Abstract
The underlying cause of resistant hypertension after adrenalectomy for primary hyperaldosteronism remains controversial. The objective of this study was to identify preoperative factors predictive of resistant hypertension in patients after undergoing retroperitoneoscopic adrenalectomy. Between 2003 and 2009, 124 patients with unilateral aldosterone-producing adenoma or unilateral adrenal hyperplasia underwent retroperitoneoscopic adrenalectomy at our institution. Clinical and biochemical data were reviewed retrospectively at baseline and after a median follow-up time of 59.2 ± 37.2 months. Adrenalectomy cured hypertension in 68 patients (54.8%) and 43 (34.8%) had persistent hypertension that was much easier to control after surgery, whereas 13 patients (10.4%) had continued hypertension and poor blood pressure control. Multivariate regression analysis revealed that the main determinants of postoperative cure were duration of hypertension less than 5 years [odds ratio (OR): 6.515, 95% confidence interval (CI) 2.278–10.293), number of antihypertensive medications ≤2 (OR: 2.939, 95% CI 1.254–5.235), preoperative response to spironolactone (OR: 3.405, 95% CI 1.681–6.985), the TT genotype of the CYP11B2 gene (344 C/T) (OR: 2.765, 95% CI 1.221–4.986), and the presence of adenoma rather than hyperplasia (OR: 5.274, 95% CI 2.150–8.141). The main determinants of surgical cure or control of hypertension in patients with primary hyperaldosteronism were duration of hypertension, number of antihypertensive medications, preoperative response to spironolactone, the presence of adenoma, and CYP11B2 (344 C/T) genotype. Consideration of these factors may help in the evaluation of patients for surgery and for the identification of patients with continued postoperative hypertension that may require more long-term monitoring and treatment.
Keywords: Primary aldosteronism, Adrenalectomy, Resistant hypertension, CYP11B2
Primary hyperaldosteronism (PHA) is a common cause of secondary hypertension and is estimated to account for between 5% and 20% of patients with hypertension.1–3 PHA is characterized by drug-resistant hypertension and hypokalemia caused by renin-independent overproduction of aldosterone. Hypertension is not only the main symptom in patients with PHA, but is also the most important factor for morbidity and reduced quality of life due to a higher rate of cardiovascular complications, target organ damage, and metabolic syndrome than essential hypertension.4,5 Thus, the control of hypertension is the most important aim for the treatment of these patients.
Retroperitoneoscopic adrenalectomy greatly benefits patients with PHA.6 Although adrenalectomy is considered the treatment of choice for patients with unilateral disease, such as aldosterone-producing adenoma (APA) and unilateral adrenal hyperplasia, cure rates for APA-associated hypertension are only 30%–60%.7 Polymorphic variation in the 5′-promoter region (−344C/T) of the gene encoding aldosterone synthase (CYP11B2) is associated with increased aldosterone metabolite excretion and hypertension associated with an elevated aldosterone-to-renin ratio.8 Genotypes of the CYP11B2 promotor polymorphism predicted a positive response to treatment.9 Despite the postoperative normalization of the biochemical abnormalities (e.g., hypokalemia) in many patients with unilateral aldosterone-secreting adenomas, some patients still remain hypertensive after curative surgery.
The aims of our study were to evaluate the long-term outcomes of patients undergoing retroperitoneoscopic adrenalectomy for PHA, with particular attention to the control of hypokalemia and hypertension, and to determine the preoperative risk factors for persistent hypertension after successful adrenalectomy.
Patients and Methods
Study population
From July 2002 to March 2009, 124 consecutive patients treated by retroperitoneoscopic adrenalectomy for primary hyperaldosteronism at Guangzhou General Hospital were enrolled in this retrospective study. Patients gave their written informed consent for genetic diagnostics, and all aspects of this study were approved by the institutional ethics committee. A single surgeon (W.-L.H.) performed all procedures to minimize operator variability.
All patients who presented with PHA were retrospectively included in this study. Patients' medical records were collected and preoperative data, including demographics, antihypertensive medications used, imaging results, and biochemical variables were compiled. Postoperative data included surgical complications, outcome for hypertension and hypokalemia, and pathologic analysis of the adrenal gland.
The preoperative diagnosis of PHA was defined as a plasma aldosterone-to-plasma renin activity ratio of 20 or more with a plasma aldosterone concentration of 15 ng/dL or more and plasma renin activity suppressed to 1 ng/mL or less per hour. Diagnosis was confirmed by a lack of aldosterone suppression after an intravenous saline load. Computed tomography (CT) was required in all patients. If the contralateral adrenal gland was enlarged or the adrenal neoplasm was smaller than 1 cm, patients were selected to undergo adrenal venous sampling to distinguish a lateralized aldosterone hypersecretion. Histopathology reports after adrenalectomy were reviewed. Histologic subtype, adrenal gland weight, and maximum tumor diameter were recorded. Results of unilateral adrenalectomy were assessed in all patients at the time of hospital discharge and after a minimal follow-up of 6 months.
For determining factors influencing surgical outcome, patients were divided into three groups with the following definitions: (1) clinical cure, normotensive (systolic blood pressure ≤140 mmHg and diastolic blood pressure ≤90 mmHg) without antihypertensive medications; (2) improved control, normotensive and equal numbers or fewer antihypertensive medications postoperatively or hypertensive but requiring fewer antihypertensives for control; and (3) no difference from preoperative state or diminished control, continued hypertension with the same number or additional antihypertensive medications postoperatively.
Hypokalemia was considered cured if serum potassium levels remained within normal limits (serum potassium >3.6 mmol/L) without potassium supplementation.
Genotyping
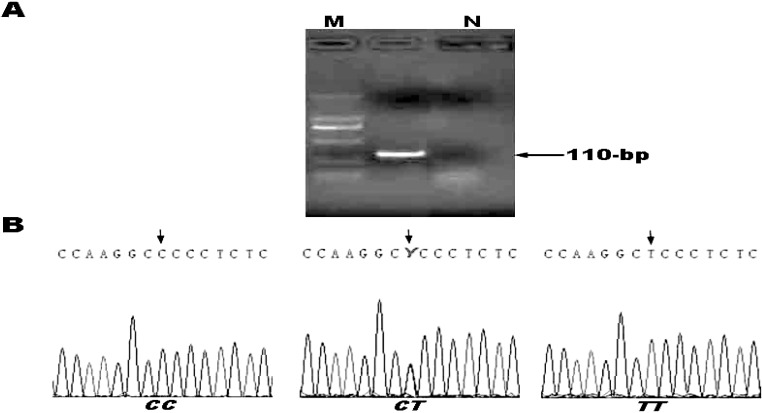
Genomic DNA was extracted from peripheral leukocytes and stored at −20°C until batch genotyping. Genotyping of the CYP11B2 C-344T polymorphism (rs1799998) was performed as described previously.10 The initial polymerase chain reaction (PCR) amplification was performed using the forward primer 5′-AATGAACTAAATCTGTGGTATAAAA-3′ and the reverse primer 5′-CCAGGGCTGAGAGGAGTAAA-3′, creating a 110-bp product (Fig. 1). The PCR reaction mixture (50 µL) contained 1 × PCR buffer II, 2.25 mmol/L MgCl2, 0.25 mmol/L of each dNTP, 0.2 µmol/L of each primer, 1 U of AmpliTaq DNA polymerase (AmpliTaq Gold, Applied Biosystems, Foster City, California) and approximately 60 ng of genomic DNA. The PCR run was performed as follows: initial denaturing step at 95°C for 10 minutes, followed by 34 cycles of 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds, and then a final elongation step at 72°C for 10 minutes (GeneAmp PCR system 9700, Applied Biosystems). These genotypes were defined by GENESCAN software (Applied Biosystems).
Figure 1.
Representative screening for CYP11B2 genotypes. (A) Products screened by PCR. Arrows indicate the size of fragment (bp). M, molecular size markers; N, negative control. (B) Results of nucleotide sequence analysis for each of the genotypes. Arrows indicate the nucleotide at the 344 C/T polymorphic site.
Statistical analysis
Results are reported as median (range) or as mean ± SD. The primary outcome measure was postoperative hypertension. In the univariate analyses, we compared each of these variables using t-tests, χ2 tests, and analysis of variance (ANOVA) where appropriate. Allele and genotype frequencies were analyzed using the gene counting method and compared to Hardy-Weinberg equilibrium by the w2-test. A stepwise multiple logistic regression analysis was performed to assess the combined predictive effects of the clinicopathologic variables. In this analysis, outcome of hypertension was the dependent variable, and the clinical, biochemical, and pathologic variables were independent candidate predictor variables.
All statistical procedures were performed using the computer software SPSS for Windows version 15.0 ((SSPS, Chicago, Illinois). Statistical significance was defined as P < 0.05.
Results
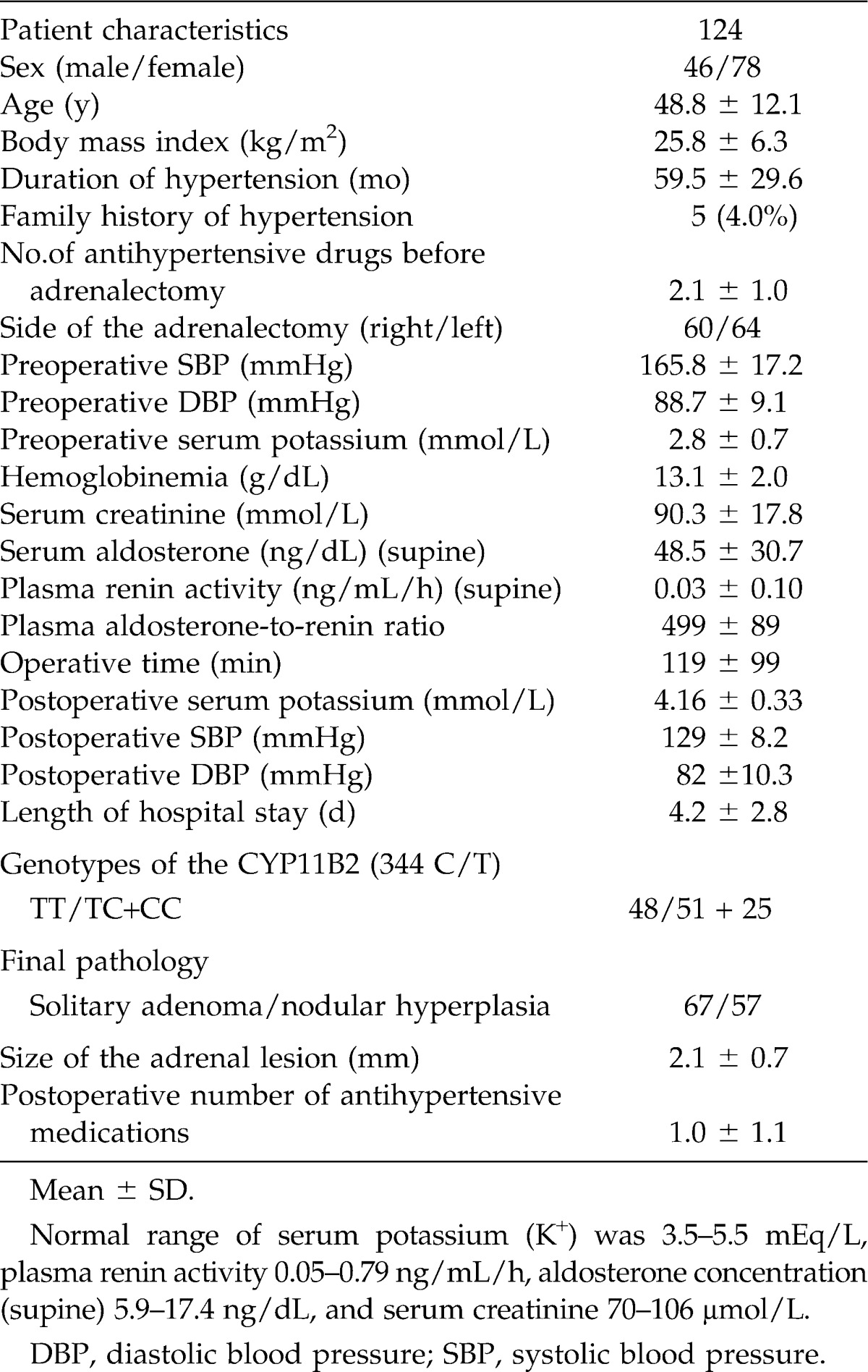
During the course of this study, 124 patients (46 men, 78 women) with a lateralized adrenal lesion on CT scan underwent a unilateral laparoscopic adrenalectomy for PHA. The demographic characteristics are summarized in Table 1. In general, most of the patients had poorly controlled hypertension, and the mean duration of hypertension before surgery was 59.5 ± 29.6 months. The most commonly used antihypertensive medications were beta-blockers, alpha-blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors.
Table 1.
Summary of study group characteristics
Retroperitoneoscopic adrenalectomy was performed on all patients. No patient required conversion to open adrenalectomy or red blood cells transfusion. Median duration from time of surgery to the first postoperative blood pressure (BP) measurement was 1 month and patients were monitored for a median time of 59.2 months. The average operating time was 119 ± 99 minutes (range, 40–240 min), and the mean postoperative stay was 4.2 ± 2.8 days (range, 1–7 days). No mortalities occurred, and the morbidity was a superficial wound infection in 5 patients treated by antibiotics and wound dressing and a cardiac ischemic event in 2 patients. Histology revealed that 67 patients (52.3%) had solitary adenoma (including 1 patient with concomitant adenoma and hyperplasia) and 57 patients (47.7%) had adrenal hyperplasia. The mean size of the adenomas was 2.1 ± 0.7 cm (range, 0.7–3.5 cm). The average (±SD) preoperative BP was systolic 165.8 ± 17.2 mmHg and diastolic 88.7 ± 9.1 mmHg. Plasma renin activity was suppressed in all patients. The mean potassium level at presentation of 2.8 ± 0.7 mmol/L (range, 1.9–3.4 mmol/L) but was 4.2 ± 2.8 mmol/L (range, 4.16–0.33 mmol/L) without potassium supplements at follow-up (P < 0.05). At follow-up, serum aldosterone also improved significantly (P < 0.0001).
Adrenalectomy cured hypertension in 68 patients (54.8%), whereas 13 patients had continued poor BP control and 43 patients had persistent hypertension that was much easier to control.
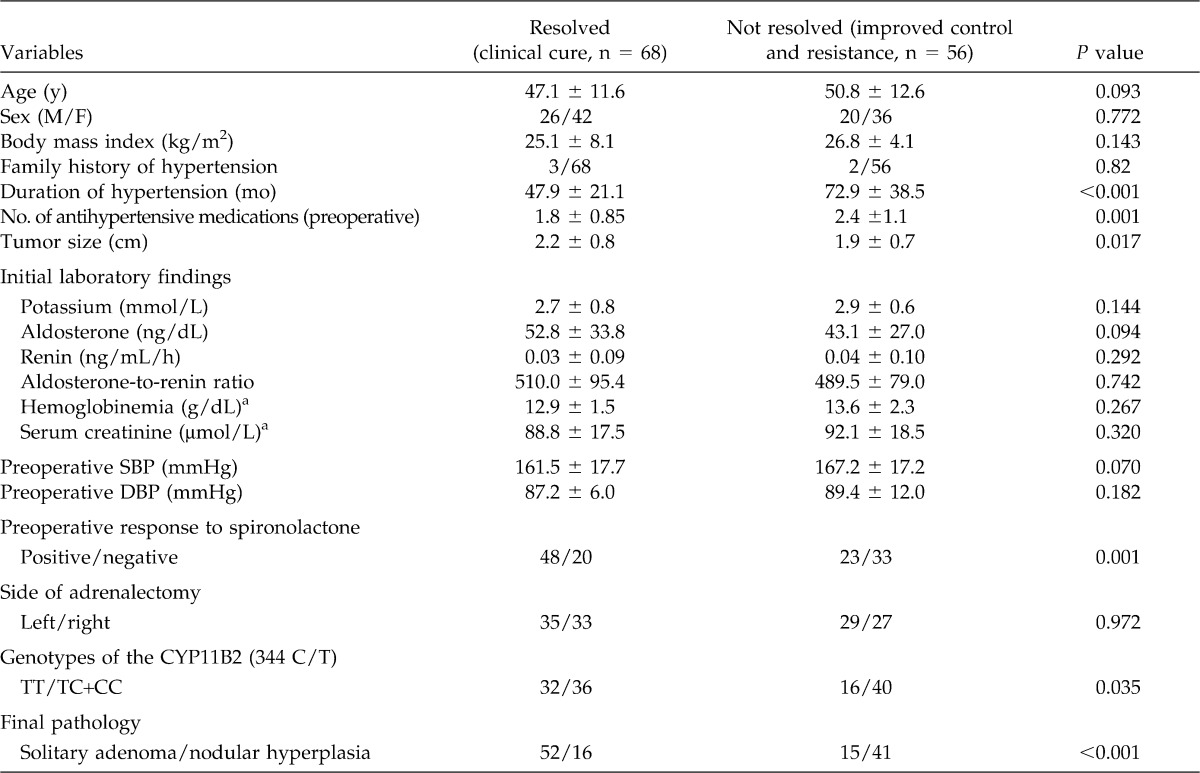
In a univariate analysis, independent preoperative predictive factors associated with cured hypertension (without the need of any antihypertensive medication) are summarized in Table 2. The mean age of patients who experienced a clinical cure after adrenalectomy was 47.1 ± 11.6 years, compared to 50.8 ± 12.6 years for those 56 patients with continued hypertension (P = 0.093). The genotype frequencies of CYP11B2 in this patient cohort were not different from the Hardy-Weinberg equilibrium. Homozygosity for the T allele of the CYP11B2 gene was significantly associated with a better response to surgery. Correspondingly, the diastolic blood pressure after treatment was significantly different between genotypes (CYP11B2 TT: 83 ± 17 mmHg, TC: 89 ± 15 mmHg, CC: 95 ± 29 mmHg, P = 0.017). Other factors, such as family history of hypertension, side of the lesion, plasma aldosterone level, aldosterone-to-renin ratio, body mass index, and serum creatinine were not correlated with positive surgical outcome (cured hypertension) (Table 2). Patients with continued postoperative hypertension had a presurgical duration of hypertension of 72.9 ± 38.5 months, which was significantly longer than the 47.9 ± 21.1 months duration of the responsive group (P < 0.001). Furthermore, patients with continued postoperative hypertension were treated with more antihypertensive drugs before surgery (2.4 ± 1.1 versus 1.8 ± 0.8; P = 0.001), exhibited a negative preoperative response to spironolactone (positive versus negative; P = 0.001), were less likely to express the TT genotype of CYP11B2 (TT versus TC+CC; P = 0.035), and more likely to present with nodular hyperplasia than solitary adenoma (P = 0.001).
Table 2.
Univariate analysis of clinical outcome according to candidate predictor variables
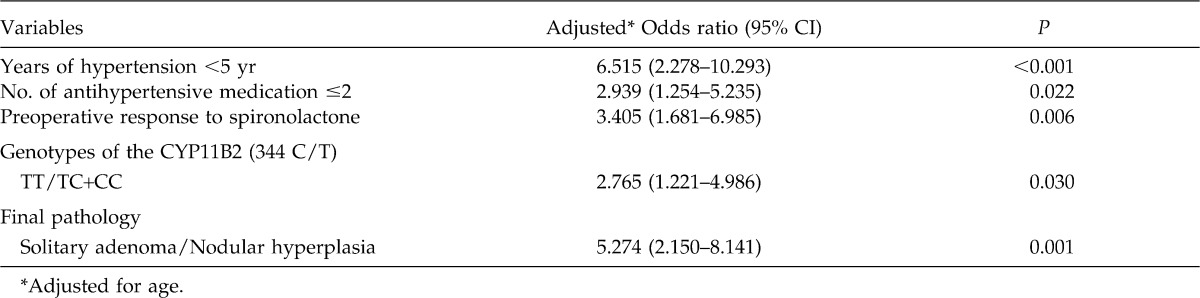
Multivariate logistic regression revealed that duration of hypertension less than 5 years, number of antihypertensive medication ≤2, preoperative response to spironolactone, TT genotype of CYP11B2, and solitary adenoma (rather than nodular hyperplasia) contributed independently to a predictive model (Table 3).
Table 3.
Results of multivariate logistic regression analysis of the major predictors
Discussion
Despite cases of hypertension recurrence, many studies have shown the benefit of unilateral adrenalectomy for PHA, even in case of hyperplasia, by reducing the level of circulating aldosterone. Chronic elevated aldosterone can lead to several cardiovascular or general complications including myocardial infarction and atrial fibrillation, left ventricular hypertrophy, arterial stiffness, metabolic syndrome, and renovascular alterations.11,12 In addition, Sywak and Pasieka13 performed a cost analysis of adrenalectomy for PHA versus the estimated cost of treating the same patient with lifelong medicinal therapy alone. They concluded that adrenalectomy, even when including the group of patients who required antihypertensive medication after surgery, was significantly less expensive than the estimated cost of lifelong drug therapy.
Our study demonstrates that retroperitoneoscopic unilateral adrenalectomy is a safe and effective treatment strategy for reducing BP in patients with PHA. Surgery reduced hypertension and serum aldosterone levels, and normalized serum potassium in patients with PHA and adrenal unilateral mass.14,15 Adrenalectomy cured hypertension in 54.8% of our patients and only 10.4% experienced continued poor BP control, whereas 34.8% of the patients had persistent hypertension that was manageable with treatment. These results are consistent with previous reports describing outcomes of patients undergoing adrenalectomy (open or laparoscopic) for hyperaldosteronism, demonstrating cure rates between 30% and 60%.
As shown in these previous studies, a significant proportion of patients not cured of hypertension by adrenalectomy, as defined by normotension, were controllable after surgery (43/56) and only a small fraction (10%) were unresponsive. The underlying cause of surgically resistant hypertension, however, is still controversial. We identified several previously described16 preoperative features that predict favorable responses to surgical treatment, duration of hypertension (<5 years), number of antihypertensive drugs (≤2), preoperative response to spironolactone, and TT genotype of CYP11B2, the gene encoding aldosterone synthase.
In our study, the mean age of the patients who did not respond to surgery was slightly older than the patients who responded positively to surgery. In addition, we found that longer duration of hypertension was associated with incomplete response to surgery. Many studies have confirmed that patients aged 50 years or younger at the time of surgery with a duration of hypertension of less than 5 years are more likely to become normotensive after adrenalectomy.17,18 Irreversible vascular damage during long-standing hypertension (despite surgical cure of the hormonal derangement) has been suggested as the primary reason why longer duration of hypertension is a risk factor for pure surgical outcome. In our treatment series, hypertensive patients responding to preoperative spironolactone with lowered BP also demonstrated a better response rate to surgery. A preoperative response to spironolactone was predictive of a complete response to surgery,19 and our results clearly support the interpretation that a response to spironolactone is predictive of better surgical outcome among patients with adenoma.
Our study also revealed that the TT genotype of the CYP11B2 C-344T polymorphism was predictive of postoperative alleviation of high BP, as 47% of responsive patients had the TT genotype versus 29% of nonresponsive patients. Polymorphisms of the CYP11B2 gene, including C-344 T, have been shown to influence aldosterone levels, arterial stiffness, and associate with hypertension and cardiovascular disease risk,16 suggesting that genetic variations might exert deleterious effects on postoperative resolution of hypertension resulting from primary aldosteronism. The polymorphisms of aldosterone synthase (CYP11B2) located −344 bp upstream of the transcription start site may influence the cardiovascular system by affecting aldosterone regulation at the genetic level as the C allele shows a higher affinity for the steroidogenic factor-1 compared to the T allele.20 The steroidogenic factor-1 represses reporter gene activity of CYP11B2 and the elevation of the steroidogenic factor-1 blocks the expression of CYP11B2 and aldosterone production.21
Consistent with previous reports revealing an association between the CYP11B2 genotype and cardiovascular disease, we propose an association of the TT genotype with resolution of hypertension after adrenolectomy. To our knowledge, this association has not been established previously, and may prove a useful surgical screening tool.
Several postoperative factors predictive of a favorable outcome were also identified in our study, specifically the average size of the nodule and the pathologic nature of the resected adrenal gland that remained. Proye et al22 found that pathologic findings were not predictive factors for alleviation of hypertension, whereas Celen et al23 found that only micronodular hyperplasia was associated with the failure of adrenalectomy to cure hypertension. Patients with adrenal adenoma were most likely to be cured, but even those showing unilateral macronodule or micronodule of the adrenal gland and hyperplasia on CT scan benefited from surgery. In fact, in our patient group, cure of hypertension without medication was significantly associated with solitary adenoma. However, when hyperplasia was diagnosed by examination of the operative specimen, the patient should be warned of the possibility that complete surgical cure is much less likely.
The reasons for inadequate BP response after adrenalectomy for primary hyperaldosteronism are not yet well understood. The duration of preoperative hypertension, patient age, and vascular remodeling are the parameters with the most important influence.24 More recent studies have suggested that resistant hypertension is associated with dysregulation of the cardiovascular and renovascular system in APA.25,26 Proposed pathogenic mechanisms include chronically high levels of aldosterone, leading to chronic intravascular fluid retention, arterial stiffness, suppression of endothelial function, induction of target organ inflammation, and fibrosis.27
As the prevalence of nonfunctioning adrenal adenoma can be as high as 10%, the simple CT and magnetic resonance imaging detection of an adrenal mass in a patient with the biochemical features of primary aldosteronism does not necessarily imply an APA. A limitation of our study is that not all patients were selected for adrenal venous sampling, which may have led to selection bias. However, in view of the limited availability of accurate and safe adrenal vein sampling, some centers have advised alternate pathways to the subtype diagnosis of the patient with primary aldosteronism. Recent studies showed that CT alone can reliably lateralize an aldosterone-producing adenoma at least 1.0 cm in diameter and is associated with excellent clinical outcomes.28 The study by Zarnegar et al28 reported that comparing cure rates between patients with and without preoperative venous sampling, for persistent hypertension were 60% versus 57%, and for reduced antihypertensive medication use they were 50% versus 38%. Certainly, if a more than 1-cm hypodense cortical adenoma with a normal appearing contralateral gland is identified with primary aldosteronism, adrenal venous sampling is not necessary. Our study found that clinical cure rates were 57% versus 52% between patients with and without preoperative venous sampling. However, when the biochemical diagnosis is secure and CT imaging shows bilaterally normal or abnormal adrenals, or adrenal limb thickening is unilateral or bilateral in the appropriate clinical scenario, adrenal venous sampling is mandatory.29,30
Our study demonstrated that 54.8% of patients experienced complete resolution of hypertension and that hypertension became more manageable in an additional 34.8% of patients. Resistant hypertension occurred in only 10.4% of patients. We found that the main determinants of surgical cure in patients with primary aldosteronism were duration of hypertension less than 5 years, number of antihypertensive medication ≤2, preoperative response to spironolactone, the presence of adenoma, and the TT genotype of the CYP11B2 gene. All of these factors could be used to evaluate patient suitability for surgery. The long-term follow-up of unresponsive patients is important because most of these patients will require life-long, regular control of the BP. Our study is limited by the lack of a control group with hypertension of alternate etiology. Larger cohort studies will be required to validate our conclusions. In the future, if we obtain data on more patients and perform the study prospectively, we can determine the exact mechanism of resistant hypertension and identify more accurate predictors.
We conclude that the majority of patients (almost 90%) will benefit from retroperitoneoscopic adrenalectomy. However, identifying these patients preoperatively is important as the minority unlikely to benefit should be warned that hypertension may persist or recur. These patients should be followed closely after surgery.
Acknowledgments
This work was partly supported by China National Natural Science Foundation (81172421, 30872577), China National Natural Science Foundation for Young Scientists (81001142, 81101822, 81101947), Natural Science Foundation of Guangdong Province (10451001002004735, 0451001002005047), China Postdoctoral Science Foundation funded project (20110490889), Scientific Research Foundation for the Returned Overseas Sasakawa Scholars (117), and Funds of Guangzhou Science and Technology Key Project (11C26090521).
References
- 1.Lim P.O, Rodgers P, Cardale K, Watson A.D, MacDonald T.M. Potentially high prevalence of primary aldosteronism in a primary-care population. Lancet. 1999;353(9146):40. doi: 10.1016/S0140-6736(05)74868-6. [DOI] [PubMed] [Google Scholar]
- 2.Fardella C.E, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, et al. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab. 2000;85(5):1863–1867. doi: 10.1210/jcem.85.5.6596. [DOI] [PubMed] [Google Scholar]
- 3.Fogari R, Preti P, Zoppi A, Rinaldi A, Fogari E, Mugellini A. Prevalence of primary aldosteronism among unselected hypertensive patients: a prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertens Res. 2007;30(2):111–117. doi: 10.1291/hypres.30.111. [DOI] [PubMed] [Google Scholar]
- 4.Milliez P, Girerd X, Plouin P.F, Blacher J, Safar M.E, Mourad J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–459. doi: 10.1210/jc.2005-1733. [DOI] [PubMed] [Google Scholar]
- 6.Walz M.K, Gwosdz R, Levin S.L, Alesina P.F, Suttorp A.C, Metz K.A, et al. Retroperitoneoscopic adrenalectomy in Conn's syndrome caused by adrenal adenomas or nodular hyperplasia. World J Surg. 2008;32(5):847–853. doi: 10.1007/s00268-008-9513-0. [DOI] [PubMed] [Google Scholar]
- 7.Meyer A, Brabant G, Behrend M. Long-term follow-up after adrenalectomy for primary aldosteronism. World J Surg. 2005;29(2):155–159. doi: 10.1007/s00268-004-7496-z. [DOI] [PubMed] [Google Scholar]
- 8.Nejatizadeh A, Kumar R, Stobdan T, Goyal A.K, Gupta M, Tyagi S, et al. CYP11B2 gene haplotypes independently and in concurrence with aldosterone and aldosterone to renin ratio increase the risk of hypertension. Clin Biochem. 2010;43(1-2):136–141. doi: 10.1016/j.clinbiochem.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 9.van Rijn-Bikker P.C, Mairuhu G, van Montfrans G.A, et al. Genetic factors are relevant and independent determinants of antihypertensive drug effects in a multiracial population. Am J Hypertens. 2009;22(12):1295–1302. doi: 10.1038/ajh.2009.192. [DOI] [PubMed] [Google Scholar]
- 10.Isaji M, Mune T, Takada N, Yamamoto Y, Suwa T, Morita H, et al. Correlation between left ventricular mass and urinary sodium excretion in specific genotypes of CYP11B2. J Hypertens. 2005;23(6):1149–1157. doi: 10.1097/01.hjh.0000170377.00591.7e. [DOI] [PubMed] [Google Scholar]
- 11.Strauch B, Petrák O, Zelinka T, Wichterle D, Holaj R, Kasalický M, et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21(10):1086–1092. doi: 10.1038/ajh.2008.243. [DOI] [PubMed] [Google Scholar]
- 12.Tuck M.L, Corry D.B. Renal damage in primary aldosteronism: results of the PAPY study. Curr Hypertens Rep. 2007;9(2):87–89. doi: 10.1007/s11906-007-0016-4. [DOI] [PubMed] [Google Scholar]
- 13.Sywak M, Pasieka J.L. Long-term follow-up and cost benefit of adrenalectomy in patients with primary hyperaldosteronism. Br J Surg. 2002;89(12):1587–1593. doi: 10.1046/j.1365-2168.2002.02261.x. [DOI] [PubMed] [Google Scholar]
- 14.Zarnegar R, Young W.F, Jr, Lee J, Sweet M.P, Kebebew E, Farley D.R, et al. The aldosteronoma resolution score: predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg. 2008;247(3):511–518. doi: 10.1097/SLA.0b013e318165c075. [DOI] [PubMed] [Google Scholar]
- 15.Kim R.M, Lee J, Soh E.Y. Predictors of resolution of hypertension after adrenalectomy in patients with aldosterone-producing adenoma. J Korean Med Sci. 2010;25(7):1041–1044. doi: 10.3346/jkms.2010.25.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arlt W. A detour guide to the Endocrine Society Clinical Practice Guideline on case detection, diagnosis and treatment of patients with primary aldosteronism. Eur J Endocrinol. 2010;162(3):435–438. doi: 10.1530/EJE-09-0869. [DOI] [PubMed] [Google Scholar]
- 17.Gockel I, Heintz A, Polta M, Junginger T. Long-term results of endoscopic adrenalectomy for Conn's syndrome. Am Surg. 2007;73(2):174–180. [PubMed] [Google Scholar]
- 18.Rossi G.P. Diagnosis and treatment of primary aldosteronism. Endocrinol Metab Clin North Am. 2011;40(2):313–332. doi: 10.1016/j.ecl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Zarnegar R, Lee J, Brunaud L, Lindsay S, Kebebew E, Clark O.H, et al. Good blood pressure control on antihypertensives, not only response to spironolactone, predicts improved outcome after adrenalectomy for aldosteronoma. Surgery. 2007;142(6):921–929. doi: 10.1016/j.surg.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Bassett M.H, Zhang Y, Clyne C, White P.C, Rainey W.E. Differential regulation of aldosterone synthase and 11b-hydroxylase transcription by steroidogenic factor-1. J Mol Endocrinol. 2002;28(2):125–135. doi: 10.1677/jme.0.0280125. [DOI] [PubMed] [Google Scholar]
- 21.Ye P, Nakamura Y, Lalli E, Rainey W.E. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology. 2009;150(3):1303–1309. doi: 10.1210/en.2008-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proye C.A, Mulliez E.A, Carnaille B.M, Lecomte-Houcke M, Decoulx M, Wémeau J.L, et al. Essential hypertension: first reason for persistent hypertension after unilateral adrenalectomy for primary aldosteronism? Surgery. 1998;124(6):1128–1133. doi: 10.1067/msy.1998.93108. [DOI] [PubMed] [Google Scholar]
- 23.Celen O, O'Brien M.J, Melby J.C, Beazley R.M. Factors influencing outcome of surgery for primary aldosteronism. Arch Surg. 1996;131(6):646–650. doi: 10.1001/archsurg.1996.01430180072015. [DOI] [PubMed] [Google Scholar]
- 24.Rossi G.P, Bolognesi M, Rizzoni D, Seccia T.M, Piva A, Porteri E, et al. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension. 2008;51(5):1366–1371. doi: 10.1161/HYPERTENSIONAHA.108.111369. [DOI] [PubMed] [Google Scholar]
- 25.Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371(9628):1921–1926. doi: 10.1016/S0140-6736(08)60834-X. [DOI] [PubMed] [Google Scholar]
- 26.Rossi G.P, Pessina A.C. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens. 2009;27(3):656–657. doi: 10.1097/HJH.0b013e3283224393. [DOI] [PubMed] [Google Scholar]
- 27.Gaddam K.K, Pimenta E, Husain S, Calhoun D.A. Aldosterone and cardiovascular disease. Curr Probl Cardiol. 2009;34(2):51–84. doi: 10.1016/j.cpcardiol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Zarnegar R, Bloom A.I, Lee J, Kerlan R.K, Jr, Wilson M.W, Laberge J.M, et al. Is adrenal venous sampling necessary in all patients with hyperaldosteronism before adrenalectomy? J Vasc Interv Radiol. 2008;19(1):66–71. doi: 10.1016/j.jvir.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Tan Y.Y, Ogilvie J.B, Triponez F, Caron N.R, Kebebew E.K, Clark O.H, et al. Selective use of adrenal venous sampling in the lateralization of aldosterone-producing adenomas. World J Surg. 2006;30(5):879–885. doi: 10.1007/s00268-005-0622-8. [DOI] [PubMed] [Google Scholar]
- 30.Toniato A, Bernante P, Rossi G.P, Pelizzo M.R. The role of adrenal venous sampling in the surgical management of primary aldosteronism. World J Surg. 2006;30(4):624–627. doi: 10.1007/s00268-005-0482-2. [DOI] [PubMed] [Google Scholar]