Abstract
Hemobilia is the process of bleeding into the biliary tree and is an unusual cause of upper gastrointestinal hemorrhage. When this event results from a cystic artery pseudoaneurysm, it is a particularly rare phenomenon; fewer than 20 cases are described in the literature. Alongside the literature review, we report a case of a 34-year-old woman presenting 3 months post laparoscopic cholecystectomy with hematemesis. Computed tomography (CT) angiography revealed a cystic artery pseudoaneurysm. Following an ineffective hyperselective arterial embolization, the patient was successfully treated by surgical ligation of the right hepatic artery. Even though this complication is uncommon, all surgeons need to be aware of its presentation and of available therapeutic options.
Keywords: Hemobilia, Cystic artery, Pseudoaneurysm
Upper gastrointestinal bleeding originating from the biliary tree (hemobilia) is an uncommon but well-described condition. Hepatic trauma and iatrogenic injury are the principal causative factors.1 Cystic artery pseudoaneurysm as the cause of hemobilia represents a rare phenomenon. The most frequent causes are acute cholecystitis and cholecystectomy-related injury. Here we present a case from our center and a review of the literature on hemobilia in association with a cystic artery pseudoaneurysm.
Case Presentation
A 34-year-old woman presented to the emergency department 3 months post laparoscopic cholecystectomy with epigastric pain, nausea, and multiple episodes of hematemesis. With the exception of recent laparoscopic cholecystectomy, the patient had no significant past medical history. On examination, she was pale; blood pressure was 105/60 mmHg and heart rate was 98 bpm. Epigastric and right upper quadrant tenderness was noted. Laboratory results were as follows: hemoglobin 8.7 g/dl, white cell count 9.2 mol/L, platelet count 256 × 109/L, with mildly prolonged coagulation times, total bilirubin 2.8 mg/dl, direct bilirubin 1.9 mg/dl, alanine aminotransferase 160 IU/L (N (normal range for these laboratory results): 13–40 IU/L), aspartate aminotransferase 232 IU/L (N: 10–42 IU/L), γ-glutamyltransferase 194 IU/L (N: <50 IU/L), and alkaline phosphatase 199 IU/L (125–240 IU/L). Serum amylase levels were normal, and serum C-reactive protein was 140 mg/dl (N: <0.5 mg/dl). Abdominal ultrasound (US) failed to reveal any hepatobiliary or digestive tract abnormality.
Emergency upper gastrointestinal endoscopy (OGD) revealed multiple blood clots over the anterior/superior wall of the first and second parts of the duodenum. In addition, fresh blood, with clots, was clearly visualized emerging from the ampulla of Vater, suggesting underlying hemobilia. A subsequent computed tomography (CT) angiogram showed postcholecystectomy changes, including high-density digestive material, suggesting recent intraluminal bleeding. The arterial phase of dynamic CT imaging revealed an ill-defined nodular lesion, measuring approximately 13 mm, with an enhancement pattern parallel to the arteries. This resulted in extravasation of contrast solution in the liver hilum area, suggesting a possible vascular pseudoaneurysm. The patient subsequently proceeded to have a formal CT angiogram with interventional radiology. This revealed a moderate to large pseudoaneurysm of the cystic artery (Fig. 1). After several unsuccessful attempts were made, flow to the pseudoaneurysm was arrested with the placement of multiple coils (Fig. 2). However, 1 month after this procedure was performed, rebleeding occurred.
Figure 1.
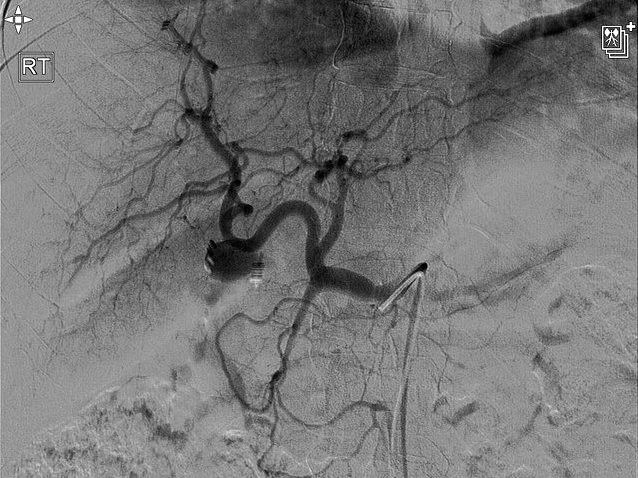
Pseudoaneurysm of the cystic artery.
Figure 2.
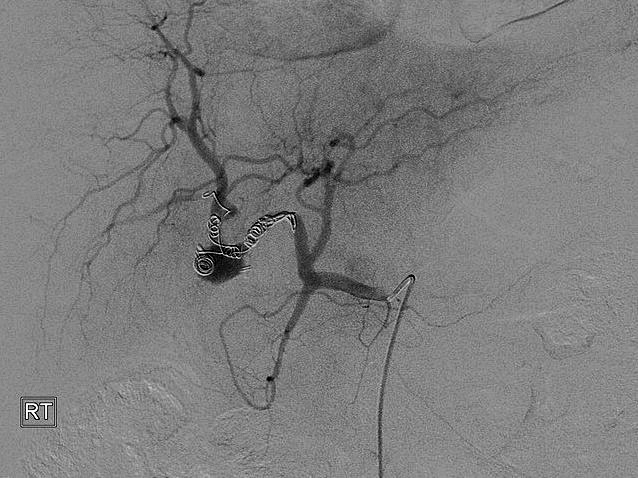
Multiple coils seen within the pseudoaneurysm arresting flow.
Following several previous radiologic attempts, further interventional radiology was considered inappropriate because the coils placed previously were seen to approach the main hepatic artery along the right. Therefore, the risk was that additional coils might occlude the left hepatic artery (Fig. 1). Clear anastomoses were seen between right and left hepatic arteries; these probably were causing back bleeding along the right hepatic artery proximal to the coils placed previously (Fig. 1). Therefore, additional coils along the right hepatic artery would have been unlikely to arrest the bleeding. Approaching the proximal right hepatic artery from the left through the anastomoses was considered too hazardous to attempt. Therefore a surgical option was chosen. Ligature and suture of the ostium of the pseudoaneurysm proved challenging, but the right hepatic artery eventually was successfully ligated. Sacrificing the right hepatic artery was considered safe because obvious anastomoses were noted between right and left hepatic arteries. Recovery was uneventful with normalization of laboratory tests and a normal follow-up CT scan. Six months after surgery, the patient is well and is complaint free.
Discussion
Hemobilia is classically associated with Quincke's triad of biliary colic, jaundice, and gastrointestinal bleeding; however, the complete triad is reported in less than 40% of patients.2 A significant majority of cases arise from iatrogenic causes such as hepatobiliary procedures and, less commonly, laparoscopic cholecystectomy. Noniatrogenic causes include blunt abdominal trauma, localized infection, gallstones, and hepatic tumors.2,3 Although laparoscopic cholecystectomy is the treatment of choice for symptomatic cholelithiasis, the incidence (range, 0.3%–1.0%) of biliary and vascular injuries is increased by this procedure.4,5 Most arterial complications occur secondary to direct injury or diathermy heat transmitted via surgical clips.6 Although very rare, pseudoaneurysms of the hepatic artery are a serious complication of laparoscopic cholecystectomy. Pseudoaneurysm formation may result from thermal or mechanical injury to the vessels during the operation.
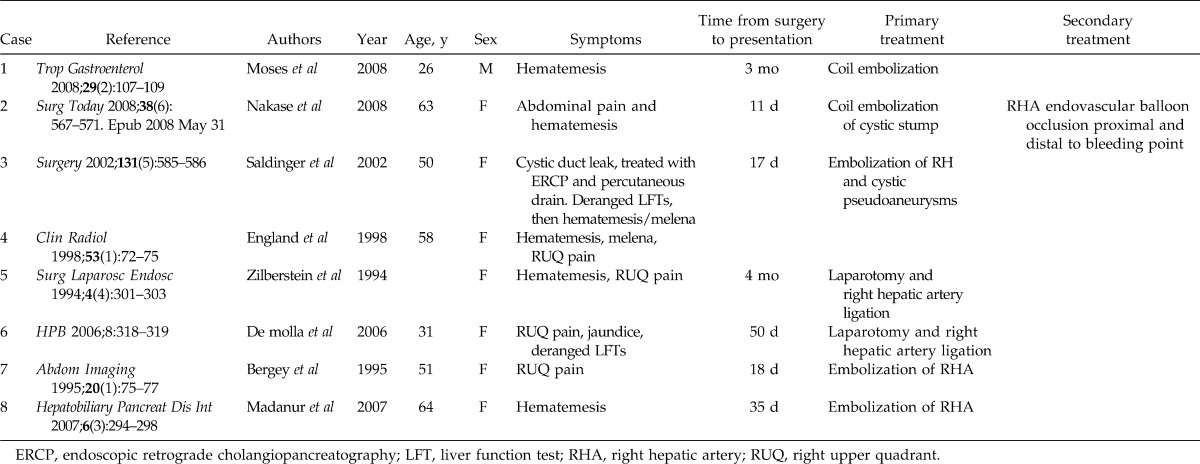
Hemobilia secondary to a cystic artery pseudoaneurysm is extremely rare; only 20 cases have been reported—only 9 cases following laparoscopic cholecystectomy—as depicted in Table 1.7–9 The mechanism of hepatic or cystic artery pseudoaneurysms post cholecystectomy is unclear but likely involves direct vascular injury, thermal injury, erosion due to clip intrusion, bile leakage, and infection.10 The cytotoxic properties of high concentrations of intracellular or extracellular bile have been postulated to cause direct weakening of the suture line and to erode the vascular wall, leading to pseudoaneurysm formation.11 Secondary infection propagated by the presence of a biloma has been discussed as a precipitant to pseudoaneurysm formation.12,13
Table 1.
Reported cases of hemobilia secondary to cystic artery pseudoaneurysm following laparoscopic cholecystectomy
Hepatic or cystic artery pseudoaneurysms most frequently present with bleeding in the form of hemobilia, hematemesis, or melena. Depending on the time of presentation, bleeding may be minimal, or massive if presenting late.14,15 Diagnosis of the source of bleeding can be made by OGD, CT angiography with three-dimensional (3D) reconstruction, or angiography. Because of its high diagnostic accuracy and therapeutic potential, angiography is considered a useful modality in the diagnosis and management of hemobilia.16,17 The reported success rate of angiographic control of hemobilia by transarterial embolization is 80% to 100%.16,17 However, its diagnostic limitations have been noted to include variable flow rate and intermittent bleeding, as well as hepatic artery abnormalities.15 In addition, angiographic treatment carries with it serious risks such as hepatobiliary necrosis, bleeding, abscess formation, and gallbladder fibrosis.15 With these risks, early surgical intervention following failed radiologic intervention for hemobilia is often suggested, especially if embolization fails, or if cholecystitis, gallstones, or resectable neoplasms are present.2,15
Conclusion
Cystic artery aneurysm with associated hemobilia is a rare, potentially life-threatening emergency wherein prompt recognition and treatment are essential. Iatrogenic causes are common, along with bile leak and infection. Any patients presenting with gastrointestinal bleeding post laparoscopic cholecystectomy need to undergo urgent investigation with endoscopy and abdominal CT followed by angiography. Radiologic embolization may be sufficient in most cases; however, early surgical intervention may be warranted if control of bleeding is not possible.
References
- 1.Green M.H.A, Johnson C.D, Jamieson N.V. Haemobilia. Br J Surg. 2001;88(6):773–786. doi: 10.1046/j.1365-2168.2001.01756.x. [DOI] [PubMed] [Google Scholar]
- 2.Golich J, Rilinger N, Brado M, Huppert P, Vogel J, Siech M, et al. Non-operative management of arterial liver hemorrhages. Eur Radiol. 1999;9(1):85–88. doi: 10.1007/s003300050633. [DOI] [PubMed] [Google Scholar]
- 3.Merrell S.W, Schneider P.D. Hemobilia: evolution of current diagnosis and treatment. West J Med. 1991;155(6):621–625. [PMC free article] [PubMed] [Google Scholar]
- 4.Fullarton G.M, Bell G. Prospective audit of the introduction of laparoscopic cholecystectomy in the west of Scotland. West of Scotland Laparoscopic Cholecystectomy Audit Group. Gut. 1994;35(8):1121–1126. doi: 10.1136/gut.35.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters J.H, Ellison E.C, Innes J.T, Liss J.L, Nichols K.E, Lomano J.M, et al. Safety and efficacy of laparoscopic cholecystectomy: a prospective analysis of 100 initial patients. Ann Surg. 1991;213(1):3–12. doi: 10.1097/00000658-199101000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart B.T, Abraham R.J, Thomson K.R, Collier N.A. Postcholecystectomy haemobilia: enjoying a renaissance in the laparoscopic era? Aust N Z J Surg. 1995;65(3):185–188. doi: 10.1111/j.1445-2197.1995.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 7.Madanur M.A, Battula N, Sethi H, Deshpande R, Heaton N, Rela M. Pseudoaneurysm following laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2007;6(3):294–298. [PubMed] [Google Scholar]
- 8.England R.E, Marsh P.J, Ashleigh R, Martin D.F. Case report: pseudoaneurysm of the cystic artery: a rare cause of hemobilia. Clin Radiol. 1998;53(1):72–75. doi: 10.1016/s0009-9260(98)80041-x. [DOI] [PubMed] [Google Scholar]
- 9.Delgadillo X, Berney T, Didier D, Morel P. Successful treatment of a pseudoaneurysm of the cystic artery with microcoil embolization. J Vasc Interv Radiol. 1999;10(6):789–792. doi: 10.1016/s1051-0443(99)70116-8. [DOI] [PubMed] [Google Scholar]
- 10.Otah E, Cushin B.J, Rozenblit G.N, Neff R, Otah K.E, Cooperman A.M. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg. 2002;137(1):55–59. doi: 10.1001/archsurg.137.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann A.F. Bile acids: the good, the bad, and the ugly. News Physiol Sci. 1999;14(Feb):24–29. doi: 10.1152/physiologyonline.1999.14.1.24. [DOI] [PubMed] [Google Scholar]
- 12.Croce M.A, Fabian T.C, Spiers J.P, Kudsk K.A. Traumatic hepatic artery pseudoaneurysm with hemobilia. Am J Surg. 1994;168(3):235–238. doi: 10.1016/s0002-9610(05)80193-x. [DOI] [PubMed] [Google Scholar]
- 13.Hagiwara A, Tarui T, Murata A, Matsuda T, Yamaguti Y, Shimazaki S. Relationship between pseudoaneurysm formation and biloma after successful transarterial embolization for severe hepatic injury: permanent embolization using stainless steel coils prevents pseudoaneurysm formation. J Trauma. 2005;59(1):49–55. doi: 10.1097/01.ta.0000171457.18637.69. [DOI] [PubMed] [Google Scholar]
- 14.Stanley J.C, Zelenock G.B. Splanchnic artery aneurysms. In: Rutherford R.B, editor. Vascular Surgery. Philadelphia, PA: Saunders; 1989. pp. 973–975. [Google Scholar]
- 15.Sibulesky L, Ridlen M, Pricolo V.E. Hemobilia due to cystic artery pseudoaneurysm. Am J Surg. 2006;191(6):797–798. doi: 10.1016/j.amjsurg.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Maeda A, Kunou T, Saeki S, Aono K, Murata T, Niinomi N, et al. Pseudoaneurysm of the cystic artery with hemofbilia treated by arterial embolization and elective cholecystectomy. J Hepatobiliary Pancreat Surg. 2002;9(6):755–758. doi: 10.1007/s005340200105. [DOI] [PubMed] [Google Scholar]
- 17.Liu T.T, Hou M.C, Lin H.C, Chang F.Y, Lee S.D. Life-threatening hemobilia caused by hepatic artery pseudoaneurysm: a rare complication of chronic cholangitis. World J Gastroenterol. 2003;9(12):2883–2884. doi: 10.3748/wjg.v9.i12.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]


