Abstract
Proximal gastrectomy (PG) has been introduced for patients who are preoperatively diagnosed with early gastric cancer located in the upper third of the stomach. In the present study, we compared the prognosis of patients who underwent PG with that of patients who underwent total gastrectomy (TG). Between 1997 and 2006, 51 patients were diagnosed with early gastric cancer located in the upper third of the stomach and underwent PG. In the same period, 35 patients were diagnosed with early gastric cancer and underwent TG. Of these, in 24 patients, the cancer was localized in the middle to upper part of the stomach, and 11 patients had multiple cancers. We compared the clinicopathologic differences and prognoses between the two groups. Significantly fewer lymph nodes were dissected in the PG group (mean, 18.2) than in the TG group (mean, 36.6;P < 0.001). Complications were detected in 17.6% of patients in the PG group and in 14.3% of patients in the TG group, which was not significant (P = 0.678). The overall and disease-specific 5-year survival rates in the 51 patients who underwent PG (88.7% and 97.1%, respectively) were not different from those in the 35 patients who underwent TG (87.6% and 93.4%; P = 0.971 and P = 0.553; respectively). These findings indicate that PG can be performed safely and may have various advantages compared with TG in terms of patients' daily lives.
Key words: Early gastric cancer, Proximal gastrectomy, Prognosis, Total gastrectomy
Total gastrectomy (TG) has been widely used as a standard treatment for gastric cancers located in the upper third of the stomach to achieve a sufficient resection margin and more radical lymphadenectomy.1 Recently, however, there has been increased interest in the use of proximal gastrectomy (PG), which can be performed with preservation of the physiologic function of the gastric remnant. Many reports have indicated that PG may be better than TG in terms of the quality of life of patients after gastrectomy.2–4
The principal difference between TG and PG is whether the lymph nodes are dissected radically or not. In patients undergoing PG, the lymph nodes located in the lesser curvature (No. 3) and the right gastroepiploic artery (No. 4d) are not dissected completely. Thus, the radicality of PG is inferior to that of TG in gastric cancer. However, Ooki et al5 reported that, in proximal gastric cancer, if the tumor invasion is limited at the muscularis propria (mp), no patients have metastatic lymph nodes at the right gastroepiploic artery (No. 4d). Thus, in our hospital, the indication for PG is limited to patients who are preoperatively diagnosed with early gastric cancer located in the upper third of the stomach. Thus, the optimal surgical strategy for proximal gastric cancer remains controversial. In the present study, to clarify the clinicopathologic characteristics of patients with gastric cancer who underwent PG, we retrospectively compared the prognosis and complications between patients who underwent PG with those who underwent TG.
Materials and Methods
Patients
Between 1997 and 2006, 51 patients were diagnosed with early gastric cancer located in the upper third of the stomach and underwent PG. In the same period, 35 patients were diagnosed with early gastric cancer and underwent TG. In those who underwent TG, the cancer was located at the middle to upper part of the stomach in 24 patients, and 11 patients had multiple cancers. We enrolled these 86 patients in our study and followed them until June 2010. Preoperative diagnosis of gastric cancer was established by endoscopic and histopathologic examinations. Also, preoperative diagnosis of early stage, without lymph node metastasis, of our cohort was confirmed by the endosonography and the computed tomography (CT).
PG was performed in patients who met the following criteria: (1) early gastric cancer; (2) tumor located in the upper third of the stomach; and (3) no lymph node metastasis. Although this study was a retrospective study and not a randomized study, clinicopathologic differences, including differences in prognosis, were compared between the two groups. Surgical morbidity and mortality rates were defined as any complication or death, respectively, associated with gastrectomy. Patients were followed until June 2010. Any deaths that occurred after surgery, including operative death, and deaths from causes other than cancer, were included in the survival analysis.
Surgical procedure
In patients with early gastric cancer, the greater omentum was preserved, and splenectomy was not performed. In conventional TG, D1 + beta lymphadenectomy was performed for early gastric cancer. The right cardiac (No. 1), the left cardiac (No. 2), lesser curvature (No. 3), along the short gastric vessels (No. 4sa), left gastroepiploic artery (No. 4sb), right gastroepiploic artery (No. 4d), suprapyloric (No. 5), infrapyloric (No. 6), left gastric artery (No. 7), common hepatic artery (No. 8a), celiac artery (No. 9), and suprapancreatic (No. 11p) lymph nodes were excised during TG. The No. 3 and No. 4d lymph nodes were not completely dissected in PG.
Various reconstruction methods were performed after PG or TG. In PG, esophagogastrostomy was performed in 15 patients, double-tract was performed in 5 patients, and jejunal-interposition was performed in 31 patients. In TG, Roux-en-Y was performed in 33 patients, and interposition and double-tract were performed in one patient each.
Clinicopathologic findings
The histopathologic findings, stage classification, depth of tumor invasion, lymph node grouping, and curability of gastric resection were reported according to the Japanese Classification of Gastric Carcinoma.6
Statistical analysis
Chi-squared and Fisher's exact probability tests were used to compare the distribution of individual variables between patient groups. Differences between the two groups were evaluated using the Mann-Whitney U test. The survival rates were estimated by the Kaplan-Meier method, and the statistical differences between survival curves were examined by the log-rank test. P values <0.05 were regarded as statistically significant.
Results
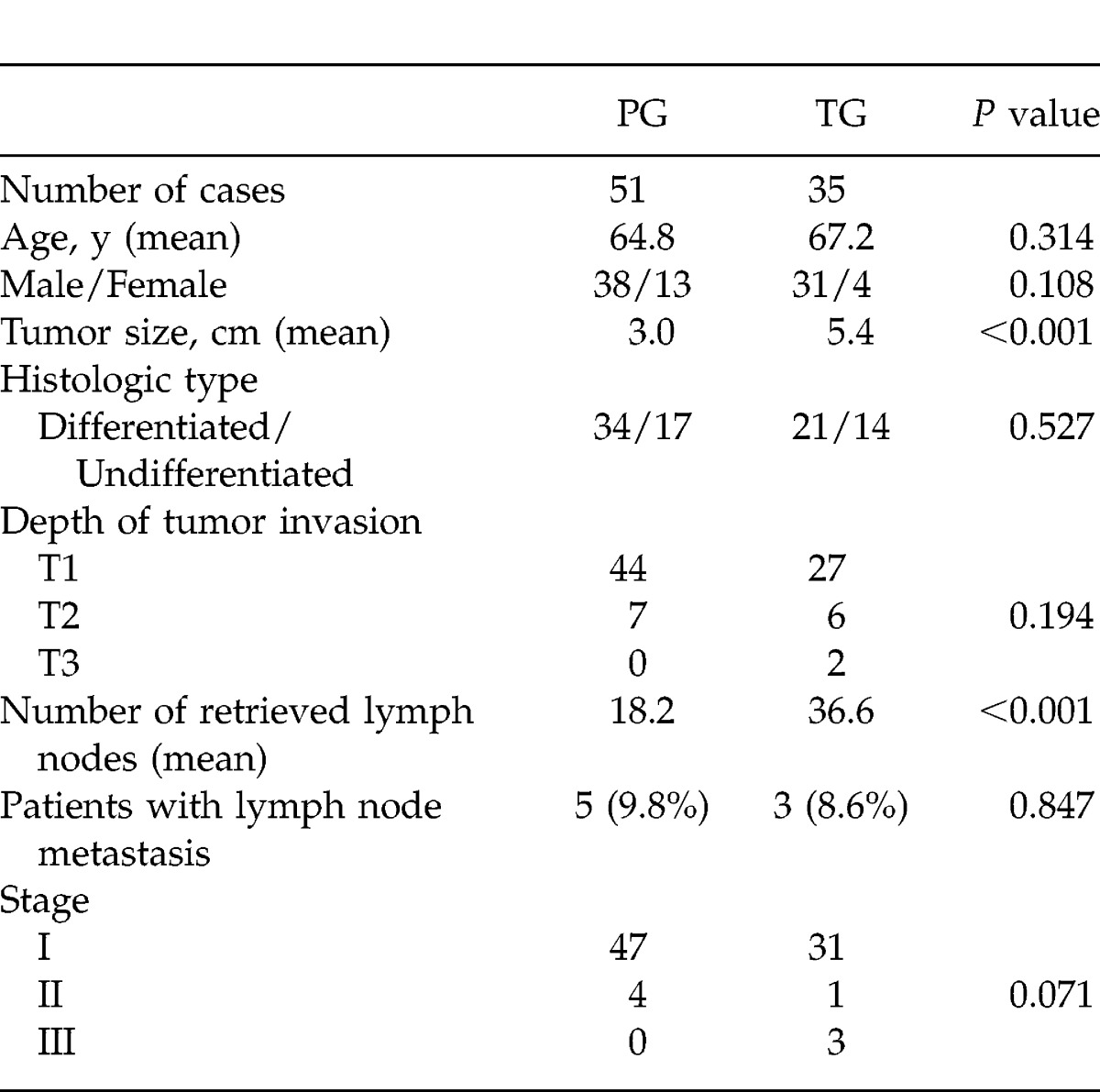
Table 1 shows the differences in clinicopathologic characteristics between the PG group (51 patients) and the TG group (35 patients). In detail, the mean tumor size was larger in the TG group than in the PG group (TG group, 5.4 ± 3.1 cm; PG group, 3.0 ± 1.5 cm; P < 0.001). The mean number of retrieved lymph nodes in the TG group (36.6 ± 17.8) was much higher than that in the PG group (18.2 ± 8.4; P < 0.001). However, the percentage of lymph node metastasis in the PG group (9.8%) was similar to that in the TG group (8.6%; P = 0.847).
Table 1.
Clinicopathological differentiation between PG and TG
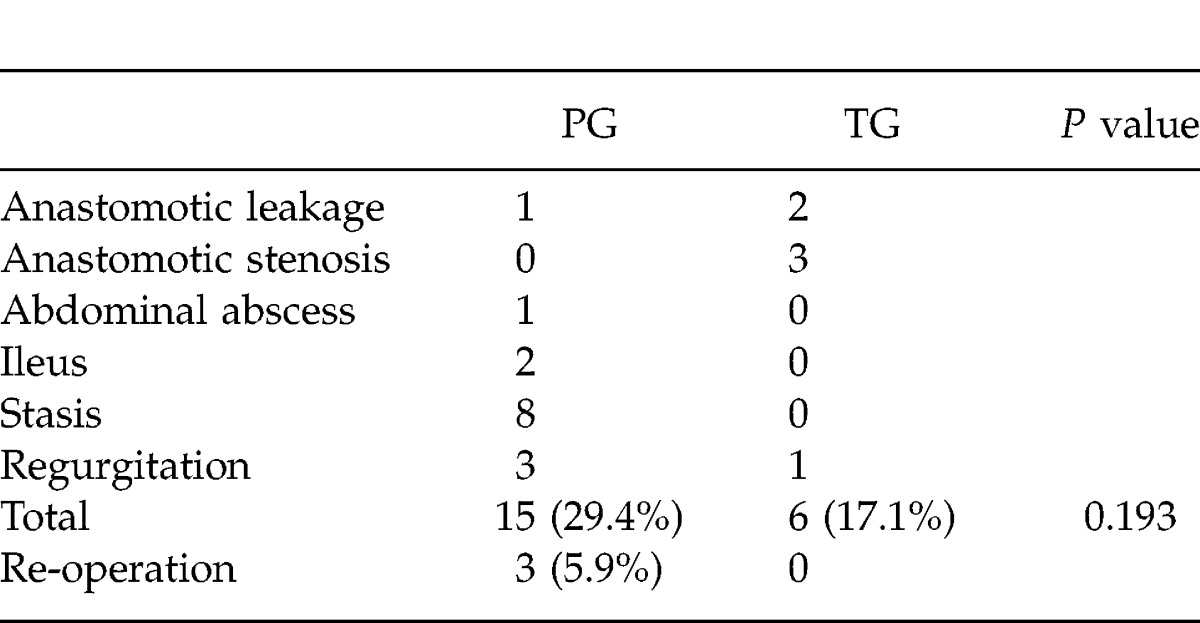
One patient died after PG as a result of operative complications. Complications were detected in 15 of 51 patients (29.4%) after PG; and in 6 of 35 patients (17.1%) after TG. Although the difference was not significant (P = 0.193), complications were more frequently detected after PG than TG (Table 2). Re-operation was performed in 3 patients after PG because of postoperative ileus (2 cases) and intra-abdominal abscess (1 case). Stasis and regurgitation were frequently detected after PG. Stasis was detected in 40% of patients after double-tract reconstruction, in 16.7% of patients after jejunal-interposition reconstruction, and in only 6.7% of patients after esophagogastrostomy. Regurgitation was detected in 13% of patients who underwent esophagogastrostomy.
Table 2.
Complications after gastrectomy
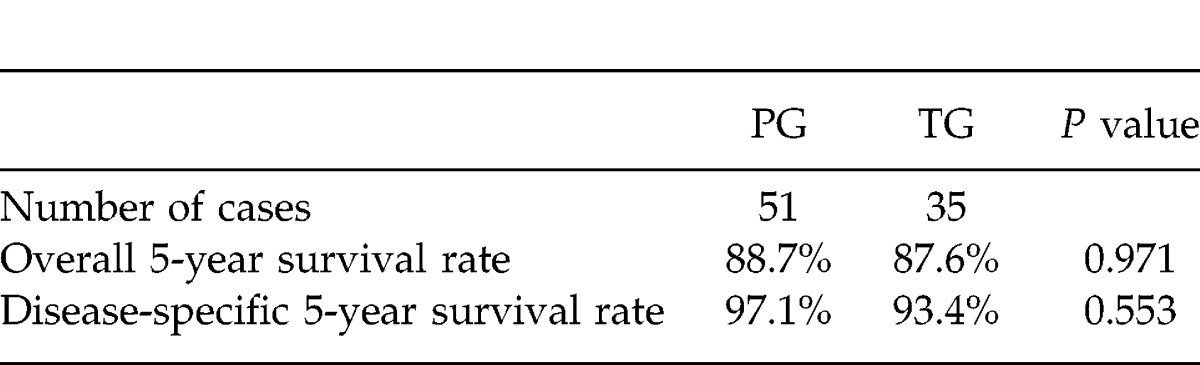
The mean postoperative follow-up period of the PG group (74.2 months) was similar to that of the TG group (82.7 months, P = 0.282). A total of 16 patients died during the follow-up period. In the PG group, 3 patients died from cancer recurrence (5.9%), and 6 patients died from another disease. In the TG group, 4 patients died from cancer recurrence, and 3 patients died from another disease. The overall 5-year survival rate of all 86 patients was 88.3%, and the disease-specific 5-year survival rate was 95.6%. The overall 5-year survival rate of the 51 patients in the PG group (88.7%) was similar to that of the 35 patients in the TG group (87.6%; P = 0.971). The disease-specific 5-year survival rates were 97.1% (PG) and 93.4% (TG), respectively (P = 0.553; Table 3).
Table 3.
Prognosis of the patients
Discussion
The prognosis of patients with advanced gastric cancer located in the upper third of the stomach was reported to be worse than for other sites.7–9 Thus, it is clear that total gastrectomy (TG) with D2 lymphadenectomy is indicated in patients with advanced gastric cancer located in the upper third of the stomach. On the other hand, PG has been introduced for early gastric cancer located in the upper third of the stomach to improve patients' quality of life after gastrectomy.10 Katai and Sano11 reported that PG should only be indicated for early gastric cancer when at least half of the stomach can be preserved to maintain both the curability of the operation and the capacity of the remnant stomach. However, lymph nodes located at the lesser curvature (No. 3) and at the right gastroepiploic artery (No. 4d) are not dissected completely in PG. Thus, the radicality of PG is incomplete. Therefore, the prognostic evaluation of patients after PG is very important. In the present study, we retrospectively evaluated the clinical benefits of PG in patients with early gastric cancer located in the upper third of the stomach. We found that the overall and disease-specific survival rates of patients after PG were similar to those of patients after TG. Kunisaki et al12 reported that the prognosis of early gastric cancer located in the upper third of the stomach was as good as that of gastric cancer in the distal two-thirds of the stomach. These findings indicate that even though the number of dissected lymph nodes is small in PG, this procedure offers high curability rates, provided the indication of PG is limited to early gastric cancer.
However, An et al13 reported that PG was associated with a high rate of complications, and they doubted the clinical benefits of PG in patients with early gastric cancer. Does the function-preserving operation, PG for gastric cancer, have benefits for patients? We need to answer this question. It is well known that patients who underwent gastrectomy for gastric cancer may continue to suffer from various symptomatic nutritional or functional problems.14 However, very few reports have compared the postoperative conditions after TG versus PG. For example, Yoo et al3 reported that PG with jejunal pouch interposition for gastric cancer in the upper third of the stomach showed better nutritional status compared with conventional total gastrectomy. To show the nutritional predominancy of PG, the random examination between PG and TG with one reconstruction type each is needed. The incidence of postoperative complication was higher in PG than in TG in our study. However, the occurrence rate of major complications, such as anastomotic leakage in PG (2%) was similar to that in TG (5.7%). Stasis was frequently detected in double-tract reconstruction or in interposition reconstruction after PG, and reflux esophagitis was mainly detected after esophagogastrostomy. These short-term complications should be followed carefully for long periods.
After PG, there are 3 major reconstructions: esophagogastrostomy, interposition reconstruction with or without a pouch, and double-tract reconstruction. Tokunaga et al15 reported that esophagogastrostomy was a better reconstruction method compared with jejunal-interposition after PG when evaluating subjective symptoms. However, the indication for esophagogastrostomy should be limited to patients in whom a sufficient volume of the residual stomach could be prepared (nearly two-thirds of the stomach). If the volume of the residual stomach is not sufficient, severe reflux of gastric or duodenal juice can occur, and patients will suffer from severe esophagitis postoperatively for many days.16
The incidence of early gastric carcinoma in the upper third of the stomach has recently increased in Japan.11 PG can be performed in such patients, and patients will have a long survival. We now need to prospectively determine which reconstruction method is best for patients after PG.
References
- 1.Papachristou D. N., Fortner J. G. Adenocarcinoma of the gastric cardia: the choice of gastrectomy. Ann Surg. 1980;192(1):58–64. doi: 10.1097/00000658-198007000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaibara N., Nishimura O., Nishidoi H. Proximal gastrectomy as the surgical procedure of choice for upper gastric carcinoma. J Surg Oncol. 1987;36(2):110–112. doi: 10.1002/jso.2930360207. [DOI] [PubMed] [Google Scholar]
- 3.Yoo C. H., Sohn B. H., Han W. K., Pae W. K. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005;29(12):1592–1599. doi: 10.1007/s00268-005-7793-1. [DOI] [PubMed] [Google Scholar]
- 4.Kim J. H., Park S. S., Kim J., Boo Y. J., Kim S. J., Mok Y. J., et al. Surgical outcomes for gastric cancer in the upper third of the stomach. World J Surg. 2006;30(10):1870–1876. doi: 10.1007/s00268-005-0703-8. [DOI] [PubMed] [Google Scholar]
- 5.Ooki A., Yamashita K., Kikuchi S., Sakuramoto S., Katada N., Hutawatari N., et al. Clinical significance of total gastrectomy for proximal gastric cancer. Anticancer Res. 2008;28(5B):2875–2883. [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma – 2nd English edition. Gastric Cancer. 1998;1(1):10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X. F., Huang C. M., Lu H. S., Wu X. Y., Wang C., Guang G. X., et al. Surgical treatment and prognosis of gastric cancer in 2613 patients. World J Gastroenterol. 2004;10(23):3405–3408. doi: 10.3748/wjg.v10.i23.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D. Y., Joo J. K., Ryu S. Y., Park Y. K., Kim Y. J., Kim S. K., et al. Clinicopathological characteristics of patients with proximal third gastric carcinoma. Acta Chir Belg. 2004;104(6):677–682. doi: 10.1080/00015458.2004.11679642. [DOI] [PubMed] [Google Scholar]
- 9.Lamb P., Sivashanmugam T., White M., Irving M., Wayman J., Raimes S., et al. Gastric cancer surgery – a balance of risk and radicality. Ann R Coll Surg Engl. 2008;90(3):235–242. doi: 10.1308/003588408X261546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katai H., Sano T., Fukagawa T., Shinohara H., Sasako M. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2003;90(7):850–853. doi: 10.1002/bjs.4106. [DOI] [PubMed] [Google Scholar]
- 11.Katai H., Sano T. Early gastric cancer: concepts, diagnosis, and management. Int J Clin Oncol. 2005;10(6):375–383. doi: 10.1007/s10147-005-0534-5. [DOI] [PubMed] [Google Scholar]
- 12.Kunisaki C., Akiyama H., Nomura M., Matsuda G., Otsuka Y., Ono H., et al. Surgical outcomes for early gastric cancer in the upper third of the stomach. J Am Coll Surg. 2005;200(1):15–19. doi: 10.1016/j.jamcollsurg.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.An J. Y., Youn H. G., Choi M. G., Noh J. H., Sohn T. S., Kim S., et al. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008;196(4):587–591. doi: 10.1016/j.amjsurg.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 14.Huang C. C., Lien H. H., Wang P. C., Yang J. C., Cheng C. Y., Huang C. S., et al. Quality of life in disease-free gastric adenocarcinoma survivors: impacts of clinical stages and reconstructive surgical procedures. Dig Surg. 2007;24(1):59–65. doi: 10.1159/000100920. [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga M., Hiki N., Ohyama S., Nunobu S., Miki A., Fukunaga T., et al. Effects of reconstruction methods on a patient's quality of life after a proximal gastrectomy: subjective symptoms evaluation using questionnaire survey. Langenbecks Arch Surg. 2009;394(4):637–641. doi: 10.1007/s00423-008-0442-z. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga M., Ohyama S., Hiki N., Hoshino E., Nunobu S., Fukunaga T., et al. Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg. 2008;32(7):1473–1477. doi: 10.1007/s00268-007-9459-7. [DOI] [PubMed] [Google Scholar]


