Abstract
To identify risk factors for recurrence in patients with stage II colon cancer, Cox proportional hazards regression analysis was performed in 194 patients with stage II colon cancer who underwent curative surgery between April 1997 and December 2008. Thirteen clinical and pathologic factors, including use of fluoropyrimidine-based adjuvant chemotherapy in 113 of the patients (58.2%), were assessed. By multivariate analysis, only obstruction, perforation, and T4-level invasion were identified as independent risk factors affecting disease-free survival (DFS) (P < 0.01). The 5-year DFS rate was 70.6% in patients with one or more risk factors (n = 68) and 96.0% in patients with no risk factors (n = 126) (P < 0.01). These results suggest that obstruction, perforation, and T4-level invasion are suitable candidates for prediction of tumor recurrence in patients with stage II colon cancer. The oxaliplatin-based adjuvant chemotherapy, which has been reported to be effective in stage III colon cancer patients, may improve the prognosis in high-risk stage II colon cancer patients.
Keywords: Colon cancer, High risk factor, Stage II
Current clinical guidelines on the management of stage II colon cancer state that the standard of care is surgical resection alone, but recommend that patients with poor prognostic features should be considered for adjuvant chemotherapy.1–3 The ability to identify a subgroup of stage II colon cancer patients at high risk of recurrence would improve treatment strategies and perhaps also subsequent outcome. Recent advances in adjuvant chemotherapy have significantly improved the prognosis in patients with stage III colon cancer, particularly with regard to the superiority of oxaliplatin-based chemotherapy over 5-fluorouracil (5-FU)-based chemotherapy.4–7 However, the effectiveness of oxaliplatin-based chemotherapy has yet to be confirmed in patients with stage II colon cancer.4,5 We conducted this retrospective study to identify risk factors for recurrence in stage II colon cancer by analyzing those patients in Japan who had undergone curative surgery before oxaliplatin-based adjuvant chemotherapy became available in clinical practice.
Methods
Patients
This study was performed in accordance with ethical guidelines for clinical research with the approval of our institutional ethics committee. All patients included in the study underwent surgery for histologically proven stage II colon cancer between January 1997 and December 2008 at our department. Exclusion criteria were as follows: postoperative mortality within 30 days; a limited follow-up period of less than 3 years in cases without recurrence; synchronous multiple cancers; positive surgical margins; concomitant inflammatory bowel disease; and familial adenomatous polyposis or previous malignancy within 5 years. No patient received preoperative chemotherapy or radiotherapy.
Methods
The relationship between various clinicopathologic variables and disease-free survival (DFS) was evaluated. The factors evaluated included the following 15 nominal variables: age (>70 years or ≤70 years), sex, tumor perforation (presence or absence), bowel obstruction (presence or absence), serum carcinoembryonic antigen (CEA) level (elevated or normal; cutoff, 6.7 ng/mL), site of tumor (right colon or left colon), size of tumor (>60 mm or ≤60 mm), number of regional lymph nodes examined (12 > or 12 ≦), histologic differentiation (well-differentiated adenocarcinoma, moderately differentiated adenocarcinoma, and poorly differentiated adenocarcinoma), depth of invasion (T3, T4a, or T4b), lymphatic invasion (no/minimal or moderate/severe), venous invasion (no/minimal or moderate/severe), perineural invasion (presence or absence), level of lymph node dissection (D1, D2, or D3), and 5-FU-based adjuvant chemotherapy (use or non-use). Obstruction was defined as complete stoppage of flatus or defecation with dilatation proximal to the tumor, or no passage of contrast medium into the proximal site of the tumor. Information on pathological data was obtained from histopathologic reports. All original slides relating to perineural invasion, however, were reviewed by a single pathologist who was blinded to clinical details and outcomes. Histologic differentiation, lymphatic and vascular invasion, and level of lymph node dissection performed were described according to the Japanese Classification of Colorectal Carcinoma.8 Specifically, lymphatic and venous invasion were classified into 4 categories each: ly0/v0, ly1/v1, ly2/v2, and ly3/v3, representing no, minimal, moderate, and severe invasion, respectively. D1-level lymph node dissection included removal of paracolic and epicolic lymph nodes; D2-level lymph node dissection included additional intermediate lymph nodes in addition to D1-level dissection; and D3-level lymph node dissection included additional removal of central lymph nodes in addition to D2-level lymph node dissection. Depth of invasion was categorized according to the seventh edition of the TNM classification.9
Follow-up
All the patients were assessed at intervals for local control and distant metastases by clinical examination, chest X-ray, liver ultrasonography (or computed tomography), and measurement of serum CEA level. In principle, these results were recorded at follow-up visits every 3 months during the first 2 years, every 6 months during the next 3 years, and then once a year when considered necessary. As adjuvant chemotherapy, 5-FU-based chemotherapy such as oral UFT (Tegafur/Uracil) for 12 months, and oral UFT/leucovorin (LV) or intravenous 5-FU/LV for 6 months was given in selected patients based on physician's recommendation and patient preference. In Japan, use of oxaliplatin-based chemotherapy for colonic cancer in an adjuvant setting was not approved by the governmental health insurance system until August 2009.
Statistical analysis
Continuous variables are expressed as median and range. Survival analysis was conducted using both the Kaplan-Meier method and Cox proportional hazards regression. The log-rank test was used to determine significance in the Kaplan-Meier analysis. Disease-free survival was calculated from the time of surgery to the date of diagnosis of recurrence, and was censored at the time of death from other causes, at the time of diagnosis of other malignant or metachronous colorectal cancer, at the time of the last visit to our hospital with no clinical or radiologic findings suggesting recurrence, or December 2011, whichever came first. Multivariate Cox proportional hazards regression was used to identify independent significant risk factors affecting DFS. All variables were initially assessed by univariate Cox proportional hazards regression, and variables with a P value < 0.10 were introduced into multivariate regression with forward stepwise selection. A P value < 0.05 was considered to be statistically significant. All the statistical analyses were performed using a statistical software package (StatFlex version 6.0; Artec, Osaka, Japan).
Results Patient characteristics and recurrence
A total of 235 patients underwent curative surgery for histologically proven stage II colon cancer during the study period. Of these, 41 patients met at least one of the exclusion criteria. Therefore, a total of 194 patients were eligible. The 5-year DFS rate was 87.2% in all patients (Fig. 1). Table 1 shows patient characteristics. There were 118 men and 76 women. Median patient age was 67 years (range, 25–92 years). Eight patients (4.1%) and 14 patients (7.2%) were associated with perforation and obstruction, respectively. The sites were the right colon (cecum, ascending colon, or transverse colon) in 83 patients (42.8%), and the left colon (descending colon, sigmoid colon, or rectosigmoid colon) in 111 patients (57.2%). Serum CEA level (cutoff, 6.7 ng/mL) was elevated in 80 patients (41.2%). The maximal tumor diameter was ≥60 mm in 67 patients (34.5%)%). Histologic differentiation revealed well-differentiated adenocarcinoma in 108 patients (55.9%), moderately differentiated adenocarcinoma in 82 patients (42.3%), and poorly differentiated adenocarcinoma in 4 patients (2.1%). The median number of regional lymph nodes harvested was 14 (range, 1–63), thus the number was 12 or greater in 119 patients (61.3%). The depth of invasion was T3 in 140 patients (72.2%), T4a in 44 patients (22.7%), and T4b in 10 patients (5.2%). Lymphatic and venous invasion was moderate to severe in 11 patients (5.8%) and 25 patients (12.9%), respectively. Perineural invasion was positive in 15 patients (7.7%). The level of lymph node dissection was D1 in 2 patients (1.0%), D2 in 21 patients (10.8%), and D3 in 171 patients (88.1%). A total of 113 patients (58.2%) received 5-FU-based adjuvant chemotherapy (Table 1).
Fig. 1 .
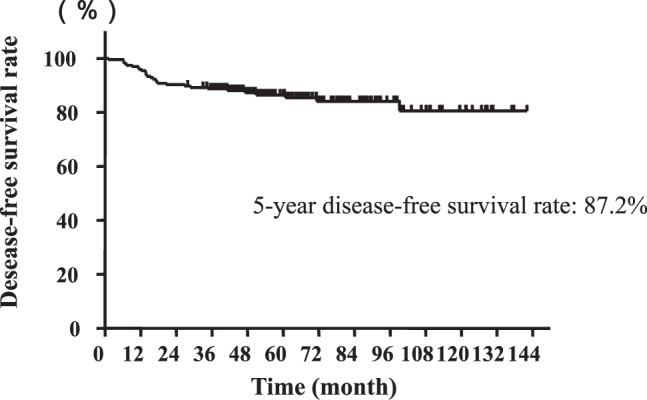
The 5-year disease-free survival curve of all patients with stage II colon cancer.
Table 1 .
Characteristics of patients with stage II colon cancer

The median follow-up period was 53 months (range, 36–142 months) in patients without recurrence and 17 months (range, 6–61 months) in patients with tumor recurrence. Of the 28 patients with tumor recurrence, 15 had hematogenous and lung metastasis, 2 had peritoneal carcinomatosis, 3 had local recurrence around the primary tumor site, and 1 patient had a combination of hematogenous metastasis and peritoneal carcinomatosis.
Univariate and multivariate analyses
On univariate analysis, 4 variables were identified as significant risk factors affecting DFS: obstruction [P = 0.01; hazard ratio, 3.323 (1.273–8.674)], perforation [P < 0.01; hazard ratio, 5.782 (2.008–16.650)], T4-level invasion [P < 0.01; hazard ratio, 3.520 (1.730–7.143)], and limited lymph node dissection (D1- and D2-level) [P = 0.048; hazard ratio, 2.494 (1.006–6.173)].
These four variables were introduced into the multivariate analysis, and the following 3 variables were retained as independent significant risk factors: obstruction [P = 0.02; hazard ratio, 3.319 (1.257–8.765)], perforation [P < 0.01; hazard ratio, 4.392 (1.482–13.015)], and T4-level invasion [P < 0.01; hazard ratio, 3.039 (1.475–6.250)] (Table 2).
Table 2 .
Univariate and multivariate analyses using the Cox proportional hazard model to identify significant risk factors affecting recurrence.

The 5-year DFS rate was significantly higher in patients with one or more risk factors (n = 68) than in patients with no risk factors (n = 126) (70.6% versus 96.0%, P < 0.01) (Fig. 2). The survival analysis was separately undertaken based on the use or non-use of 5-FU-based adjuvant chemotherapy. Among patients with one or more risk factors, DFS showed no significant difference between patients with adjuvant chemotherapy and those without (P = 0.85) (Fig. 3). This was also the case in patients with no risk factors (P = 0.56).
Fig. 2 .

Comparison of the 5-year disease-free survival curves between high-risk patients and low-risk patients.
Fig. 3 .
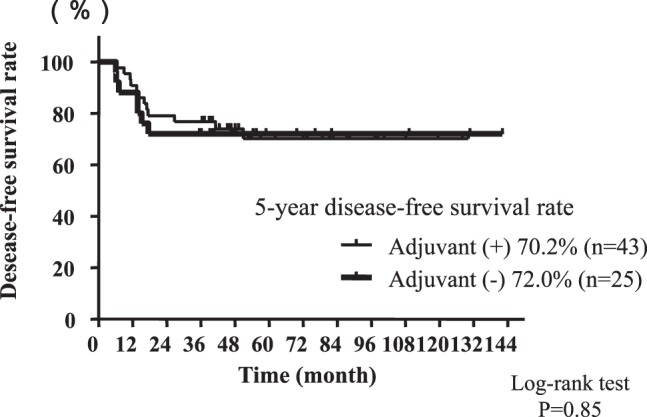
Comparison of disease-free survival curves of patients with high-risk factors for recurrence between patient groups with and without adjuvant chemotherapy.
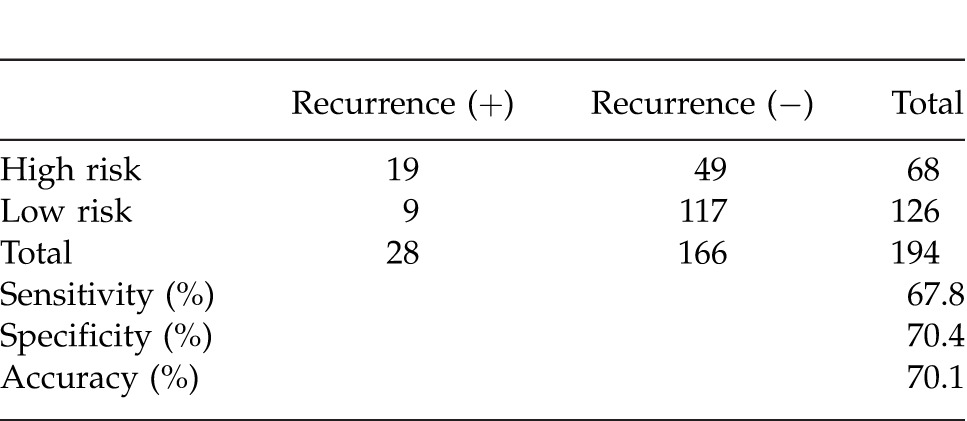
With regard to prediction of tumor recurrence with one or more risk factors, sensitivity, specificity, and accuracy were 67.8%, 70.4%, and 70.1%, respectively (Table 3).
Table 3 .
Sensitivity, specificity, and accuracy regarding the prediction of tumor recurrence with one or more risk factors.
Discussion
This study focused on DFS rather than overall survival (OS), as our aim was to identify stage II colon cancer patients at high risk of recurrence who might benefit from oxaliplatin-based chemotherapy rather than 5-FU-based chemotherapy in an adjuvant setting. It should also be noted that prolonged survival after recurrence, possibly as a result of effective chemotherapy, is generally known to reduce the association between DFS and OS. Using the 3 independent prognostic factors (T4-level invasion, obstruction, and perforation) determined in the present study, the authors were able to identify favorable and poor groups, with cumulative 5-year DFS rates of 96.0% and 70.6%, respectively. Limited lymph node dissection was significantly associated with shorter DFS by univariate, but not by multivariate analysis. The reason for this is unclear, but limited lymph node dissection was selectively performed in patients with tumor perforation or obstruction requiring emergency surgery, and/or in those with severe morbidity. Thus, the impact of a shorter DFS may be associated with the tumor-specific and/or patient-specific characteristics rather than the extent of lymph node dissection itself.
The clinical guidelines of the National Comprehensive Cancer Network,1 the American Society of Clinical Oncology,2 and the European Society of Medical Oncology3 do not recommend routine administration of adjuvant chemotherapy in patients with stage II colon cancer and suggest that adjuvant chemotherapy should be considered in patients with at least one of the following factors: inadequate lymph node sampling,1–3 obstruction,1–3 tumor perforation,1–3 poorly or undifferentiated histology,1–3 lymph-vascular invasion,1,3 perineural invasion,3 and T4-level invasion.1–3 The 3 independent factors identified in the present study offer a simple set of selection criteria for use in a clinical setting and are partly in agreement with these guidelines.
The number of lymph nodes harvested was reported to be an important prognostic factor,10,11 and several reports and clinical guidelines1–3,12 recommend that at least 12 or 13 lymph nodes should be evaluated for accurate staging. The median number (14) of lymph nodes examined in the present study was, therefore, within the recommended sample size for accurate staging, although 40.0% of the patients had fewer than 12 lymph nodes harvested. There are two possible explanations for the poorer prognosis observed in stage II colorectal cancer patients showing a lower number of harvested lymph nodes. First, the lower number may signify inadequate excision, and thus have an association with poor prognosis. In our series, lymph node dissection at the D3-level, which included additional removal of the central lymph nodes, was performed in 88.7%, while more limited dissection (D1- or D2-level) was performed in 12.3% of patients. This suggests that the extent of resection was oncologically sufficient in these patients. The second explanation involves host-specific response. It was previously demonstrated that lymphocystic infiltration was significantly associated with paracortical hyperplasia in the regional lymph nodes and favorable prognosis in patients with stage II colon cancer.13 It is possible that lack of inflammatory reaction yields a lower number of lymph nodes and a worse prognosis. It is worth noting here that, although 40.0% of the patients in the present study had 11 or fewer nodes examined, they did not have a significantly worse DFS.
Poorly differentiated histology was not associated with shorter DFS, but the number of cases was extremely limited (2.1%), resulting in little impact on the percentage of high-risk patients, even though poorly differentiated histology may be of significant importance. Other histologic features, including lymphatic, venous, and perineural invasion and elevated CEA level, showed no association with DFS, although some previous reports14–17 and guidelines1–3 defined these factors as risk factors for recurrence or poor survival in stage II colorectal cancer. These factors may be important, but reports have been conflicting. In addition, evaluation of such factors, excluding CEA level, has potential drawbacks in that the results appear to sometimes differ among pathologists.
In terms of adjuvant chemotherapy for colonic cancer, several predictive factors have been examined, including biomarkers such as microsatellite instability (MSI), chromosome 18q loss of heterozygosity (18qLOH), K-ras mutation, and P53, TGFBR2, DCC, and thymidylate synthase gene expression.18–20 Of these, the most promising risk factors clinically available at the present time are represented by high-frequency MSI (MSI-H positive for prognosis) and 18q deletion (negative for prognosis). According to a recent meta-analysis,21 which included 7 studies representing 3690 patients with stage II (25%) or III (75%) disease and known MSI status, 5-FU-based adjuvant chemotherapy had no effect on DFS or OS in MSI-H (high-frequency MSI) patients. Although the predictive value of MSI status for resistance to 5-FU is supported by many studies, few have assessed its predictive value under polychemotherapy regimens. In metastatic colorectal cancer, FOLFOX appears to yield similar results in both MSI-H and low frequency MSI (MSI-L)/microsatellite stability (MSS) tumors.22 A retrospective study assessing adjuvant chemotherapy for stage III colon cancer reported that addition of oxaliplatin to 5-FU significantly improved DFS in patients with non-MSI-H tumors in comparison with 5-FU alone.23 The long arm of chromosome 18 (18q) contains several suppressor genes (SMAD-2, SMAD-4, CABLES1, and DCC) thought to be important in the development of colorectal cancer.24 In a translational study in the PETACC-3 trial,25 a subset analysis for stage II colon cancer revealed that patients with 18qLOH had a better DFS according to univariate, but not multivariate, analysis regardless of the regimen randomized (infusional 5-FU/leucovorin or infusional 5-FU/leucovorin plus irinotecan). The combination of the 3 independent risk factors identified in the present study with MSI and/or 18q-LOH status should be evaluated in further clinical studies assessing the usefulness of oxaliplatin-based adjuvant chemotherapy for high-risk stage II colon cancer.
One limitation of the present study is that no results were obtained from patients who were followed up without adjuvant chemotherapy or those who were randomized to receive adjuvant chemotherapy or not. However, the univariate analysis showed that 5-FU-based adjuvant chemotherapy did not significantly reduce the risk of recurrence. Actually, this factor could not be introduced into the multivariate analysis. In addition, when analysis was restricted to patients with one or more risk factors, DFS showed no difference between patients with or without 5-FU-based adjuvant chemotherapy. Thus, 5-FU-based adjuvant chemotherapy does not appear to reduce the risk of recurrence in patients with stage II colon cancer, regardless of risk stratification. This assumption bids us examine the efficacy of oxaliplatin-based adjuvant chemotherapy in selected patients with stage II colon cancer at high risk of recurrence.
In the MOSAIC trial,4,5 which first demonstrated the efficacy of oxaliplatin-based adjuvant chemotherapy for colon cancer, 40% of the patients were stage II. In high-risk stage II patients (22.4% of all patients) in the MOSAIC study (T4 level invasion, obstruction, perforation, poorly differentiated tumor, venous invasion, or ≦10 examined lymph nodes), the relative risk of relapse was insignificantly reduced by 26% with FOLFOX4 as compared with infusional 5-FU/leucovorin ( LV5FU2), even though no such tendency was observed in OS. In addition, no benefit was observed in either DFS or OS in low-risk stage II patients. The results of the present study suggest that the definition of high-risk stage II patients in the MOSAIC study should have been stricter.
In conclusion, the risk factors identified in the present study suggest that oxaliplatin-based adjuvant chemotherapy might be considered in high-risk patients with stage II colon cancer. However, the decision to use oxaliplatin-based adjuvant chemotherapy in such patients should be made carefully: while offering the advantage of an expected 5-year DFS of 6.3% to 8.2% as demonstrated in stage III patients,4–7 it also carries a 0.5% to 1.2% risk of chemotherapy-related mortality,4–7 in addition to chemotherapy-related morbidities such as an 8.2% to 12.4% incidence of grade 3 peripheral neurotoxicity.4–7
References
- 1.Engstrom PF, Arnoletti JP, Benson AB, III, Chen YJ, Choti MA, Cooper HS, et al. NCCN clinical practice guidelines in oncology: colon carcinoma. J Natl Compr Canc Netw. 2009;7(8):838–881. doi: 10.6004/jnccn.2009.0057. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB, III, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO clinical practice guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010;21((suppl 5)):70–77. doi: 10.1093/annonc/mdq168. [DOI] [PubMed] [Google Scholar]
- 4.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. The multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators. N Engl J Med. 2004;350((XX)):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 5.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27((XX)):3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25((XX)):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 7.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer [published online ahead of print October 15, 2011] Int J Clin Oncol. doi: 10.1007/s10147-011-0315-2. doi:xx.xxxx. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Gospodarowicz MK, Wittekind C. UICC. International Union Against Cancer, TNM Classification of Malignant Tumors, 7th ed. New York, NY: Wiley-Blackwell; 1516 N. Lake Shore Drive, Chicago, IL 60610 USA: 2009. [Google Scholar]
- 10.Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10(1):65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99(6):433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 12.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54(6):295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 13.Pihl E, Malahy MA, Khankhanian N, Hersh EM, Mavligit GM. Immunomorphological features of prognostic significance in Dukes' class B colorectal carcinoma. Cancer Res. 1977;37(11):4145–4149. [PubMed] [Google Scholar]
- 14.Takagawa R, Fujii S, Ohta M, Nagano Y, Kunisaki C, Yamagishi S, et al. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann Surg Oncol. 2008;15((XX)):3433–3439. doi: 10.1245/s10434-008-0168-8. [DOI] [PubMed] [Google Scholar]
- 15.Sun LC, Chu KS, Cheng SC, Lu CY, Kuo CH, Hsieh JS, et al. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. 2009;20((XX)):288–295. doi: 10.1186/1471-2407-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010;101(5):396–400. doi: 10.1002/jso.21495. [DOI] [PubMed] [Google Scholar]
- 17.Ogata Y, Murakami H, Sasatomi T, Ishibashi N, Mori S, Ushijima M, et al. Elevated preoperative serum carcinoembrionic antigen level may be an effective indicator for needing adjuvant chemotherapy after potentially curative resection of stage II colon cancer. Surg Oncol. 2009;99((XX)):65–70. doi: 10.1002/jso.21161. [DOI] [PubMed] [Google Scholar]
- 18.Gangadhar T, Schilsky RL. Molecular markers to individualize adjuvant therapy for colon cancer. Nat Rev Clin Oncol. 2010;7(6):318–325. doi: 10.1038/nrclinonc.2010.62. [DOI] [PubMed] [Google Scholar]
- 19.Javle M, Hsueh C-T. Recent advances in gastrointestinal oncology-updates and insights from the 2009 annual meeting of the American Society of Clinical Oncology. J Hematol Oncol. 2010 doi: 10.1186/1756-8722-3-11. Mar 23;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez RO. São Julião GP, Habr-Gama A, Kiss D, Proscurshim I, Campos FG et al. I. The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon Rectum. 2009;52(6):1137–1143. doi: 10.1007/DCR.0b013e31819ef76b. [DOI] [PubMed] [Google Scholar]
- 21.Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45((XX)):1890–1896. doi: 10.1016/j.ejca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Chua W, Goldstein D, Lee CK, Dhillon H, Michael M, Mitchell P, et al. Molecular markers of response and toxicity to FOLFOX chemotherapy in metastatic colorectal cancer. Br J Cancer. 2009;101((XX)):998–1004. doi: 10.1038/sj.bjc.6605239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaanan A, Cuilliere-Dartigues P, Guilloux A, Parc Y, Louvet C, de Gramont A, et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann Oncol. 2010;21(4):772–780. doi: 10.1093/annonc/mdp383. [DOI] [PubMed] [Google Scholar]
- 24.Chun P, Wainberg ZA. Adjuvant chemotherapy for stage II colon cancer: the role of molecular markers in choosing therapy. Gastrointest Cancer Res. 2009;3((XX)):191–196. [PMC free article] [PubMed] [Google Scholar]
- 25.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Molecular markers in colon cancer have a stage specific prognostic value: results of the translational study on the PETACC 3-EORTC 40993-SAKK 60-00 trial. J Clin Oncol. 2010;28(3):466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]


