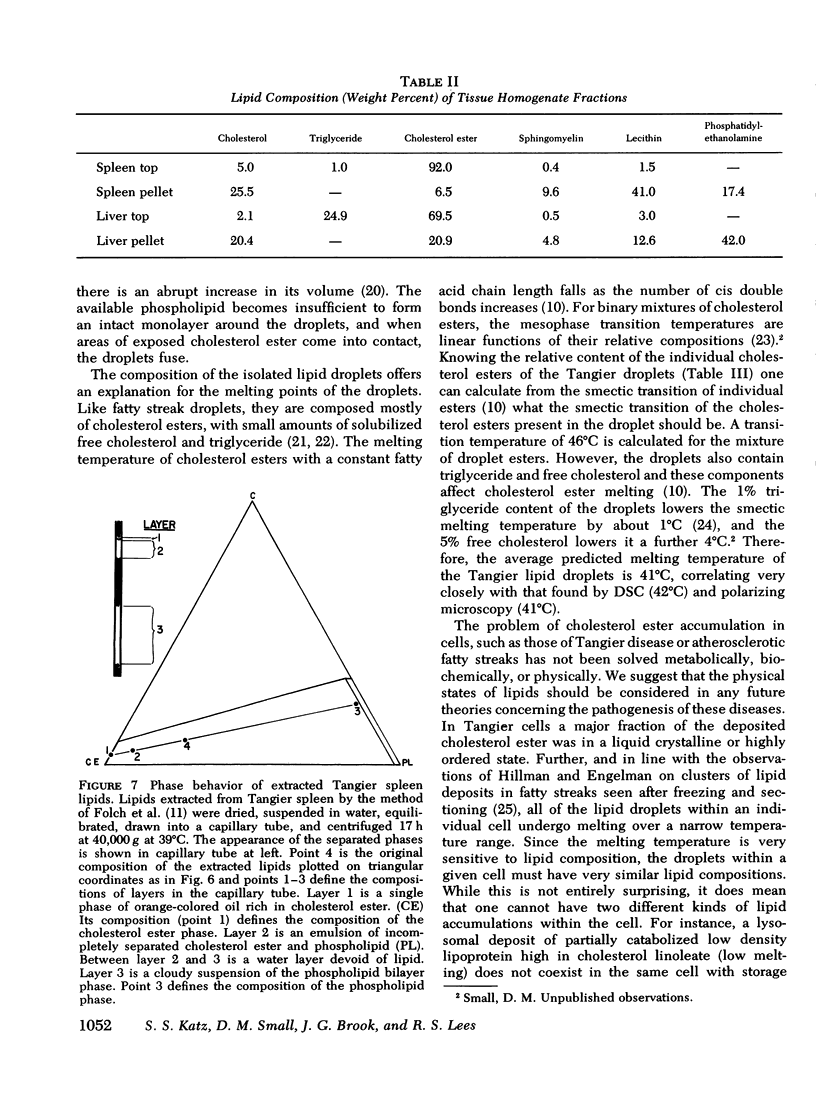
Lipid compositions of spleen and liver were determined, and when plotted on the cholesterol-phospholipid-cholesterol ester phase diagram, fell within the two phase zone. The two phases, cholesterol ester droplets and phospholipid bilayers were isolated by ultracentrifugation of tissue homogenates. Lipid compositions of the separated phases approximated those predicted by the phase diagram. Extracted lipids from the spleen, when dispersed in water and ultracentrifuged, underwent phase separation in a similar way. Thus (a) most of the storage lipids in the liver and spleen of this patient were in the liquid crystalline state at body temperature, (b) the phase behavior of the storage lipids conformed to that predicted by lipid model systems indicating lipid-lipid interactions predominate in affected cells, (c) lipid droplets within individual cells have similar compositions, whereas droplet composition varies from cell to cell, and (d) cholesterol ester does not accumulate in the splenic artery. Since Tangier patients lack high density lipoprotein, we conclude that high density lipoprotein-mediated cholesterol removal from cells is essential only for those cells which have an obligate intake of cholesterol (macrophages).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale P. M., Clifton-Bligh P., Benjamin B. N., Whyte H. M. Pathology of Tangier disease. J Clin Pathol. 1971 Oct;24(7):609–616. doi: 10.1136/jcp.24.7.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S. R., Rothblat G. H. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974 Jul 26;360(1):38–55. doi: 10.1016/0005-2760(74)90178-7. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M., Lees R. S., George P. K. Thermal transitions in human plasma low density lipoproteins. Science. 1975 Oct 24;190(4212):392–394. doi: 10.1126/science.170681. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M. Structure and interactions of lipids in human plasma low density lipoproteins. J Biol Chem. 1977 Jan 25;252(2):744–754. [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Ferrans V. J., Fredrickson D. S. The pathology of Tangier disease. A light and electron microscopic study. Am J Pathol. 1975 Jan;78(1):101–158. [PMC free article] [PubMed] [Google Scholar]
- Gerschenson L. E., Berliner J., Davidson M. B. The isolation and culture of liver cells. Methods Enzymol. 1974;32:733–740. doi: 10.1016/0076-6879(74)32076-9. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Hoffman H. N., Fredrickson D. S. Tangier disease (familial high density lipoprotein deficiency). Clinical and genetic features in two adults. Am J Med. 1965 Oct;39(4):582–593. doi: 10.1016/0002-9343(65)90081-1. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P. D., Insull W., Jr Lipid droplets in atherosclerotic fatty streaks of human aorta. J Clin Invest. 1970 Aug;49(8):1479–1488. doi: 10.1172/JCI106365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., Levy R. I., Gotto A. M., Fredrickson D. S. Studies on the protein defect in Tangier disease. Isolation and characterization of an abnormal high density lipoprotein. J Clin Invest. 1972 Oct;51(10):2505–2519. doi: 10.1172/JCI107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M., Shipley G. G. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974 Jul 19;185(4147):222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]
- Stein O., Vanderhoek J., Stein Y. Cholesterol content and sterol synthesis in human skin fibroblasts and rat aortic smooth muscle cells exposed to lipoprotein-depleted serum and high density apolipoprotein/phospholipid mixtures. Biochim Biophys Acta. 1976 May 27;431(2):347–358. doi: 10.1016/0005-2760(76)90155-7. [DOI] [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Cholesterol metabolism in the macrophage. 3. Ingestion and intracellular fate of cholesterol and cholesterol esters. J Exp Med. 1972 Jan;135(1):21–44. doi: 10.1084/jem.135.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]