Abstract
Malignant phyllodes tumors are an uncommon breast tumor in clinical practice of surgery. The study population consisted of five consecutive patients. Each patient had complete clinical follow-up with annual mammograms and physical examination in a specialized breast clinic. They were surgically treated for with malignant phyllodes tumor of the breast. All patients are alive and well with a complete follow-up. The first 2 patients had a fine needle aspiration cytology and were surgically treated by a simple mastectomy. The remaining 3 patients were preoperatively diagnosed with core needle biopsy. These 3 patients were treated with a wide excision of the phyllodes tumor with at least a 1-cm margin of normal breast tissue. Mammography was 100% accurate in demonstrating a dense breast mass. In each patient ultrasound suggested heterogeneous internal echoes present in each malignant phyllodes tumor. Fine needle aspiration cytology was of no value in the diagnosis of a phyllodes tumor. Core needle biopsy is highly reliable in establishing a preoperative diagnosis. The most helpful clinical observation of a malignant phyllodes tumor was rapid growth and enlargement, which is frequently noted by the patient.
Keywords: Malignant, Phyllodes, Tumor
The tumor initially named cystosarcoma phyllodes is now generally known as phyllodes tumor.1 The phyllodes tumor is a rare breast tumor, accounting for less than 1% of all breast neoplasms.1 Histologically, phyllodes tumors are classified as benign, borderline, or malignant based on characteristics of the stroma.2 They can be found as a breast lump at any age including adolescence.1 The peak incidence of this tumor in women is between 35 and 55 years of age, with only a few cases reported in men.3 A large series from the M.D. Anderson Cancer Center reported the incidence of phyllodes tumors histologically as benign (58%), intermediate (12%), and malignant (30%).4 Wide local excision is usually the primary approach to treatment. Reoccurrence rate after surgical excision is variously described as between 14% and 21% with recurrence more likely in those with the tumor at the margins of excision.5 Malignant phyllodes tumors behave like sarcomas and can develop blood-borne metastases with a poor prognosis.6 Because of the rarity of these tumors, all large series are reported from major cancer medical centers. This is a retrospective study of 5 patients with malignant phyllodes tumors diagnosed in a private practice clinic during the past 30 years. The purpose of this study is to assess in young patients the accuracy of the various pre-excisional diagnostic modalities used in distinguishing these phyllodes tumors from the common breast mass, which are typically fibroadenomas.
Materials and Methods
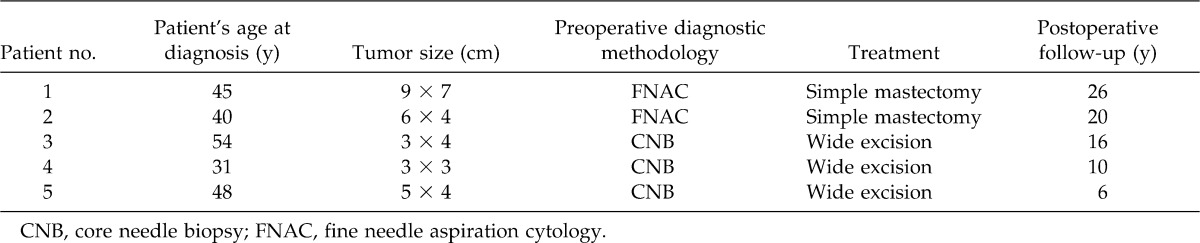
During the period from 1981 to 2011, 5 women with malignant phyllodes tumors were diagnosed and treated (Table 1). Patients 1, 2, and 4 were referred and examined for the first time in our clinic with a large breast mass. Patients 3 and 5 were previously examined yearly for several years before the development of a large breast mass. During the early period, patients with breast masses were evaluated by fine needle aspiration cytology (FNAC) after mammogram evaluation. During the past 15 years, core needle biopsies (CNB) were obtained of the breast mass before wide excision of each tumor. Initially, each patient has mammographic examination performed with contemporary equipment before initial physical evaluation. Ultrasound has been used to assist in the guidance of a CNB. For the lesions in the present study, core biopsies were performed under ultrasound guidance or using a prone table stereotactic device in selected difficult cases. Four to 10 14-G cores were taken per patient.
Table 1.
Summary of patients with malignant phyllodes tumors
Results
Each patient in this series (Table 1) related the rapid development of a breast mass. Each patient had a breast size of a C or D bra cup. Some of the patients in this series performed monthly breast examinations. None of the patients were meticulous and regular with monthly breast examination. Patients 1, 2, and 4 were referred after a breast mass was found during an annual physical examination. Patients 3 and 5 were long-term patients who had yearly mammograms and breast examination in our clinic. Both patients had a normal mammogram and breast examination 1 year prior before the discovery and subsequent evaluation of the breast mass.
FNAC was found to be of no assistance in the preoperative diagnosis of a phyllodes tumor. In the first patient FNAC was histologically diagnosed as a fibroadenoma with only the suggestion of a possible phyllodes tumor. Patient 2 had a FNAC histologic report of a fibroadenoma with mild stromal atypia. CNB was very accurate in the last 3 patients in making the diagnosis of a malignant phyllodes tumor preoperatively. The age range of women with phyllodes tumors was 31–54 years, and 3 of the 5 women were aged between 40 and 48 years (Table 1). All patients are continually seen yearly with mammograms and physical examination. All patients are currently alive and well. A 1-cm margin of normal breast tissue was excised in the 3 patients treated by wide excision. A simple mastectomy was the operative treatment selected for the first 2 patients because of their relative small breasts in relationship to the large size of the malignant phyllodes tumor.
Discussion
This is a report of 5 cases of malignant phyllodes tumors from a single clinical practice during a 30-year period. CNB has been quite accurate in the last 3 patients for making a preoperative diagnosis compared with other reports.7 Clinically, we have found phyllodes tumors to grow rapidly as opposed to the very common fibroadenoma.
In this series, mammography contributed to the diagnosis by demonstrating a dense mass abnormality in 100% of mammograms. Several investigators have noted phyllodes tumors to be of a higher density by mammography than the surrounding parenchyma.1 In the present study, mammography demonstrated a very dense mass compared with the surrounding parenchyma. All of the patients with malignant phyllodes tumor in this study were round or oval with lobulated margins. In each of our 5 patients ultrasound suggested that there were heterogeneous internal echoes present in each malignant phyllodes tumor.1 None of our patients had cysts in the malignant phyllodes tumor detected by ultrasound.1
FNAC was used in the first 2 patients in this series. FNAC was of no value in distinguishing a phyllodes tumor from a fibroadenoma during the preoperative pathologic evaluation.1,8 Once CNB were routinely available, the last 3 patients in our series were accurately preoperatively diagnosed using a stereotactic CNB. Four to 10 cores were usually taken from each patient.
The average size of the phyllodes tumor in this series (Table 1) was quite large. The range was 30 to 90 mm.1 The most helpful symptom described by the patient was a rapidly developing mass that quickly enlarged on monthly physical examination by the patient.1,9
The diagnostic difficulty is to determine between the common fibroadenoma and a patient who may have a phyllodes tumor. Typically fibroadenomas are 20 to 30 mm in size.1 They tend to stabilize or even regress during serial ultrasound examinations. Fibroadenoma typically occur in patients between the age of 18 and 35 years, whereas phyllodes tumors tend to occur in patients in an older age group.1 All of the patients in this series had a malignant phyllodes tumor occur between the ages of 31 and 54 years. A rapid growth rate is extremely helpful in differentiating between the two types of tumors.1 During the past 10 years magnetic resonance imaging has been helpful in the evaluation of a difficult mass in a very dense breast.
Recent research has been carefully evaluating the expression of many biological markers in phyllodes tumors.2 The expression of many biological markers, including P53, hormone receptors, proliferation markers, angiogenesis group of markers, c-kit, CD10, and epidermal growth factor receptor, have been explored.2 These markers are of limited value in predicting the behavior of the phyllodes tumor.2 Recently investigators have reported a plethora of genetic changes in malignant phyllodes tumor, the most consistent of which was found to be 1q gain by competitive genomic hybridization.9 Most malignant phyllodes tumors will express estrogen and progesterone receptor presence.9 The progesterone receptor is most common.9 Interestingly, it has been reported that histologically, malignant phyllodes tumors tend to show more chromosomal changes than the benign and borderline counterparts.2 Among these changes, 1q gain, 7q gain, 5p gain, 3p loss, 6q loss, 9p loss, 10p loss, and 3q loss are the most common changes detected.2,10 There is also evidence that 1q gain and 4q12 gain may be associated with recurrent disease in malignant phyllodes tumors.11
The diagnosis of phyllodes tumors and the assignment of a grading based on histologic characteristics are still associated with much uncertainty and variability. Histologic grading can be used to predict biological behavior to some extent, but accurate data or specific markers are lacking.12 At present assessment of biological markers by immunohistochemistry does not significantly improve prognostic prediction. Furthermore, in-depth assessment of phyllodes tumors at the molecular level may provide more insight into the biology of this unusual breast tumor.12
Patients with phyllodes tumors require careful follow-up after the surgical procedure. Recurrence of the tumor in the breast or elsewhere by distant metastasis varies depending on the reported series, but can be as high as 27%.13,14 Recurrence or distant metastasis is related to poor histopathologic factors such as stromal overgrowth, infiltrative margins, and pleomorphism.13,14 Metastases or recurrence tend to develop within the first 2 years after diagnosis and represent a hematogenous pattern of spread.15 In one series16 tumor size exceeding 5 cm was related to be the only prognostic indicator of decreased long-term survival. Another recent retrospective series17 found higher grade tumors to occur more often in Hispanic patients. Our clinic follows all patients with phyllodes tumor with repeat mammograms and physical examination every 6 months postoperatively for the first 3 years and then annually.
References
- 1.Foxcroft L.M, Evans E.B, Porter A.J. Difficulties in the preoperative diagnosis of phyllodes tumors of the breast. Breast. 2007;16(1):27–37. doi: 10.1016/j.breast.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Tse G.M.K, Niu Y, Shi H. Phyllodes tumor of the breast: an update. Breast Cancer. 2010;17(5):29–34. doi: 10.1007/s12282-009-0114-z. [DOI] [PubMed] [Google Scholar]
- 3.Benhassouna J, Damak T, Gamoudi A, Charqui R, Khomsi F, Mahjoub S, et al. Phyllodes tumors of the breast: a case series of 106 patients. Am J Surg. 2006;192(2):141–147. doi: 10.1016/j.amjsurg.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Chaney A.W, Pollack A, Mcneese M, Zagers K, Pisters P, Pollock R.E, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89(7):1502–1511. doi: 10.1002/1097-0142(20001001)89:7<1502::aid-cncr13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Shabahang M, Francheschi D, Sundaram M, Castillo M.H, Moffat F.L, Frank D.S, et al. Surgical management of primary breast sarcoma. Am Surg. 2002;68(8):673–677. [PubMed] [Google Scholar]
- 6.Stebbing J.F, Nash A.G. Diagnosis and management of phyllodes tumor of the breast: experience of 33 cases at a specialist center. Ann R Coll Surg Engl. 1995;77(3):181–184. [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon M.F, Quinn C.M, McDermott E.W, Doherty M.B, O'Higgins N, Hill A. Needle core biopsy in the diagnosis of phyllodes neoplasm. Surgery. 2006;140(5):779–784. doi: 10.1016/j.surg.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K, Masawa N, Yamada T. Cytologic evaluation of phyllodes tumors as compared to fibroadenomas of the breast. Acta Cytol. 1994;36(7):691–697. [PubMed] [Google Scholar]
- 9.Gordon P, Gagnon F, Lanzkowsky L. Solid breast masses diagnosed as fibroadenomas at fine needle aspiration biopsy: acceptable rates of growth at long term follow-up. Radiology. 2003;229(1):233–238. doi: 10.1148/radiol.2291010282. [DOI] [PubMed] [Google Scholar]
- 10.Lv S, Niu Y, Wei L, Wang X, Chen Y. Chromosomal aberrations and genetic relations in benign, borderline, and malignant phyllodes tumors of the breast: a comparative genomic hybridization study. Breast Cancer Res Treat. 2008;112(3):411–418. doi: 10.1007/s10549-007-9876-1. [DOI] [PubMed] [Google Scholar]
- 11.Jee K.V, Gong G, Ahn S.H, Park J.M, Knoutila S. Gain in 1q is a common abnormality in phyllodes tumors of the breast. Anal Cell Pathol. 2003;25(1):89–93. doi: 10.1155/2003/803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karim R.Z, Gerega S.K, Yang Y.H, Spillane A, Carmalt H, Scolyer R.A, et al. Phyllodes tumors of the breast. A clinicopathological analysis of 63 cases from a single institution. Breast. 2009;18(3):165–170. doi: 10.1016/j.breast.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Gullett N.P, Rizzo M, Johnson P.A. National surgical patterns of care for primary care and axillary staging of phyllodes tumors. Breast J. 2009;15(1):41–44. doi: 10.1111/j.1524-4741.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 14.Sahin A.A, Sneige N, Singletary S.E. Phyllodes tumors of the breast. The Cancer Bulletin. 1995;47(5):392–395. [Google Scholar]
- 15.Barrio A.V, Clark B.D, Goldberg J.I. Clinopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol. 2007;14(10):2961–2970. doi: 10.1245/s10434-007-9439-z. [DOI] [PubMed] [Google Scholar]
- 16.Fields R.C, Aft R.L, Gillanders W.E, Eberlein T.J, Margenthaler J.A. Treatment and outcomes of patients with primary breast sarcoma. Am J Surg. 2008;196(4):559–561. doi: 10.1016/j.amjsurg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Pimiento J.M, Godgil P.V, Santillan A.A, Lee M.C, Esposito N.N, Kiluk J.V, et al. Phyllodes tumors: race-related differences. J Am Coll Surg. 2011;213(4):537–542. doi: 10.1016/j.jamcollsurg.2011.07.012. [DOI] [PubMed] [Google Scholar]


