Abstract
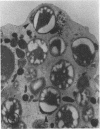
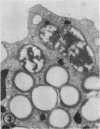
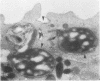
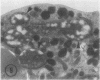
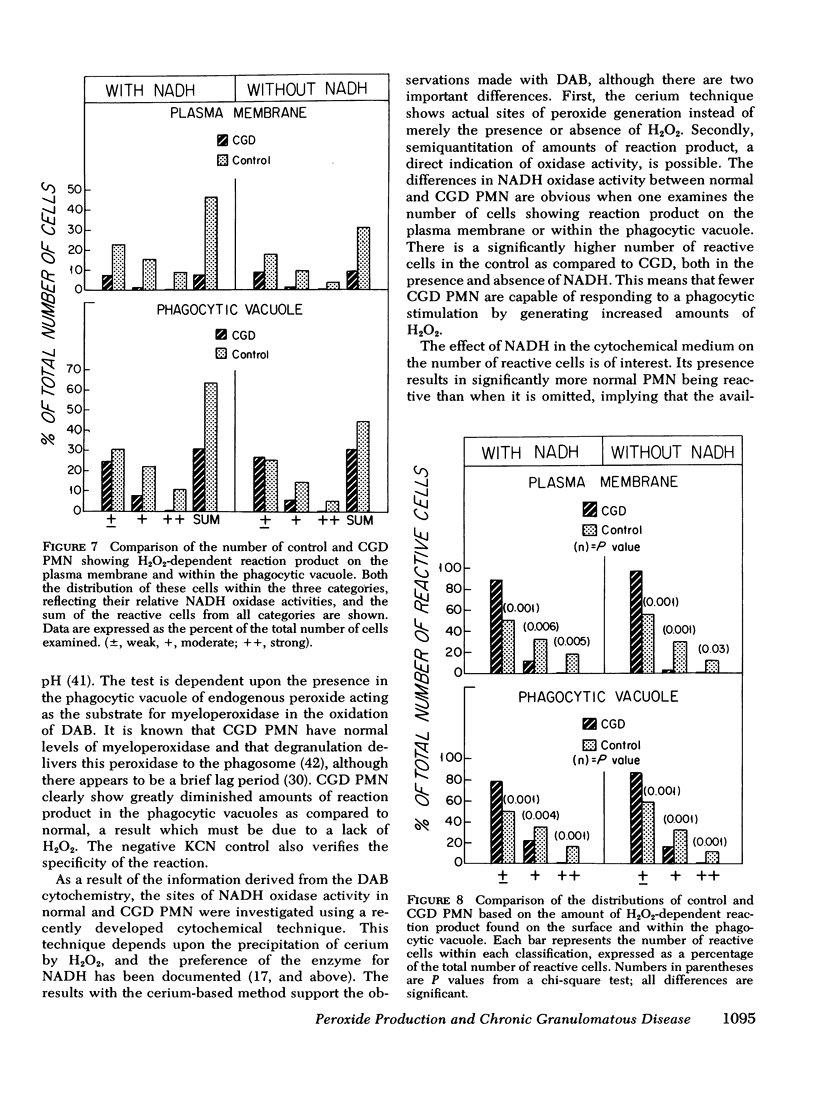
The ability of polymorphonuclear leukocytes (PMN) to produce H2O2 in response to phagocytic stimulation was examined cytochemically using leukocytes from normal individuals and patients with chronic granulomatous disease (CGD). Normal PMN oxidized diaminobenzidine within the phagocytic vacuole by a reaction dependent upon endogenous H2O2 and myeloperoxidase. CGD PMN failed to oxidize diaminobenzidine, which is consistent with the biochemical data showing a lack of H2O2-generating capacity. A plasma membrane enzyme (oxidase) activated by phagocytosis is capable of H2O2 production in PMN. The localization of this oxidase activity was explored in CGD PMN using a cytochemical technique specific for H2O2. The enzyme activity is stimulated by exogenous NADH, but not NADPH. Reaction product formation, indicative of activity of the oxidase, is dependent upon precipitation of cerium ions by the enzymatically generated H2O2. The advantage of this approach is that enzyme activity of individual cells can be assessed, allowing determination of numbers of reactive cells in the population and their relative degrees of reactivity. NADH oxidase was found to be active both on the plasma membrane and within the phagocytic vacuoles of control PMN, whereas those cells from three CGD patients showed greatly reduced activity in both these sites. Assessment of the reactivity of individual cells showed the number of cells with oxidase activity in CGD to be significantly reduced when compared to control values. Additionally, of those cells that do react, a higher percentage of them are only weakly reactive. Omission of NADH from the incubation medium reduced the percentage of control cells showing enzyme activity but had no effect on CGD PMN, implying that the enzyme is not saturated with substrate in control cells, but in CGD the diminished enzyme is fully saturated. The defect may lie in the fact that in CGD patients there are fewer cells capable of peroxide generation, and a majority of these reactive cells produce only reduced amounts of this bactericidal agent.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. L. Deficiency of reduced nicotinamide-adenine dinucleotide oxidase in chronic granulomatous disease. Science. 1968 Dec 13;162(3859):1277–1279. doi: 10.1126/science.162.3859.1277. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Karnovsky M. L., Karnovsky M. J. Cytochemical demonstration of hydrogen peroxide in polymorphonuclear leukocyte phagosomes. J Cell Biol. 1975 Jan;64(1):254–260. doi: 10.1083/jcb.64.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAGAN R. H., KARNOVSKY M. L. ENZYMATIC BASIS OF THE RESPIRATORY STIMULATION DURING PHAGOCYTOSIS. Nature. 1964 Oct 17;204:255–257. doi: 10.1038/204255a0. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Karnovsky M. L., Babior B. M. Manganese-dependent NADPH oxidation by granulocyte particles. The role of superoxide and the nonphysiological nature of the manganese requirement. J Clin Invest. 1976 Apr;57(4):1059–1067. doi: 10.1172/JCI108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Gold S. B., Hanes D. M., Stites D. P., Fudenberg H. H. Abnormal kinetics of degranulation in chronic granulomatous disease. N Engl J Med. 1974 Aug 15;291(7):332–337. doi: 10.1056/NEJM197408152910704. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- HARRIS H. Chemotaxis of granulocytes. J Pathol Bacteriol. 1953 Jul;66(1):135–146. doi: 10.1002/path.1700660117. [DOI] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Baehner R. L. Improvement of leukocyte bactericidal activity in chronic granulomatous disease. Blood. 1970 Mar;35(3):350–355. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Baehner R. L. Disorders of phagocytic cell function. Prog Hematol. 1971;7(0):235–254. [PubMed] [Google Scholar]
- Nathan D. G., Baehner R. L., Weaver D. K. Failure of nitro blue tetrazolium reduction in the phagocytic vacuoles of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Oct;48(10):1895–1904. doi: 10.1172/JCI106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Dri P., Fant L., Basford R. E., Rossi F. NADPH oxidizing activity in rabbit polymorphonuclear leukocytes: localization in azurophilic granules. Biochem Biophys Res Commun. 1973 Aug 6;53(3):830–837. doi: 10.1016/0006-291x(73)90168-x. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Dri P., Kakinuma K., Tedesco F., Rossi F. Studies on the mechanism of metabolic stimulation in polymorphonuclear leucocytes during phagocytosis. I. Evidence for superoxide anion involvement in the oxidation of NADPH2. Biochim Biophys Acta. 1975 Apr 7;385(2):380–386. doi: 10.1016/0304-4165(75)90367-0. [DOI] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Sbarra A. J., Paul B. B., Jacobs A. A., Strauss R. R., Mitchell G. W., Jr Biochemical aspects of phagocytic cells as related to bactericidal function. J Reticuloendothel Soc. 1972 May;11(5):492–502. [PubMed] [Google Scholar]
- Segal A. W., Peters T. J. Characterisation of the enzyme defect in chronic granulomatous disease. Lancet. 1976 Jun 26;1(7974):1363–1365. doi: 10.1016/s0140-6736(76)93021-x. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaka K., O'Brien P. J. Mechanisms of H2O2 formation by leukocytes. Evidence for a plasma membrane location. Arch Biochem Biophys. 1975 Aug;169(2):428–435. doi: 10.1016/0003-9861(75)90184-8. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]