Abstract
Synbiotics are combinations of probiotics and prebiotics that have recently been used in the context of various gastrointestinal diseases, including infectious enteritis, inflammatory bowel disease, and bowel obstruction. We encountered a patient with recurrent D-lactic acidosis who was treated successfully for long periods using synbiotics. The patient was diagnosed as having short bowel syndrome and had recurrent episodes of neurologic dysfunction due to D-lactic acidosis. In addition to fasting, the patient had been treated with antibiotics to eliminate D-lactate–producing bacteria. After the failure of antibiotic treatment, a stand-alone synbiotic treatment was started, specifically Bifidobacterium breve Yakult and Lactobacillus casei Shirota as probiotics, and galacto-oligosaccharide as a prebiotic. Serum D-lactate levels declined, and the patient has been recurrence-free for 3 years without dietary restriction. Synbiotics allowed the reduction in colonic absorption of D-lactate by both prevention of D-lactate–producing bacterial overgrowth and stimulation of intestinal motility, leading to remission of D-lactate acidosis.
Keywords: D-lactic acidosis, Short bowel syndrome, Synbiotics, Antibiotics
D-lactate exists in extremely small amounts in human serum. Generally, in healthy adults serum D-lactate concentration ranges from 11 to 70 nmol/L.1 It is produced by bacterial fermentation in the intestinal tract and is metabolized by D-2-hydroxyacid dehydrogenase, contained abundantly in the liver. D-lactic acid accumulates when its production and absorption in the intestinal tract exceed the capacity of hepatic metabolism.2
D-lactic acidosis is one of the complications sometimes seen in patients with short bowel syndrome (SBS).3 D-lactate accumulates because of unusual overgrowth of D-lactate–producing bacteria in the colon and dysfunction of metabolic systems.2 D-lactic acidosis is associated with neurotoxic effects characterized by ataxia, slurred speech, and confusion, and symptoms manifests at serum concentrations >2.5 to 3.0 mmol/L.1 Standard management consists of restriction of oral carbohydrates or fasting and elimination of D-lactate–producing bacteria by nonabsorbable antibiotics. However, D-lactic acidosis is usually recurrent, and patients are therefore forced to fast repeatedly, leading to an impaired quality of life.
We encountered a patient with SBS who had recurrent episodes of D-lactic acidosis. After pretreatment with oral nonabsorbable antibiotics, we used only synbiotics consisting of Bifidobacterium breve Yakult and Lactobacillus casei Shirota as probiotics, and galacto-oligosaccharide as a prebiotic. The patient has been recurrence-free for 3 years without dietary restriction, such as carbohydrate restriction or fasting. We propose a stand-alone therapy using synbiotics for the prevention of D-lactic acidosis in patients with SBS.
Case Report
A 28-year-old man was diagnosed with SBS after a massive small bowel resection and right hemicolectomy due to intestinal volvulus at age 23 years. The duodenum and transverse colon were directly anastomosed. After the operation, the patient was dependent on total parenteral nutrition without dietary restriction. His bowel movement showed tendency toward constipation rather than diarrhea. He experienced recurrent dizziness but had been followed for benign paroxysmal positional vertigo for several years.
He was referred to our hospital because of somnolence. He did not have any history of drug or alcohol abuse. The serum biochemical panel showed severe metabolic acidosis (pH, 7.18; PO2, 121 mmHg; PCO2, 20.8 mmHg; HCO3, 7.6 mEq/L; base excess, −19.6 mmol/L; l-lactate, 0.9 mmol/L) and slight liver dysfunction (asparate aminotransferase, 50 U/L; alanine aminotransferase, 104 U/L). Serum electrolytes, blood sugar, NH3, and vitamin B1 levels were within normal range. Serum D-lactate level was elevated to as high as 9.8 mmol/L. Based on the findings above, the patient was diagnosed with D-lactic acidosis. Treatment was started with intravenous administration of sodium bicarbonate and maintenance fluid therapy. The patient fully recovered his consciousness within 12 hours, and the acid-base imbalance was normalized. After recovery, before an oral diet was begun, the standard treatment approach was followed (i.e., fasting and oral administration of nonabsorbable antibiotics). The patient received kanamycin (Kanamycin Capsules, Meiji Seika Pharma, Tokyo, Japan) 1000 mg/d. Five days after commencing an oral diet, he had a relapse of D-lactate acidosis.
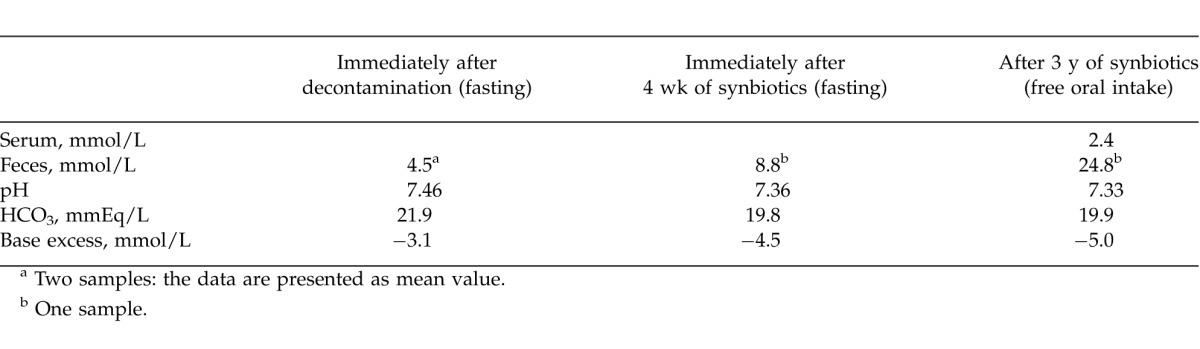
To eliminate D-lactate–producing bacteria completely, thorough intestinal decontamination was carried out. Specifically, metronidazole (Flagyl, Shionogi & Co, Ltd, Osaka, Japan) 500 mg/d and kanamycin 2000 mg/d were administered for 5 days under fasting conditions. Polymyxin B (Polymyxin B Sulfate, Pfizer Japan Inc, Tokyo, Japan) 500 × 103 U/d and vancomycin (Vancomycin Hydrochloride Powder, Lilly, Kobe, Japan) 1000 mg/d were administered over the subsequent 5 days. After the use of antibiotics, a purgative (Niflec, Ajinomoto Pharmaceuticals Co, Ltd, Tokyo, Japan) was used. After these decontaminations, although two Gram-stained samples of stool showed that microorganisms like Candida constituted nearly all of the intestinal flora, the fecal concentration of D-lactate in 2 samples of stool remained detectable (4.5 and 4.4 mmol/L, respectively), which indicated impossibility of eliminating D-lactate–producing bacteria completely using this methodology (Table 1). We understood that it was more important to suppress the overgrowth of D-lactate–producing bacteria than to eliminate them.
Overgrowth suppression was approached by starting synbiotics, specifically B breve Yakult (prepared by Yakult Co, Ltd, Tokyo, Japan) 3.0 g/d and L casei Shirota (Biolactis Powder, Yakult Co, Ltd, Tokyo, Japan) 3.0 g/d as probiotics, and galacto-oligosaccharide 8.4 g/d as a prebiotic. Additionally, an overall review of total parenteral nutrition was performed to prevent progression to parenteral nutrition–associated cholestasis and liver disease; total daily calories of 1200 kcal with fat emulsion of 60 g/wk were administered intravenously.
Before the treatment, the patient's height, body weight, and body mass index were 168.0 cm, 43.1 kg, and 15.3 kg/m2, respectively. After the above-mentioned treatment, body weight was maintained at around 45 kg and serum albumin levels were kept around 3.5 g/dL. Serum levels of alanine aminotransferase were maintained at close to normal range. He also noticed a change in bowel habits after starting the treatment (i.e., frequent defecation 3–5 times per day and loose stool). Fecal concentrations of D-lactate were as high as 8.8 mmol/L after 4 weeks of synbiotic treatment under fasting conditions, and the concentrations showed an additional increase with free oral intake (Table 1). The most recent fecal concentration of D-lactate increased up to 24.8 mmol/L; however, the most recent serum concentration of D-lactate declined to as low as 2.4 mmol/L. The patient was free from recurrence for 3 years without fasting or restriction of oral ingestion after the introduction of synbiotics.
Discussion
In recent years, clinicians may have encountered D-lactic acidosis more frequently because of increased survival of patients with SBS, due to improvements in total parenteral nutrition. In patients with SBS, the resection of large segments of the intestine enables massive inflow of carbohydrates directly into the colon. Carbohydrates are fermented into organic acids by colonic bacteria. The resulting acid load lowers the intraluminal pH of the colon, which allows the excessive proliferation of acid-resistant D-lactate–producing bacteria, such as Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus delbrueckii subsp lactis, Lactobacillus buchneri, and Streptococcus bovis.4 Diminished peristalsis in these patients also increases the time necessary for intestinal absorption of D-lactate. Furthermore, impaired hepatic metabolism due to parenteral nutrition–associated cholestasis and liver disease may impair the metabolism of D-lactate. Standard management for D-lactic acidosis has consisted of restricting oral carbohydrates or fasting and administration of nonabsorbable antibiotics to eliminate intestinal D-lactate–producing bacteria; however, this approach frequently results in an impaired quality of life for patients. Several reports have described the development of D-lactic acidosis in patients with SBS as a result of the administration of various antibiotics, including tetracycline,5 metronidazole,5 and vancomycine.6 In the present case, it was confirmed that complete elimination of D-lactate–producing bacteria by antibiotics was impossible. After the failure of antibiotics administration, we had no choice but to implement other strategies. Although surgical intervention for the prevention of D-lactic acidosis remained an option,7 a synbiotic treatment was chosen as a promising alternative because of synbiotics' safety and compliance rates.
Synbiotics involve the combined use of probiotics and prebiotics. Probiotics are used as live microbial supplements that beneficially affect the host by improving intestinal microbial balance. Prebiotics refer to nondigestive food ingredients that selectively target the growth and activity of a limited number of bacteria in the colon, which improves host health. In recent years, there have been several reports of probiotics or synbiotics used in the management of SBS. Kanamori et al8 reported a dramatic improvement in intestinal absorptive function and motility in a patient with SBS who was treated with synbiotics. Uchida et al9 described the successful management of recurrent D-lactic acidosis in SBS by combination treatment with antibiotics and probiotics for 8 months. On the other hand, Godey et al10 reported that enteral treatment with Lactobacillus should be avoided because it increases D-lactic production. In their study, they used L acidophilus, which is known to also produce D-lactate. In the present case, because of the inability to eliminate D-lactate–producing bacteria, we chose a stand-alone synbiotic treatment in order to achieve long-term suppression of D-lactate–producing bacteria overgrowth. We used as probiotics B breve Yakult and L casei Shirota, which are known to produce only L-lactate. D-lactic acidosis was successfully controlled by this treatment for 3 years (i.e., the patient was free of recurrence and had a good quality of life, such as having unlimited oral intake). There were no reports that described successful management of D-lactic acidosis continuously for such a long period.
Bustos et al11 reported that fecal concentrations of D-lactate tended to be high in patients with SBS even when serum D-lactate levels were undetectable. The authors pointed out that the development of D-lactic acidosis requires not only overproduction of D-lactate in the colon, but also other factors, such as absorption or impaired D-lactic acid metabolism. Synbiotics have been shown to produce a large amount of short-chain fatty acids by correcting intestinal flora.5 These fatty acids promote proliferation of the intestinal epithelium and stimulate intestinal motility.12 Through active bowel movements, D-lactate produced by colonic fermentation can be expelled with feces prior to excessive absorption in the colon. In the present case, although fecal concentration of D-lactate was high (24.8 mmol/L), serum D-lactate levels (2.4 mmol/L) maintained a level lower than the threshold of symptom onset (>2.5 mmol/L), which allowed the patient to remain asymptomatic. Actually, the patient had frequent defecation and persistent loose stools after starting synbiotic treatment. Therefore, it was likely that synbiotics increased intestinal peristalsis, resulting in prevention of excessive absorption of D-lactic acid.
In conclusion, a stand-alone synbiotic treatment was effective in maintaining long-term remission of D-lactic acidosis in patients with SBS. The underlying mechanism might be the reduction in colonic absorption of D-lactate by both suppression of D-lactate–producing bacterial overgrowth and stimulation of intestinal motility. A stand-alone synbiotic treatment is a workable alternative for the management of D-lactate acidosis in SBS.
Acknowledgments
The authors thank Yakult Co, Ltd, for its kind provision of probiotic agents.
Table 1 .
Serum and fecal concentrations of D-lactic acid and acid-base balance
References
- 1.Ewaschuk JB, Naylor JM, Zello GA. D-lactate in human and ruminant metabolism. J Nutr. 2005;135(7):1619–1625. doi: 10.1093/jn/135.7.1619. [DOI] [PubMed] [Google Scholar]
- 2.Petersen C. D-lactic acidosis. Nutr Clin Pract. 2005;20(6):634–645. doi: 10.1177/0115426505020006634. [DOI] [PubMed] [Google Scholar]
- 3.Oh MS, Phelps KR, Traube M, Barbosa-Saldivar JL, Boxhill C, Carroll HJ. D-Lactic acidosis in a man with the short-bowel syndrome. N Engl J Med. 1979;301(5):249–252. doi: 10.1056/NEJM197908023010505. [DOI] [PubMed] [Google Scholar]
- 4.Satoh T, Narisawa K, Konno T, Katoh T, Fujiyama J, Tomoe A, et al. D-lactic acidosis in two patients with short bowel syndrome: bacteriological analyses of the fecal flora. Eur J Pediatr. 1982;138(4):324–326. doi: 10.1007/BF00442509. [DOI] [PubMed] [Google Scholar]
- 5.Coronado BE, Opal SM, Yoburn DC. Antibiotic-induced D-lactic acidosis. Ann Intern Med. 1995;122(11):839–842. doi: 10.7326/0003-4819-122-11-199506010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Gavazzi C, Stacchiotti S, Cavalletti R, Lodi R. Confusion after antibiotics. Lancet. 2001 doi: 10.1016/S0140-6736(00)04565-7. 357(9266):1410. [DOI] [PubMed] [Google Scholar]
- 7.Modi BP, Langer M, Duggan C, Kim HB, Jaksic T. Serial transverse enteroplasty for management of refractory D-lactic acidosis in short-bowel syndrome. J Pediatr Gastroenterol Nutr. 2006;43(3):395–397. doi: 10.1097/01.mpg.0000228116.52229.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanamori Y, Hashizume K, Sugiyama M, Morotomi M, Yuki N. Combination therapy with Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides dramatically improved the intestinal function in a girl with short bowel syndrome: a novel synbiotics therapy for intestinal failure. Dig Dis Sci. 2001;46(9):2010–2016. doi: 10.1023/a:1010611920750. [DOI] [PubMed] [Google Scholar]
- 9.Uchida H, Yamamoto H, Kisaki Y, Fujino J, Ishimaru Y, Ikeda H. D-lactic acidosis in short-bowel syndrome managed with antibiotics and probiotics. J Pediatr Surg. 2004;39(4):634–636. doi: 10.1016/j.jpedsurg.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Godey F, Bouasria A, Ropert M, Diakite M, Le Treut A, Balençon M. Don't forget to test for D-lactic acid in short bowel syndrome. Am J Gastroenterol. 2000;95(12):3675–3677. doi: 10.1111/j.1572-0241.2000.03416.x. [DOI] [PubMed] [Google Scholar]
- 11.Bustos D, Pons S, Pernas JC, Gonzalez H, Caldarini MI, Ogawa K, et al. Fecal lactate and short bowel syndrome. Dig Dis Sci. 1994;39(11):2315–2319. doi: 10.1007/BF02087644. [DOI] [PubMed] [Google Scholar]
- 12.Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Effects of short-chain fatty acids on gastrointestinal motility. Scand J Gastroenterol Suppl. 1997;222:58–61. doi: 10.1080/00365521.1997.11720720. [DOI] [PubMed] [Google Scholar]


